Abstract
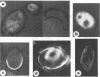
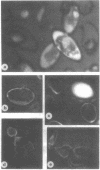
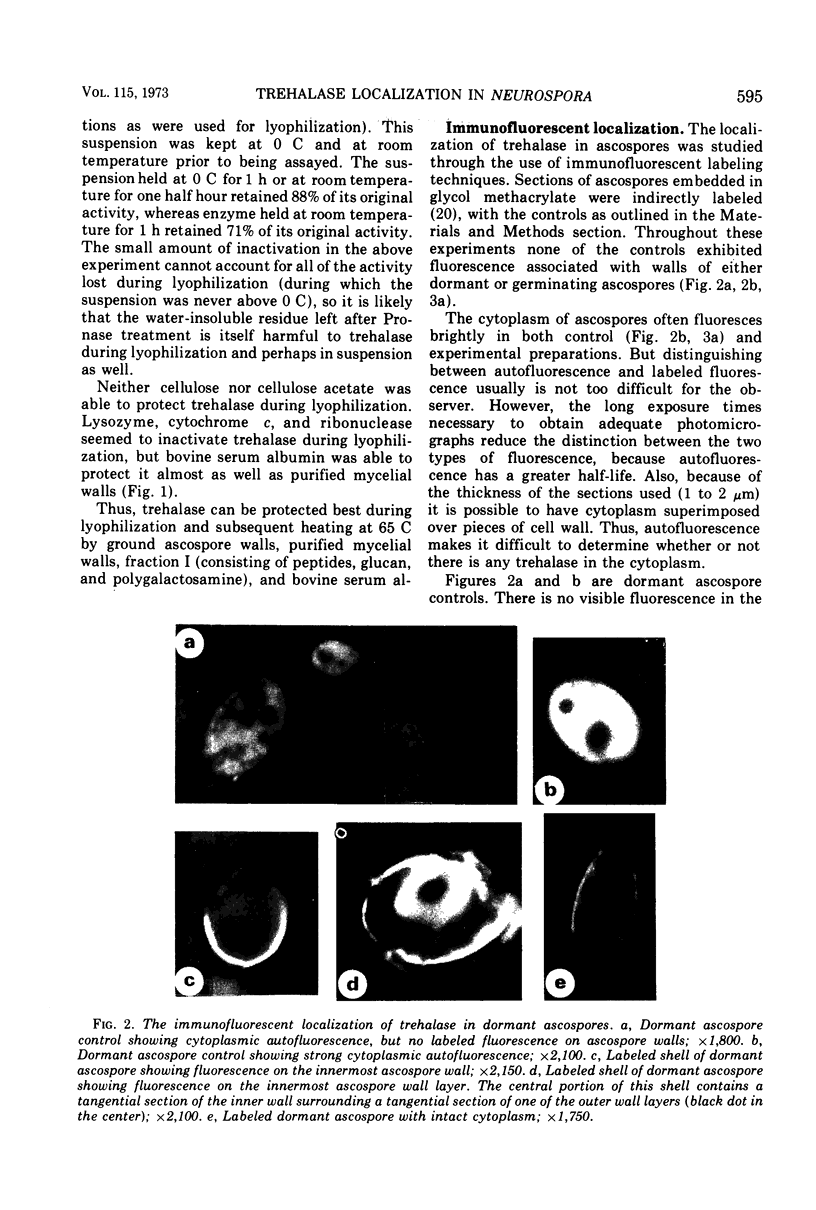
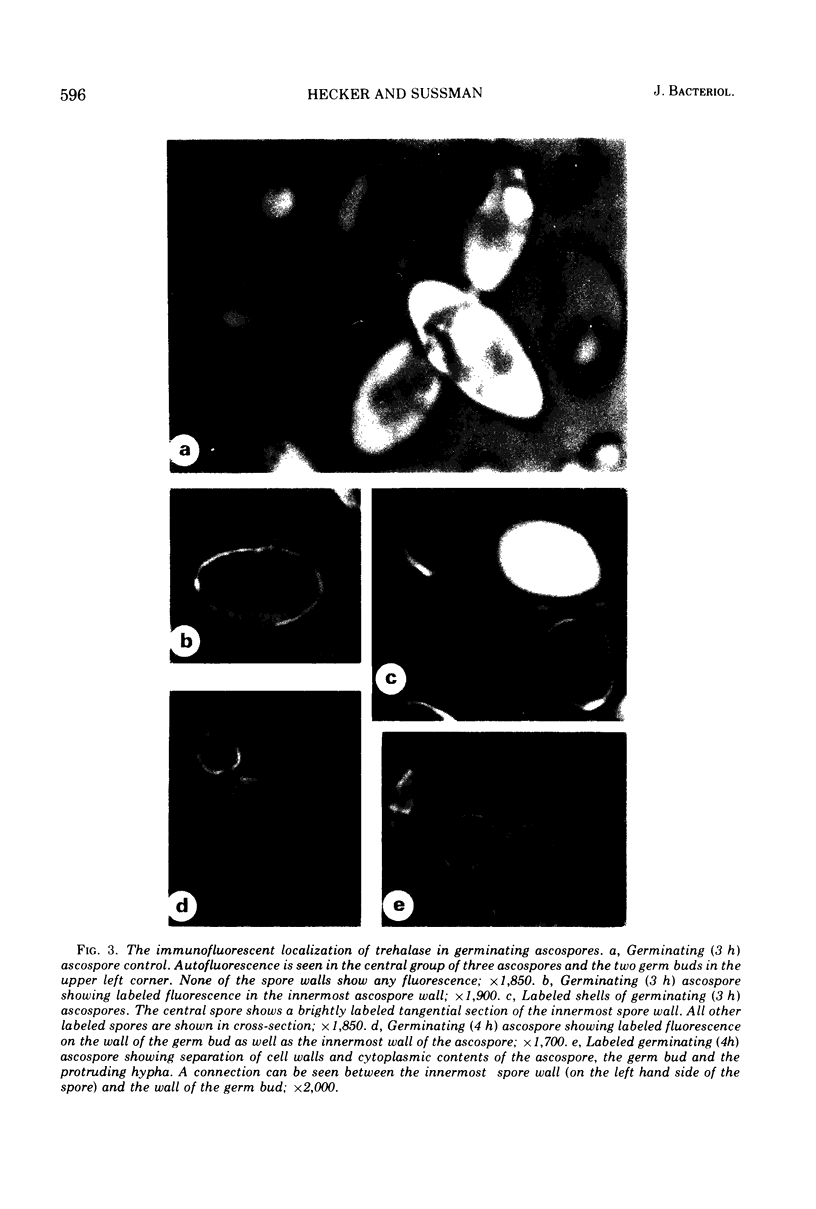
An association of trehalase with the innermost wall (endosporium) of ascospores of Neurospora is suggested, because this enzyme could be lyophilized in the presence of various wall components and heated in this dried state at 65 C without loss of activity. Ground ascospore walls, purified mycelial walls, a wall fraction consisting of protein, glucan and polygalactosamine, or bovine serum albumin stabilize trehalase under these conditions. No other substances tested protected as well as the above materials. Immunofluorescent labeling of trehalase shows that it is localized in the endosporium. Therefore, it is most probable that in dormant ascospores of Neurospora, trehalase, and its substrate, trehalose, are physically separated. Trehalose is located in the cytoplasm, whereas trehalase resides within the protein and carbohydrate matrix of the innermost major cell wall layer of the ascospore. The association with the cell wall protects the enzyme against the heating which is necessary to activate germination. Activation, whether by heat or chemical treatment (furfural), probably involves an increase in the permeability of the ascospore plasma membrane allowing trehalose to diffuse to the vicinity of its hydrolase, thereby providing the energy and intermediates for germination.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buck K. W., Obaidah M. A. The composition of the cell wall of Fusicoccum amygdali. Biochem J. 1971 Nov;125(2):461–471. doi: 10.1042/bj1250461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd K., Sussman A. S., Eilers F. I. Glucose-C14 metabolism of dormant and activated ascospores of Neurospora. J Bacteriol. 1966 Feb;91(2):551–561. doi: 10.1128/jb.91.2.551-561.