Abstract
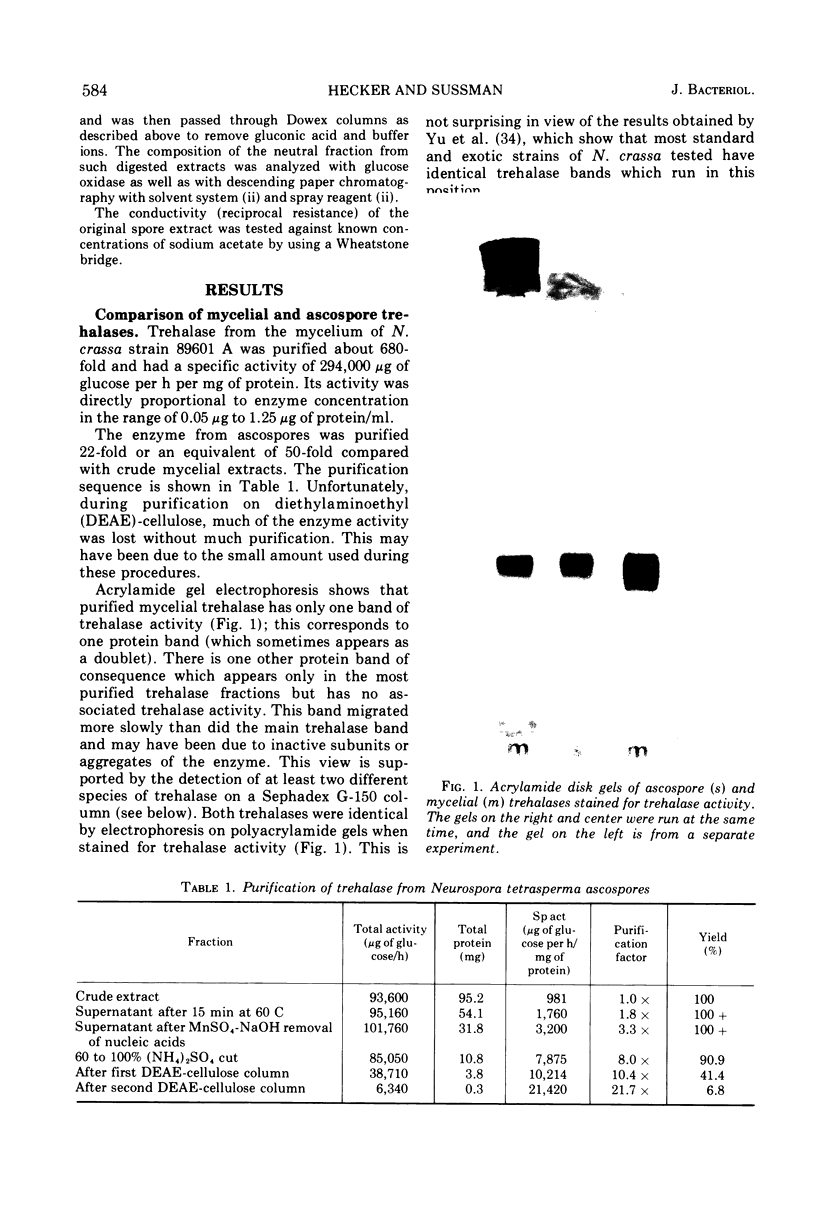
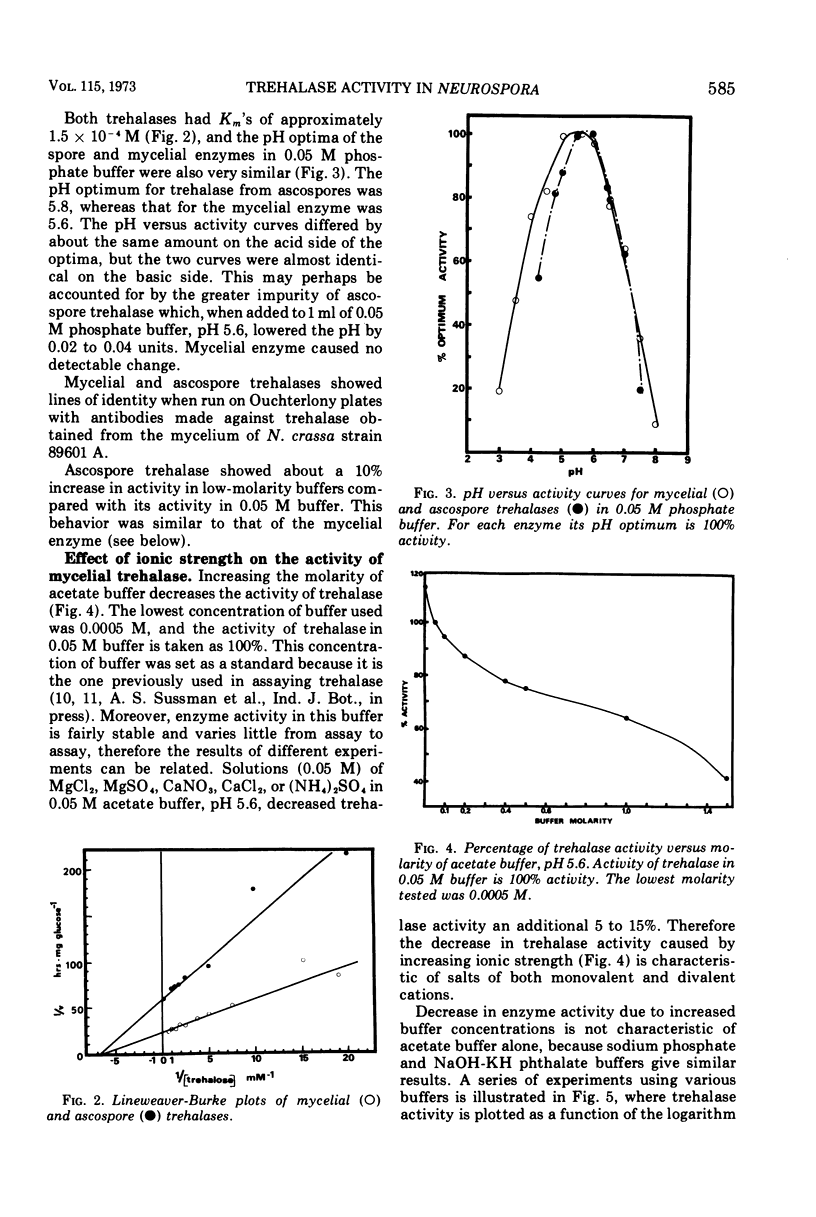
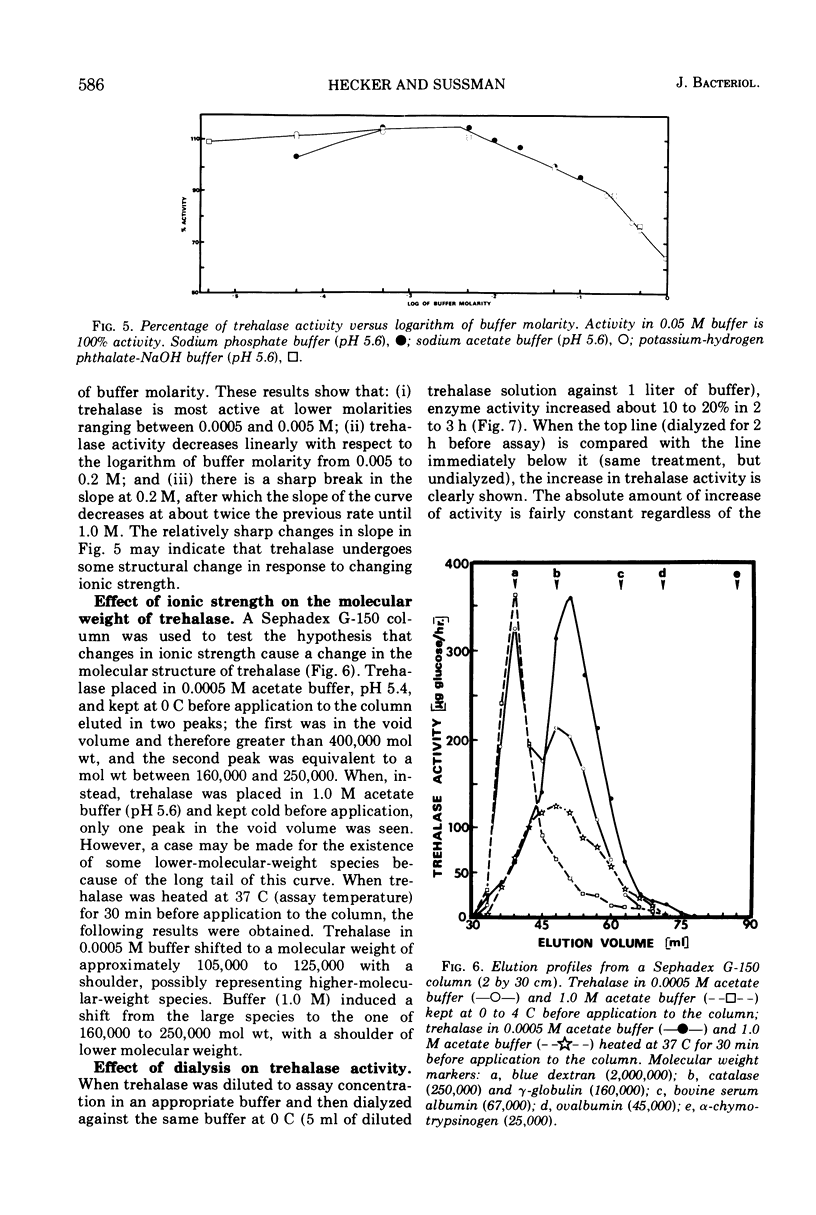
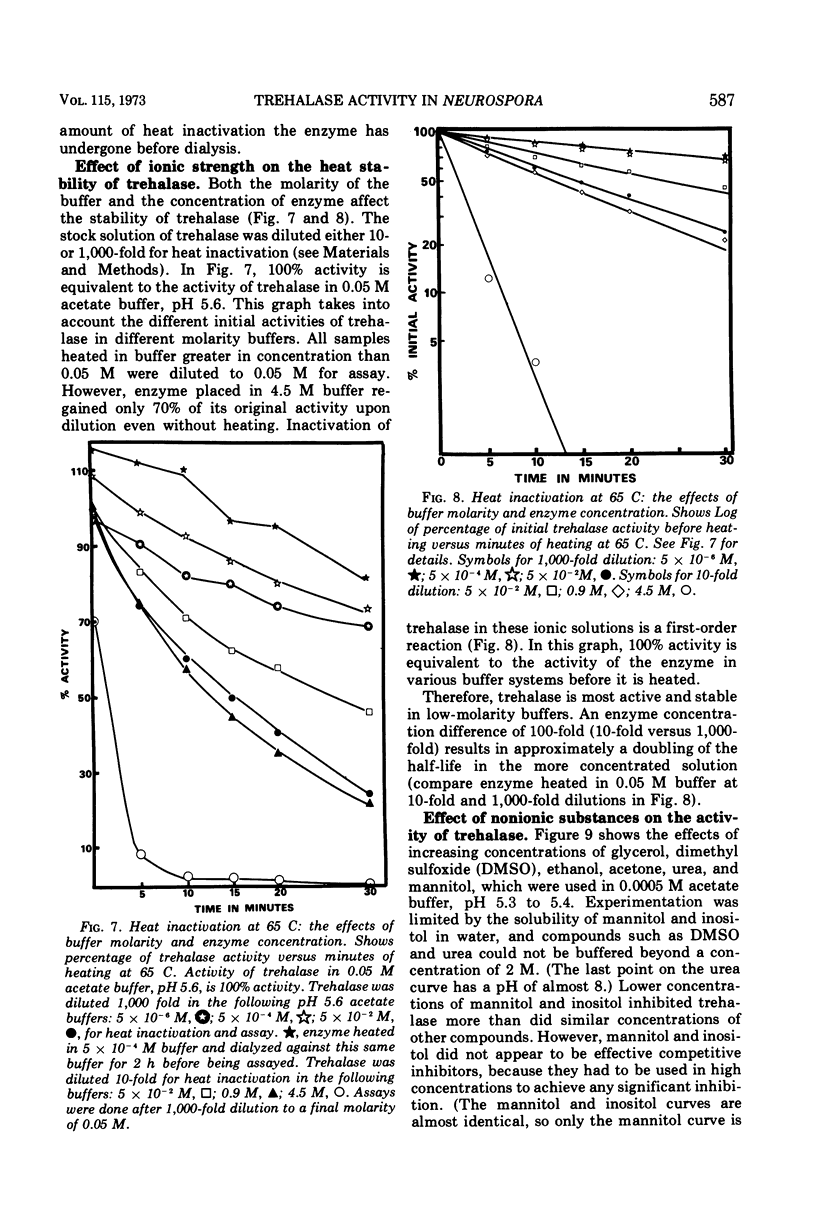
Trehalases from the ascospores of Neurospora tetrasperma and the mycelium of N. crassa were compared. Enzymes from both sources have identical electrophoretic mobilities, Km's, responses to pH, immunological reactions, and activities in low-molarity buffers. Because both enzymes are so similar, conclusions about the properties of the ascospore enzyme may, be made by studying mycelial trehalase. Mycelial trehalase is most active and stable in low-molarity buffers. The enzyme exists in at least three species; the smallest has a molecular weight between 105,000 and 125,000 and is predominant in low-molarity buffers at 37 C. The stability of trehalase to heating at 65 C can be increased by increasing enzyme concentration and by the addition of polyols. Ascospores contain large amounts of trehalose, which protects trehalase from heat inactivation at 65 C. The importance of this phenomenon in vivo and its relationship to the localization of