Abstract
It is shown that restoration of photoinduced electron flow and O2 evolution with Mn2+ in Mn-depleted photosystem II (PSII) membrane fragments isolated from spinach chloroplasts is considerably increased with bicarbonate in the region pH 5.0–8.0 in bicarbonate-depleted medium. In buffered solutions equilibrated with the atmosphere (nondepleted of bicarbonate), the bicarbonate effect is observed only at pH lower than the pK of H2CO3 dissociation (6.4), which indicates that HCO3− is the essential species for the restoration effect. The addition of just 2 Mn2+ atoms per one PSII reaction center is enough for the maximal reactivation when bicarbonate is present in the medium. Analysis of bicarbonate concentration dependence of the restoration effect reveals two binding sites for bicarbonate with apparent dissociation constant (Kd) of ≈2.5 μM and 20–34 μM when 2,6-dichloro-p-benzoquinone is used as electron acceptor, while in the presence of silicomolybdate only the latter one remains. Similar bicarbonate concentration dependence of O2 evolution was obtained in untreated Mn-containing PSII membrane fragments. It is suggested that the Kd of 20–34 μM is associated with the donor side of PSII while the location of the lower Kd binding site is not quite clear. The conclusion is made that bicarbonate is an essential constituent of the water-oxidizing complex of PSII, important for its assembly and maintenance in the functionally active state.
Keywords: oxygen evolution, manganese, donor side
Bicarbonate was discovered to stimulate electron flow in Hill reaction in chloroplasts (1). Now is is well established that bicarbonate ion is essential for the maximal activity of photosystem II (PSII) (for recent reviews, see refs. 2 and 3 and references therein). In the early 1970s the water-oxidizing side of PSII was considered as the acting site of bicarbonate (4, 5). Later, strong evidence for location of bicarbonate binding at the acceptor side of PSII between QA and QB, the primary and secondary plastoquinone electron acceptors, was first presented by Wydrzynski and Govindjee (6). This idea was then supported by numerous experimental data (for reviews, see refs. 2 and 3). The nonheme Fe acting between QA and QB seems to play an essential role in bicarbonate binding (7–9). However, it was shown that bicarbonate depletion may effect both the electron acceptor and the donor side of PSII (10). El-Shintinawy and Govindjee found that bicarbonate has two sites of action: the first accelerates the electon flow beyond QA, and the other stimulates it between hydroxilamine donation site (electron donor Z or D) and QA. The site between the primary electron acceptor, pheophytin and QA, was speculated for the latter case (11).
Recently (12–14), bicarbonate requirement for the donor side of PSII was shown. It was found that in O2-evolving PSII membrane fragments, bicarbonate reversible inhibition of electron flow at the donor side requires much lower (1,000 times) concentrations of formate than that at the acceptor side (12–14). The bicarbonate effect was especially pronounced in Mn-depleted PSII preparations—i.e., sufficient reconstitution of electron flow with exogenous Mn2+ (0.1–0.2 μM) was achieved only in the presence of bicarbonate (12, 13). It was suggested that bicarbonate takes part in the formation of the Mn center capable of water oxidation as an obligatory ligand or through modification of the binding site(s) of Mn (12, 13). On the other hand, it was recently shown (15) that formation of the Mn-bicarbonate complexes, Mn(HCO3−)+ and Mn(HCO3−)2, resulted in a considerable lowering of the redox potential for oxidation of Mn2+ to Mn3+ in solution. Therefore, bicarbonate converts the aqua-ions of Mn2+ into a form that can be oxidized much more easily by PSII. It is possible that this effect [rather than formation of the water-oxidizing complex (WOC)] is responsible for the bicarbonate requirement in Mn-depleted PSII (12).
In this paper we provide evidence for a bicarbonate requirement during reassembly of the Mn-containing center capable of water oxidation and O2 evolution. These results were obtained in Mn-depleted PSII preparations that along with the results obtained in Mn-containing preparations show that bicarbonate is an essential constituent of the WOC.
