Abstract
Cell wall-membrane preparations of Escherichia coli, prepared by the ethylenediaminetetraacetic acid-lysozyme method, contain enzymes which catalyze the oxidation of d-alanine and, to a lesser extent, l-alanine into pyruvate and ammonia without the formation of hydrogen peroxide. The kinetic parameters were (i) pH optima of 8.3 to 8.4 for l- and d-alanine and (ii) a Km value of 6.6 ± 0.2 mM for d-alanine. Several coenzymes were without effect when added to the reaction mixture. The participation of d-alanine oxidase in the oxidation of l-alanine was demonstrated. The evidence is based on (i) results of cellular fractionation; (ii) labeling experiments; (iii) inhibition studies with aminooxyacetate and cycloserine; (iv) denaturation experiments; and (v) demonstration of the presence of an active racemase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBIERI P., DI MARCO A., FUOCO L., RUSCONI A. Investigations on the mode of action of cycloserine upon protein synthesis in Escherichia coli. Biochem Pharmacol. 1960 May;3:101–109. doi: 10.1016/0006-2952(60)90026-5. [DOI] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- GREENSTEIN J. P., BIRNBAUM S. M., OTEY M. C. Optical and enzymatic characterization of amino acids. J Biol Chem. 1953 Sep;204(1):307–321. [PubMed] [Google Scholar]
- Gordon A. S., Lombardi F. J., Kaback H. R. Solubilization and partial purification of amino acid-specific components of the D-lactate dehydrogenase-coupled amino acid-transport systems (E. coli-cell membranes-sephadex-detergent-solubilized-vesicles). Proc Natl Acad Sci U S A. 1972 Feb;69(2):358–362. doi: 10.1073/pnas.69.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
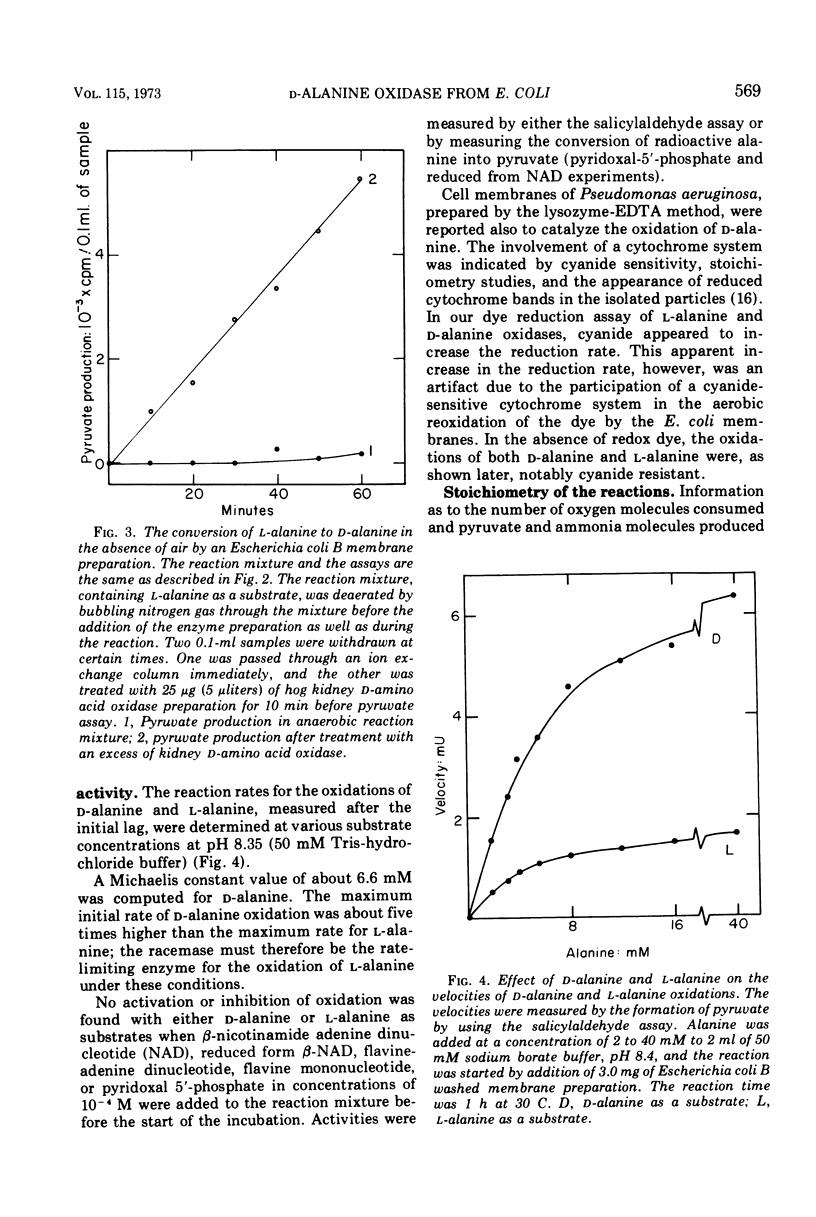
- Gryder R. M., Adams E. Inducible degradation of hydroxyproline in Pseudomonas putida: pathway regulation and hydroxyproline uptake. J Bacteriol. 1969 Jan;97(1):292–306. doi: 10.1128/jb.97.1.292-306.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAKOBY W. B. Aldehyde oxidation. I. Dehydrogenase from Pseudomonas fluorescens. J Biol Chem. 1958 May;232(1):75–87. [PubMed] [Google Scholar]