trehalase in ascospores is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Budd K., Sussman A. S., Eilers F. I. Glucose-C14 metabolism of dormant and activated ascospores of Neurospora. J Bacteriol. 1966 Feb;91(2):551–561. doi: 10.1128/jb.91.2.551-561.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung P. L., Trevithick J. R. Biochemical and histochemical localization of invertase in Neurospora crassa during conidial germination and hyphal growth. J Bacteriol. 1970 May;102(2):423–429. doi: 10.1128/jb.102.2.423-429.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELHOCH H. The properties of thyroglobulin. I. The effects of alkali. J Biol Chem. 1960 May;235:1326–1334. [PubMed] [Google Scholar]
- EILERS F. I., ALLEN J., HILL E. P., SUSSMAN A. S. LOCALIZATION OF DISACCHARIDASES IN EXTRACTS OF NEUROSPORA AFTER ELECTROPHORESIS IN POLYACRYLAMIDE GELS. J Histochem Cytochem. 1964 Jun;12:448–450. doi: 10.1177/12.6.448. [DOI] [PubMed] [Google Scholar]
- HILL E. P., SUSSMAN A. S. DEVELOPMENT OF TREHALASE AND INVERTASE ACTIVITY IN NEUROSPORA. J Bacteriol. 1964 Dec;88:1556–1566. doi: 10.1128/jb.88.6.1556-1566.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL E. P., SUSSMAN A. S. PURIFICATION AND PROPERTIES OF TREHALASE (S) FROM NEUROSPORA. Arch Biochem Biophys. 1963 Sep;102:389–396. doi: 10.1016/0003-9861(63)90246-7. [DOI] [PubMed] [Google Scholar]
- Hanks D. L., Sussman A. S. The relation between growth, conidiation and trehalase activity in Neurospora crassa. Am J Bot. 1969 Nov-Dec;56(10):1152–1159. [PubMed] [Google Scholar]
- Hoebeke J. J., Elliott F. G. Dissociation of Pila haemocyanin at low ionic strength. Eur J Biochem. 1971 Nov 11;23(1):171–177. doi: 10.1111/j.1432-1033.1971.tb01605.x. [DOI] [PubMed] [Google Scholar]
- Kaplan J. G., Messmer I. The combined effects of temperature and dilution on the activity and feedback inhibition of yeast aspartate transcarbamylase. Can J Biochem. 1969 Apr;47(4):477–479. doi: 10.1139/o69-074. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lingappa B. T., Sussman A. S. Endogenous Substrates of Dormant, Activated and Germinating Ascospores of Neurospora Tetrasperma. Plant Physiol. 1959 Jul;34(4):466–472. doi: 10.1104/pp.34.