MATERIALS AND METHODS
PSII membrane fragments, designated here as DT-20, were isolated from spinach chloroplasts using 0.4% digitonin and 0.15% Triton X-100 as described (16) with some modifications (17) (pH 6.5 was used instead of pH 7.8). Under saturating illumination in the presence of 0.3 mM phenyl-p-benzoquinone, the DT-20 fragments evolved 140–220 μmol O2/mg chlorophyll (Chl) per hour. They contained 1 PSII reaction center (RC) per 200–220 Chl molecules, and 1 molecule of P700 per 10,000 Chl molecules (16, 17). The BBY [subchloroplast preparation isolated by the method worked out by Berthold, Babcock, and Yocum (18)] PSII membrane fragments were prepared as described (18, 19). A complete (>95%) removal of Mn from the membrane fragments was carried out using 1 M Tris⋅HCl (pH 8.0) plus 0.5 M MgCl2 (17) or N,N,N′,N′-tetramethylethylenediamine (TEMED) (20, 21) treatments. The preparations were stored in liquid nitrogen or at −80°C at a Chl concentration of 10 mg/ml after the addition of 10% glycerol or 0.4 M sucrose to the medium. Removal of bicarbonate from DT-20 or BBY preparations was achieved as described (12, 13), by a 1,000-fold (in case of ΔF measurements; where ΔF is the photoinduced change of Chl fluorescence yield) or a 250-fold (in case of O2-evolution measurements) dilution of concentrated PSII preparations into a medium depleted of endogenous bicarbonate by means of 60-min flushing with air (CO2 depleted by passage through a solution of 50% NaOH and a 20-cm layer of ascarite) or with N2. The sample was subsequently incubated in this medium for 30 min at 4°C.
ΔF values were measured in a closed (sealed) 10-mm cuvette at 20°C using a phosphoroscopic set-up as described (12, 13). The rate of O2 evolution was measured in a tightly closed 3-ml cell using a Clark-type electrode under continuous illumination with white light (2500 μE/m2⋅s) in the presence of 0.5 mM 2,6-dichloro-p-benzoquinone (DCBQ) or 0.1 mM silicomolybdate (SiMo). The slope for the initial 20 s of illumination was used for determination of the rate of O2 evolution. Restoration of O2 evolution in TEMED-treated (Mn-depleted) BBY membrane fragments was done as described (21): after addition of MnCl2 to a sample suspended in the medium containing 5 mM CaCl2, 35 mM NaCl, 0.4 M sucrose, and 50 mM Mes⋅NaOH (pH 5.0–7.0) or 50 mM Tris⋅HCl (pH 7.0–8.0) at Chl concentration of 40 μg/ml, it was incubated in a glass tube for 5 min at 4°C in the dark, then 3 ml of this suspension was put in the O2-evolution measuring cell and incubated for 3 min at 20°C (in the presence or absence of NaHCO3). O2 evolution was measured under the first 1-min illumination by the continuous actinic light. Therefore, we did not separate the process at photoreactivation of the WOC [including the steps of Mn2+ binding, its photooxidation, ligation of the formed Mn3+, and assembly of tetranuclear complex (22–24)] and revealing of its capacity to evolve oxygen. The photoinduced ΔF were measured in buffer containing 50 mM Mes⋅NaOH (pH 5.0–7.0) or 50 mM Tris⋅HCl (pH 7.0–8.0), 35 mM NaCl, and 2 mM MgCl2.
RESULTS
Dependence of Mn-Restored QA Photoreduction on pH and Bicarbonate.