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung P. L., Trevithick J. R. Biochemical and histochemical localization of invertase in Neurospora crassa during conidial germination and hyphal growth. J Bacteriol. 1970 May;102(2):423–429. doi: 10.1128/jb.102.2.423-429.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A spectrophotometric method for the microdetermination of hexosamines. J Biol Chem. 1950 Jun;184(2):517–522. [PubMed] [Google Scholar]
- Fulcher R. G., Holland A. A. Fluorescent antibody staining of 1-2 mu-m sections of hyphae of Ophiobolus graminis Sacc. embedded in glycol methacrylate. Arch Mikrobiol. 1971;75(4):281–284. doi: 10.1007/BF00407689. [DOI] [PubMed] [Google Scholar]
- HILL E. P., SUSSMAN A. S. DEVELOPMENT OF TREHALASE AND INVERTASE ACTIVITY IN NEUROSPORA. J Bacteriol. 1964 Dec;88:1556–1566. doi: 10.1128/jb.88.6.1556-1566.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks D. L., Sussman A. S. The relation between growth, conidiation and trehalase activity in Neurospora crassa. Am J Bot. 1969 Nov-Dec;56(10):1152–1159. [PubMed] [Google Scholar]
- Hecker L. I., Sussman A. S. Activity and heat stability of trehalase from the mycelium and ascospores of Neurospora. J Bacteriol. 1973 Aug;115(2):582–591. doi: 10.1128/jb.115.2.582-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katchalski E., Silman I., Goldman R. Effect of the microenvironment on the mode of action of immobilized enzymes. Adv Enzymol Relat Areas Mol Biol. 1971;34:445–536. doi: 10.1002/9780470122792.ch7. [DOI] [PubMed] [Google Scholar]
- Lingappa B. T., Sussman A. S. Endogenous Substrates of Dormant, Activated and Germinating Ascospores of Neurospora Tetrasperma. Plant Physiol. 1959 Jul;34(4):466–472. doi: 10.1104/pp.34.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry R. J., Sussman A. S. Ultrastructural changes during germination of ascospores of Neurospora tetrasperma. J Gen Microbiol. 1968 May;51(3):403–409. doi: 10.1099/00221287-51-3-403. [DOI] [PubMed] [Google Scholar]