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METZENBERG R. L. ENZYMICALLY ACTIVE SUBUNITS OF NEUROSPORA INVERTASE. Biochim Biophys Acta. 1964 Aug 26;89:291–302. doi: 10.1016/0926-6569(64)90217-2. [DOI] [PubMed] [Google Scholar]
- METZENBERG R. L. THE LOCALIZATION OF BETA-FRUCTOFURANOSIDASE IN NEUROSPORA. Biochim Biophys Acta. 1963 Nov 8;77:455–465. doi: 10.1016/0006-3002(63)90521-3. [DOI] [PubMed] [Google Scholar]
- McFeters G. A., Sandine W. E., Becker R. R., Elliker P. R. Some factors affecting association-dissociation of beta-galactosidase from Streptococcus lactis 7962. Can J Microbiol. 1969 Jan;15(1):105–110. doi: 10.1139/m69-016. [DOI] [PubMed] [Google Scholar]
- REITHEL F. J. THE DISSOCIATION AND ASSOCIATION OF PROTEIN STRUCTURES. Adv Protein Chem. 1963;18:123–226. doi: 10.1016/s0065-3233(08)60269-7. [DOI] [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E., CAPUTO A. Studies on the relations between molecular and functional properties of hemoglobin. I. The effect of salts on the molecular weight of human hemoglobin. J Biol Chem. 1961 Feb;236:391–396. [PubMed] [Google Scholar]
- Ray P. M. Sugar composition of oat-coleoptile cell walls. Biochem J. 1963 Oct;89(1):144–150. doi: 10.1042/bj0890144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff H. L. Symposium on bacterial spores: X. Heat resistance of spore enzymes. J Appl Bacteriol. 1970 Mar;33(1):130–140. doi: 10.1111/j.1365-2672.1970.tb05238.x. [DOI] [PubMed] [Google Scholar]
- Sussman A. S., Lingappa B. T. Role of Trehalose in Ascospores of Neurospora Tetrasperma. Science. 1959 Nov 13;130(3385):1343–1343. doi: 10.1126/science.130.3385.1343. [DOI] [PubMed] [Google Scholar]
- Sussman A. S. The dormancy and germination of fungus spores. Symp Soc Exp Biol. 1969;23:99–121. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Yu S. A., Garrett K., Sussman A. S. Genetic control of multiple forms of trehalase in Neurospora crassa. Genetics. 1971 Aug;68(4):473–481. doi: 10.1093/genetics/68.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S. A., Sussman A. S., Wooley S. Mechanisms of protection of trehalase against heat inactivation in Neurospora. J Bacteriol. 1967 Nov;94(5):1306–1312. doi: 10.1128/jb.94.5.1306-1312.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]