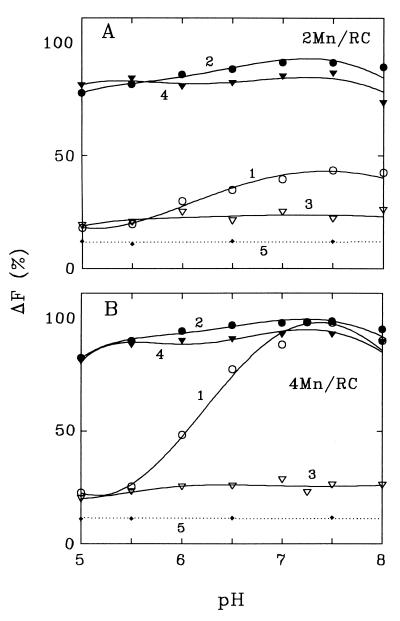
Fig. 1 shows that nearly 15% of the initial photoinduced ΔF related to photoreduction of QA remains after removal of Mn from PSII membrane fragments. At pH 7.0–7.5, MnCl2 added alone (curves 1) at a concentration of 0.1 μM or 0.2 μM (which corresponds to a ratio of 2 Mn/RC or 4 Mn/RC) restores nearly 30% and 80–85% of ΔF, respectively, while at pH 5.0–5.5 only an additional 10–15% of ΔF is restored in both cases. If MnCl2 is added together with 0.4 mM NaHCO3, a 80–90% restoration is observed at all pH for both 2 Mn/RC and 4 Mn/RC conditions (curves 2). Therefore, the stimulating effect of NaHCO3 addition (“bicarbonate effect”) is clearly seen even without special procedures for bicarbonate depletion, and it is especially pronounced at pH lower than the pK for H2CO3 dissociation which is near 6.4. If the Mn-depleted PSII preparations were reactivated with MnCl2 in a medium previously depleted of bicarbonate, then the bicarbonate effect was significant regardless of the pH. In other words, at any pH, the photoinduced ΔF remains equally small if MnCl2 is added in the absence of NaHCO3 and it becomes maximal if MnCl2 is added jointly with 0.4 mM NaHCO3 (Fig. 1, curves 3 and 4).
Figure 1.
Effect of bicarbonate on the amplitude of ΔF in Mn-restored PSII membrane fragments. The membranes (DT-20) were depleted of Mn by washing in 1 M Tris⋅HCl/0.5 M MgCl2 (17). The fragments were then suspended in media at different pH before (curves 5) and after (curves 1–4) addition of MnCl2 at 0.1 μM (2 Mn/RC) (A) or 0.2 μM (4 Mn/RC) (B). For curves 1 and 2, the PSII membranes were suspended in non-bicarbonate-depleted medium. ΔF was measured before (curves 1) and after (curves 2) addition of 0.4 mM NaHCO3. For curves 3 and 4, the membranes were placed in bicarbonate-depleted medium. ΔF was measured before (curves 3) and after (curves 4) addition of 0.4 mM NaHCO3. [Chl] = 10 μg/ml; 20°C. The value of photoinduced ΔF in untreated preparations at a given pH is taken as 100%.
Dependence of Mn-Restored O2 Evolution on pH and Bicarbonate.
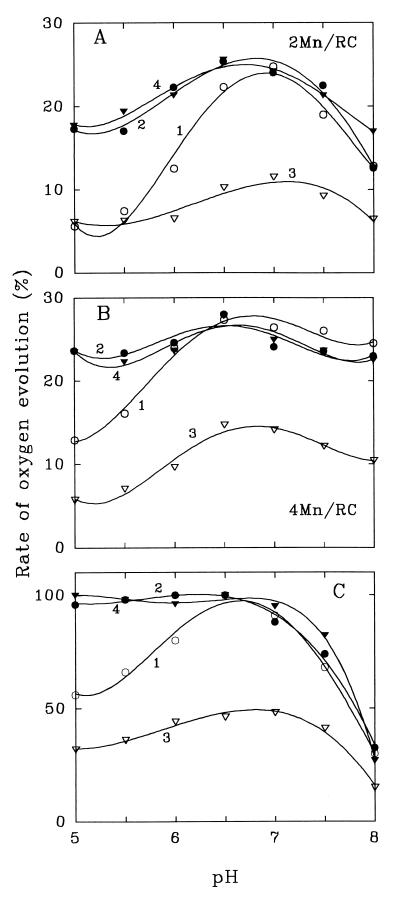
Quite similar effects were found for restoration of the O2-evolving activity in the PSII membrane fragments (Fig. 2 A and B) that had lost this activity as a result of Mn removal. In non-bicarbonate-depleted medium, the restoration was maximal at pH 6.5–7.0 (curves 1); nearly 25% and 30% of the initial activity is restored upon the addition of 2 Mn/RC and 4 Mn/RC, respectively. [These values correspond to a restoration of nearly 70–80% of the activity if the rate of O2 evolution in the BBY membrane fragments that lack the extrinsic proteins 18, 23, and 33 kDa as a result of 1 M CaCl2 treatment (25) is taken as 100%. The treatments we use for Mn depletion also result in removal of these proteins and we did not add them in the reconstitution experiments.] At pH 5.0–5.5 this activity was nearly 2 times lower than that at pH 7.0. However, if 0.4 mM NaHCO3 is added to the medium, the rate of O2 evolution increased (curves 2) and the bicarbonate effect was again especially clear at pH lower than 6.5. Preliminary depletion of bicarbonate from the medium significantly suppressed the restoration of O evolution with Mn2+ at any pH, while subsequent addition of 0.4 mM NaHCO3 again increased it to the nearly same maximal level (curves 3 and 4). On the other hand, in agreement with our earlier data (12), an increase of NaCl concentration in the medium up to 240 mM (instead of NaHCO3 addition) did not cause any restoration of the PSII activities (data not shown).