- Mahadevan P. R., Tatum E. L. Relationship of the major constituents of the Neurospora crassa cell wall to wild-type and colonial morphology. J Bacteriol. 1965 Oct;90(4):1073–1081. doi: 10.1128/jb.90.4.1073-1081.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari R., Sussman A. S. The Nature of Cold-induced Dormancy in Urediospores of Puccinia graminis tritici. Plant Physiol. 1971 Feb;47(2):289–295. doi: 10.1104/pp.47.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandels G. R., Vitols R., Parrish F. W. Trehalose as an endogenous reserve in spores of the fungus Myrothecium verrucaria. J Bacteriol. 1965 Dec;90(6):1589–1598. doi: 10.1128/jb.90.6.1589-1598.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret R. Chimie et morphologie submicroscopique des parois cellulaires de l'ascomycète Chaetomium globosum. Arch Mikrobiol. 1972;81(1):68–90. [PubMed] [Google Scholar]
- Mattingly S. J., Best G. K. Effect of temperature on the integrity of Bacillus psychrophilus cell walls. J Bacteriol. 1972 Feb;109(2):645–651. doi: 10.1128/jb.109.2.645-651.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville M. M., Suskind S. R., Roseman S. A derepressible active transport system for glucose in Neurospora crassa. J Biol Chem. 1971 Mar 10;246(5):1294–1301. [PubMed] [Google Scholar]
- Scarborough G. A. Sugar transport in Neurospora crassa. J Biol Chem. 1970 Apr 10;245(7):1694–1698. [PubMed] [Google Scholar]
- Souza N. O., Panek A. D. Location of trehalase and trehalose in yeast cells. Arch Biochem Biophys. 1968 Apr;125(1):22–28. doi: 10.1016/0003-9861(68)90633-4. [DOI] [PubMed] [Google Scholar]
- Sussman A. S. The dormancy and germination of fungus spores. Symp Soc Exp Biol. 1969;23:99–121. [PubMed] [Google Scholar]