- KEILIN D., HARTREE E. F. Catalase, peroxidase and metmyoglobin as catalysts of coupled peroxidatic reactions. Biochem J. 1955 Jun;60(2):310–325. doi: 10.1042/bj0600310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M. P., Neuhaus F. C. Factors affecting the level of alanine racemase in Escherichia coli. J Bacteriol. 1972 Mar;109(3):1156–1161. doi: 10.1128/jb.109.3.1156-1161.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGILVERY R. W., MOKRASCH L. C. Purification and properties of fructose-1, 6-diphosphatase. J Biol Chem. 1956 Aug;221(2):909–917. [PubMed] [Google Scholar]
- Martinez-Carrion M., Jenkins W. T. D-Alanine-D-glutamate transaminase. I. Purification and characterization. J Biol Chem. 1965 Sep;240(9):3538–3546. [PubMed] [Google Scholar]
- NORTON J. E., BULMER G. S., SOKATCH J. R. THE OXIDATION OF D-ALANINE BY CELL MEMBRANES OF PSEUDOMONAS AERUGINOSA. Biochim Biophys Acta. 1963 Oct 8;78:136–147. doi: 10.1016/0006-3002(63)91619-6. [DOI] [PubMed] [Google Scholar]
- Neims A. H., Hellerman L. Flavoenzyme catalysis. Annu Rev Biochem. 1970;39:867–888. doi: 10.1146/annurev.bi.39.070170.004251. [DOI] [PubMed] [Google Scholar]
- Raunio R. P., Jenkins W. T. D-alanine oxidase form Escherichia coli: localization and induction by L-alanine. J Bacteriol. 1973 Aug;115(2):560–566. doi: 10.1128/jb.115.2.560-566.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso G., Takashima K., Adams E. Coenzyme content of purified alanine racemase from Pseudomonas. Biochem Biophys Res Commun. 1969 Jan 6;34(1):134–140. doi: 10.1016/0006-291x(69)90539-7. [DOI] [PubMed] [Google Scholar]
- SAITO M. [Assimilatory mechanism of D-amino acid of Mycobacterium avium. I. Oxidation of amino acid]. Kekkaku. 1956 May;31(5):267–271. [PubMed] [Google Scholar]
- Soda K. Microdetermination of D-amino acids and D-amino acid oxidase activity with 3,methyl-2-benzothiazolone hydrazone hydrochloride. Anal Biochem. 1968 Oct 24;25(1):228–235. doi: 10.1016/0003-2697(68)90095-x. [DOI] [PubMed] [Google Scholar]
- VYSHEPAN E. D., IVANOVA K. I., LEDNEVA R. K. [Formation and deamination of alanine in E. coli]. Biokhimiia. 1961 Jul-Aug;26:758–765. [PubMed] [Google Scholar]
- WOOD W. A., GUNSALUS I. C. D-Alanine formation; a racemase in Streptococcus faecalis. J Biol Chem. 1951 May;190(1):403–416. [PubMed] [Google Scholar]