Figure 2.
Effect of removal and readdition of bicarbonate on the rate of O2 evolution in Mn-restored PSII membrane fragments as a function of pH. BBY membranes were depleted of Mn by TEMED treatment (20, 21). In A, 0.4 μM MnCl2 (2 Mn/RC) was added. In B, 0.8 μM MnCl2 (4 Mn/RC) was added. All samples were given 0.5 mM DCBQ before illumination. Curves 1 and 2, bicarbonate-nondepleted medium, before and after addition of 0.4 mM NaHCO3; curves 3 and 4, bicarbonate-depleted medium, before and after addition of 0.4 mM NaHCO3. [Chl] = 40 μg/ml; 20°C. The rate of O2 evolution in untreated control preparations at pH 6.5 (400 μmol/mg Chl⋅h) is taken as 100%. The rate of O2 evolution in the Mn-depleted preparations was near zero if MnCl2 was not added to the medium. (C) The same pH dependence for O2-evolution rate in untreated (Mn-containing) BBY membrane fragments.
Similar effects were observed for O2 evolution in untreated O2-evolving PSII membranes (Fig. 2C). In the medium equilibrated with air, the rate of O2 evolution was maximal at pH 6.5 [in agreement with a previous observation for PSII preparations (26)], dropped at pH 5–5.5, and it was significantly restored by NaHCO3 addition in this region of pH (curves 1 and 2). In bicarbonate-depleted medium, the stimulating effect of bicarbonate was seen at all pH values (curves 3 and 4).
Dependence of Mn-Restored O2 Evolution and ΔF on Bicarbonate Concentration.
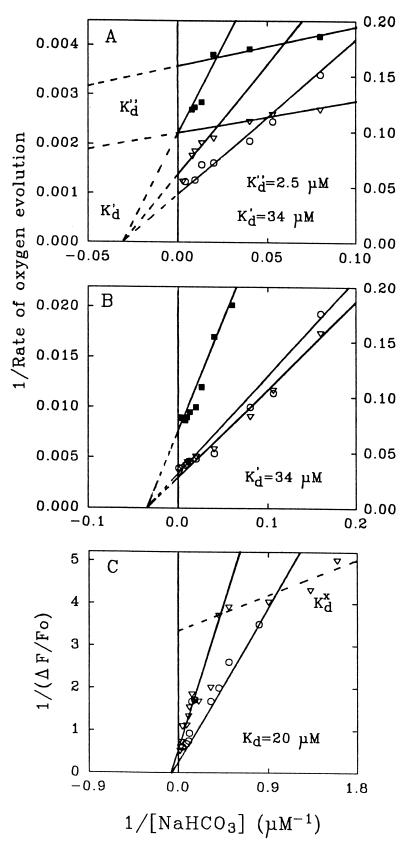
Bicarbonate concentration dependence on PSII activities at pH 5.5 (where the effect was most pronounced) showed that a 50% and nearly 100% recovery of both O2 evolution and ΔF are achieved after addition of 250 and 800 μM NaHCO3, respectively. If the equilibrium concentrations of bicarbonate at pH 5.5 were used, these values corresponded to 30 and 100 μM HCO3−, respectively. Fig. 3 shows the double reciprocal plots of this dependence at pH 5.5. For O2 evolution, it reveals two components with two different apparent dissociation constants (Kd) corresponding to 34 μM and 2.5 μM (K′d and K”d) if DCBQ is added as an electron acceptor and bicarbonate-depleted medium is used (Fig. 3A). When the electron acceptor SiMo and/or the medium equilibrated with the atmosphere are used, then only one component corresponding to Kd′ = 34 μM is revealed (Fig. 3 A and B). The effects were essentially the same for both Mn-depleted (in the presence of 4 μM MnCl2 corresponding to 2 Mn/RC) and untreated (Mn-containing) PSII membrane fragments (Fig. 3 A and B). The double reciprocal plot of ΔF dependence on bicarbonate concentration clearly reveals a component with Kd of 20 μM (Fig. 3C) in the medium both depleted and nondepleted of bicarbonate. Similar Kd (21.4 μM) was found if ΔF were measured after addition of 5 mM CaCl2 and 0.4 M sucrose to the medium (data not shown). A component with a lower (<10 μM) Kd value (Kdx) was also seen in the medium previously depleted of bicarbonate (Fig. 3C), although we could not determine its definitive value.
Figure 3.
Double reciprocal plot of bicarbonate concentration dependence for the O2-evolution rate measured in the presence of 0.5 mM DCBQ (A) or 0.1 mM SiMo (B) as electron acceptors and for the photoinduced ΔF (C) in TEMED- treated (Mn-depleted) BBY membrane fragments after addition of MnCl2 at ratio of 2 Mn/RC at pH 5.5 in non-bicarbonate-depleted (○) and previously depleted (▿) medium (right scales). The same plot for O2 evolution in untreated (Mn-containing) BBY membrane fragments in bicarbonate-depleted medium is given on A and B by ▪ (left scales). A component with Kd lower than 10 μM (we could not determine its definitive value) is designated as Kdx in C. The equilibrium bicarbonate concentration after the addition of an aliquote of NaHCO3 and 3-min incubation at 20°C in the dark in a tightly closed cell was calculated using the equation for the equilibrium between carbonic species in aquous solution at a given pH (27).
Dependence of O2 Evolution and ΔF on Concentration of Added Mn2+.
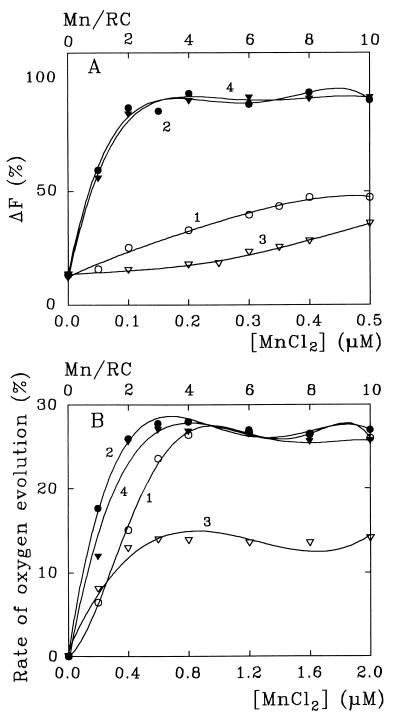
The number of Mn atoms per PSII RC required for maximal restoration of both ΔF and O2 evolution depends on the presence of bicarbonate in the medium (Fig. 4). At pH 6.0 even 10 Mn/RC restore less than 50% of the maximal activity in the absence of NaHCO3 while after the addition of 0.4 mM NaHCO3 just 2 Mn/RC is enough for maximal reactivation of both ΔF and (what is especially important) O2 evolution. The bicarbonate effect is especially accentuated in the medium previously depleted of bicarbonate.
Figure 4.
Magnitude of photoinduced ΔF (A) and the rate of O2 evolution (B) in Mn-depleted (see Figs. 1 and 2) PSII membrane fragments as a function of concentration of added MnCl2 at pH 6.0. Curves 1 and 2, non-bicarbonate-depleted medium, before and after addition of 0.4 mM NaHCO3; curves 3 and 4, bicarbonate-depleted medium, before and after addition of 0.4 mM NaHCO3.
DISCUSSION
The results on reactivation of photoinduced ΔF with Mn2+ in Mn-depleted PSII (Figs. 1 and 4) are in a good agreement with previous suggestions (12, 13) that bicarbonate is an essential component for reconstitution of the Mn center at the donor side of PSII. Our data also show that the stimulating effect of bicarbonate cannot be explained simply by converting Mn2+ into an easier oxidizable form as a result of formation of Mn-bicarbonate complexes, Mn(HCO3−)+ or Mn(HCO3−)2, [which is accompanied by both lowering the redox potential of Mn2+ oxidation (15) and decreasing or losing its positive charges]. This conclusion is drawn from the data (Figs. 2, 3, 4) showing that the reactivation of ΔF upon combined addition of Mn2+ and bicarbonate is accompanied by restoration of O2 evolution. This means that bicarbonate is required to assemble or activate the Mn cluster capable of water oxidation and O2 evolution.
The restoration of O2 evolution and ΔF reactivation by added Mn2+, as measured in non-bicarbonate-depleted media, is pH-dependent (Figs. 1 and 2). It is optimal at pH near pH 6.5–7.0 and considerably decreases at lower pH, in agreement with a previous observation (28). What is new in our present work is that (due to a considerable stimulating effect of bicarbonate) this pH dependence is practically eliminated in the region pH 5.0–7.0 if NaHCO3 is added to the medium. So, bicarbonate stimulates more significantly at pH lower than the pK of H2CO3 dissociation. This indicates that HCO3− (rather than CO2, H2CO3, or CO3−) is the essential species for the reassembly of the Mn cluster. It is noteworthy that similar effects are observed for O2 evolution in untreated (Mn-containing) membrane fragments (Fig. 2C). This shows that the effects are also characteristic of already-assembled O2-evolving complex that loses its activity as a result of bicarbonate removal. Therefore, bicarbonate is necessary for keeping the Mn-containing WOC in the functionally active state. In earlier work done with chloroplasts (29) or Synechocystis cells (30), the bicarbonate effect was much lower at pH 5.0–5.5 than at pH 6.5. It can be explained by the use of high (25–100 mM) concentration of formate in those experiments that evidently can outcompete HCO3− for its binding sites (especially at lower pH values) (31).
Analysis of the bicarbonate concentration dependence of O2-evolution restoration at pH 5.5 (Fig. 3) reveals two binding sites for bicarbonate with Kd of 2.5 μM (high-affinity site) and 34 μM (lower-affinity site). The Kd value of 20 μM revealed from the ΔF reactivation (Fig. 3C) is close to the Kd value of 34 μM found from the O2-evolution measurements and evidently both of them are related to the same (“lower-affinity”) binding site for bicarbonate. This site (with Kd of 20–34 μM) is evidently associated with reactivation of the donor side of PSII since its filling is accompanied by restoration of ΔF (with little or no change in the Fo level). Furthermore, it remains (and its value is not changed) when DCBQ [taking electrons from QA and QB (19, 32)] is replaced by SiMo [accepting electrons from pheophytin and probably from QA (33)]. The Kd value of 80–100 μM (which corresponds to 40–50 μM if equilibrium concentrations of bicarbonate at a given pH are used) was found earlier (34, 35) for bicarbonate binding to the acceptor side of PSII [although the electron transfer between QA and QB seems to occur even when this binding site is empty (36)].
The high-affinity binding site (with Kd of 2.5 μM) is not seen in non-bicarbonate-depleted medium (Fig. 3A). This is consistent with the value of HCO3− concentration in the medium equilibrated with the atmosphere at pH 5.5 (near 2 μM) which is enough to occupy this binding site while the site with a Kd of 20–34 μM can be “filled up” with HCO3− only at higher pH values. This is why the bicarbonate-stimulating effect is clearly seen at low pH even without special procedures to remove bicarbonate from the medium (Figs. 1 and 2). The high-affinity (2.5 μM) binding site revealed from the O2-evolution measurements (Fig. 3) is eliminated upon SiMo replacement for DCBQ (in contrast to the lower-affinity one) which may indicate that this site is related to the acceptor side of PSII (although we realize that the SiMo effect cannot be considered as a strong evidence for this conclusion due to complicated interaction of SiMo with PSII (for review, see refs. 2 and 3 and references therein). A possibility for the existence of such a high-affinity bicarbonate binding site in PSII was suggested earlier (35). The difference in the Kd values, 20–34 μM vs. 2.5 μM, can be responsible for the difference in formate concentrations required for revealing the bicarbonate effects on the donor and acceptor sides of PSII reported earlier (12–14). On the other hand, a binding site for bicarbonate with Kd less than 10 μM (Kdx) is revealed during the ΔF reactivation (Fig. 3C), which can imply that it is associated with the donor side. It is possible that this site is different from the site with Kd of 2.5 μM revealed from the O2-evolution measurements.
It has been shown earlier (21, 28) that 3–4 Mn/RC are required for the photoreactivation of the WOC in Mn-depleted PSII preparations while 2 Mn/RC is enough for reactivation of photoinduced ΔF (12, 17, 21). In our experiments the addition of near 2 Mn/RC is enough to recover both O2 evolution and ΔF (Fig. 4), which is evidently due to a more efficient assembling of the WOC in the presence of bicarbonate.
Acknowledgments
We thank Profs. M. Seibert and A. Stemler for helpful discussion of this work. This work was supported by the Russian Foundation of Basic Research (Grant 96-04-50394) to V.V.K. and by the Dirección General de Investigación Científica y Técnica (Grant PB 92-0125) to R.P. V.V.K. and S.I.A. are most grateful to the Ministerio de Educación y Ciencia of Spain for financial support through its sabbatical program.
ABBREVIATIONS
- PSII
photosystem II
- RC
reaction center
- Chl
chlorophyll
- QA and QB
primary and secondary plastoquinone electron acceptors of PSII
- SiMo
silicomolybdate
- DCBQ
2,6-dichloro-p-benzoquinone
- TEMED
N,N,N′,N′-tetramethylethylenediamine
- ΔF
photoinduced changes of Chl fluorescence yield
- WOC
water-oxidizing complex of PSII
- BBY
PSII membrane fragments isolated by the method worked out by Berthold, Babcock, and Yocum (18)
References
- 1.Warburg O W, Krippahl G. Z Naturforsch B. 1958;13:509–514. [PubMed] [Google Scholar]
- 2.Blubaugh D J, Govindjee Photosynth Res. 1988;19:85–128. doi: 10.1007/BF00114571. [DOI] [PubMed] [Google Scholar]
- 3.Govindjee, van Rensen J J S. In: The Photosynthetic Reaction Center. Deisenhofer J, Norris J R, editors. Vol. 1. New Yrok: Academic; 1993. pp. 357–389. [Google Scholar]
- 4.Stemler A, Govindjee Plant Physiol. 1973;52:119–123. doi: 10.1104/pp.52.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stemler A. Biochim Biophys Acta. 1980;593:103–112. doi: 10.1016/0005-2728(80)90011-0. [DOI] [PubMed] [Google Scholar]
- 6.Wydrzynski T, Govindjee Biochim Biophys Acta. 1975;387:403–408. doi: 10.1016/0005-2728(75)90121-8. [DOI] [PubMed] [Google Scholar]
- 7.Diner B A, Petrouleas V. Biochim Biophys Acta. 1990;1015:141–149. [Google Scholar]
- 8.Petrouleas V, Deligiannakis Y, Diner B A. Biochim Biophys Acta. 1994;1188:271–277. [Google Scholar]
- 9.Hienerwadel R, Berthomieu C. Biochemistry. 1995;34:16288–16297. doi: 10.1021/bi00050a008. [DOI] [PubMed] [Google Scholar]
- 10.Mende D, Weissner W. J Plant Physiol. 1985;118:259–266. doi: 10.1016/S0176-1617(85)80227-3. [DOI] [PubMed] [Google Scholar]
- 11.El-Shintinawy F, Govindjee Photosynth Res. 1990;24:189–200. doi: 10.1007/BF00032306. [DOI] [PubMed] [Google Scholar]
- 12.Klimov V V, Allakhverdiev S I, Feyziev Ya M, Baranov S V. FEBS Lett. 1995;363:251–255. doi: 10.1016/0014-5793(95)00327-6. [DOI] [PubMed] [Google Scholar]
- 13.Klimov V V, Allakhverdiev S I, Baranov S V, Feyziev Ya M. Photosynth Res. 1995;46:219–225. doi: 10.1007/BF00020434. [DOI] [PubMed] [Google Scholar]
- 14.Wincencjusz H, Allakhverdiev S I, Klimov V V, van Gorkom H J. Biochim Biophys Acta. 1996;1273:1–3. [Google Scholar]
- 15.Kozlov, Yu. N., Kazakova, A. A. & Klimov, V. V. (1996) Biol. Membr. (Moscow), in press.
- 16.Shutilova, N. I., Klimov, V. V., Shuvalov, V. A. & Kutyurin, V. M. (1975) Biofizika (Russian) 20, 844–847. [PubMed]
- 17.Klimov V V, Allakhverdiev S I, Shuvalov V A, Krasnovski A A. FEBS Lett. 1982;148:307–312. doi: 10.1016/0014-5793(82)80830-2. [DOI] [PubMed] [Google Scholar]
- 18.Berthold D A, Babcock G T, Yocum C F. FEBS Lett. 1981;134:231–234. [Google Scholar]
- 19.Yruela I, Montoya G, Alonso P J, Picorel R. J Biol Chem. 1991;266:22847–22850. [PubMed] [Google Scholar]
- 20.Ananyev G M, Wydrzynski T, Renger G, Klimov V V. Biochim Biophys Acta. 1992;1100:303–311. [Google Scholar]
- 21.Allakhverdiev S I, Karacan M, Somer G, Karacan N, Khan E M, Rane S Y, Padhye S, Klimov V V, Renger G. Biochemistry. 1994;33:12210–12214. doi: 10.1021/bi00206a025. [DOI] [PubMed] [Google Scholar]
- 22.Tamura N, Cheniae G M. Biochim Biophys Acta. 1987;890:179–194. [Google Scholar]
- 23.Miller A F, Brudvig G M. Biochemistry. 1990;29:1385–1392. doi: 10.1021/bi00458a007. [DOI] [PubMed] [Google Scholar]
- 24.Miyao-Tokutomi M, Inoue Y. Biochemistry. 1992;31:526–532. doi: 10.1021/bi00117a032. [DOI] [PubMed] [Google Scholar]
- 25.Ono T, Inoue Y. FEBS Lett. 1983;164:225–260. [Google Scholar]
- 26.Seibert M, Lavorel J. Biochim Biophys Acta. 1983;723:160–168. [Google Scholar]
- 27.Blubaugh D J, Govindjee Biochim Biophys Acta. 1986;848:147–152. doi: 10.1016/0005-2728(86)90170-2. [DOI] [PubMed] [Google Scholar]
- 28.Chen Ch, Cheniae G M. In: Photosynthesis: From Light to Biosphere. Mathis P, editor. I. Dordrecht, The Netherlands: Kluwer; 1995. pp. 329–332. [Google Scholar]
- 29.Khanna R, Govindjee, Wydrzynski T. Biochim Biophys Acta. 1977;462:208–214. doi: 10.1016/0005-2728(77)90203-1. [DOI] [PubMed] [Google Scholar]
- 30.Cao J, Govindjee . In: Current Research in Photosynthesis. Baltscheffsky H, editor. Vol. 1. Dordrecht, The Netherlands: Kluwer; 1990. pp. 515–518. [Google Scholar]
- 31.Robinson H H, Eaton-Rye J J, van Rensen J J S, Govindjee Z Naturforsch C. 1984;39:382–385. [Google Scholar]
- 32.Cao J, Govindjee Biochim Biophys Acta. 1990;1015:180–188. doi: 10.1016/0005-2728(90)90018-y. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi Y, Satoh K, Itoh S. FEBS Lett. 1989;255:133–138. [Google Scholar]
- 34.Snel J F H, van Rensen J J S. Plant Physiol. 1984;75:146–150. doi: 10.1104/pp.75.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jursinic P A, Stemler A. Photochem Photobiol. 1986;43:205–212. [Google Scholar]
- 36.Jursinic P A, Stemler A. Biochim Biophys Acta. 1992;1098:359–367. [Google Scholar]