Abstract
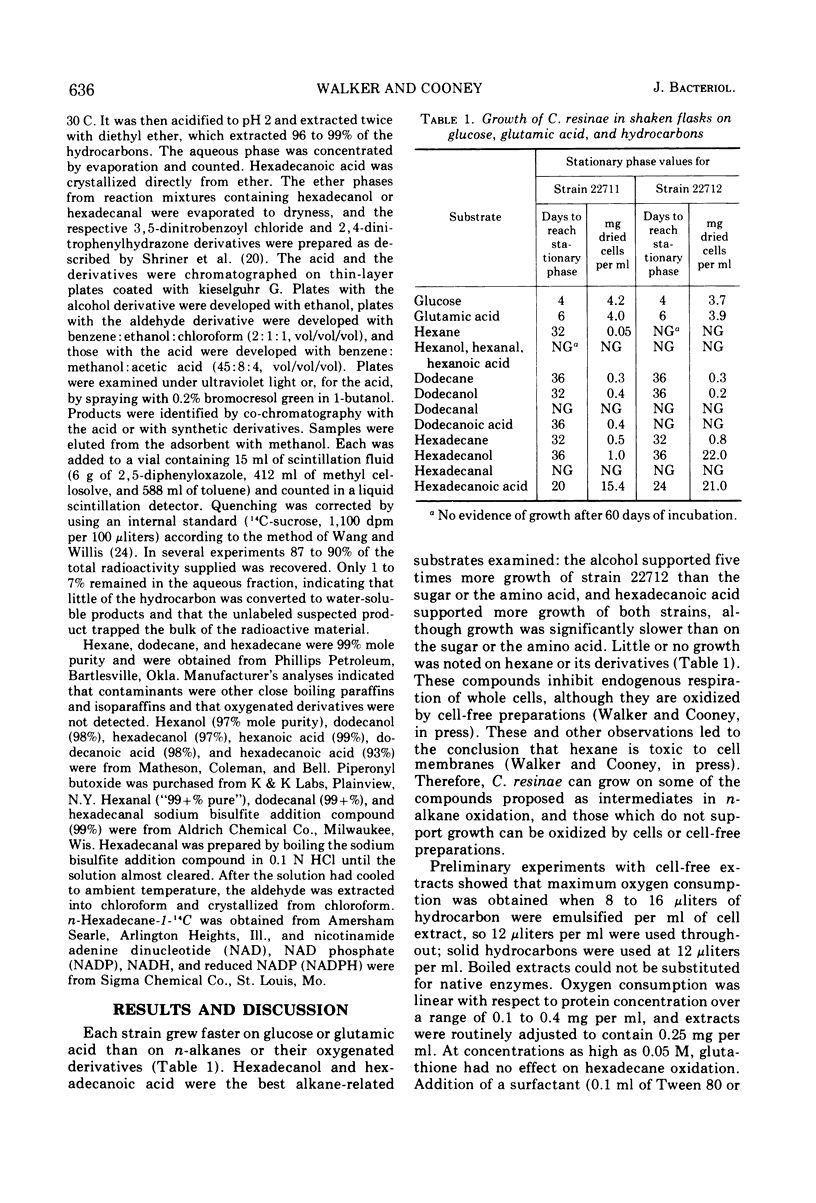
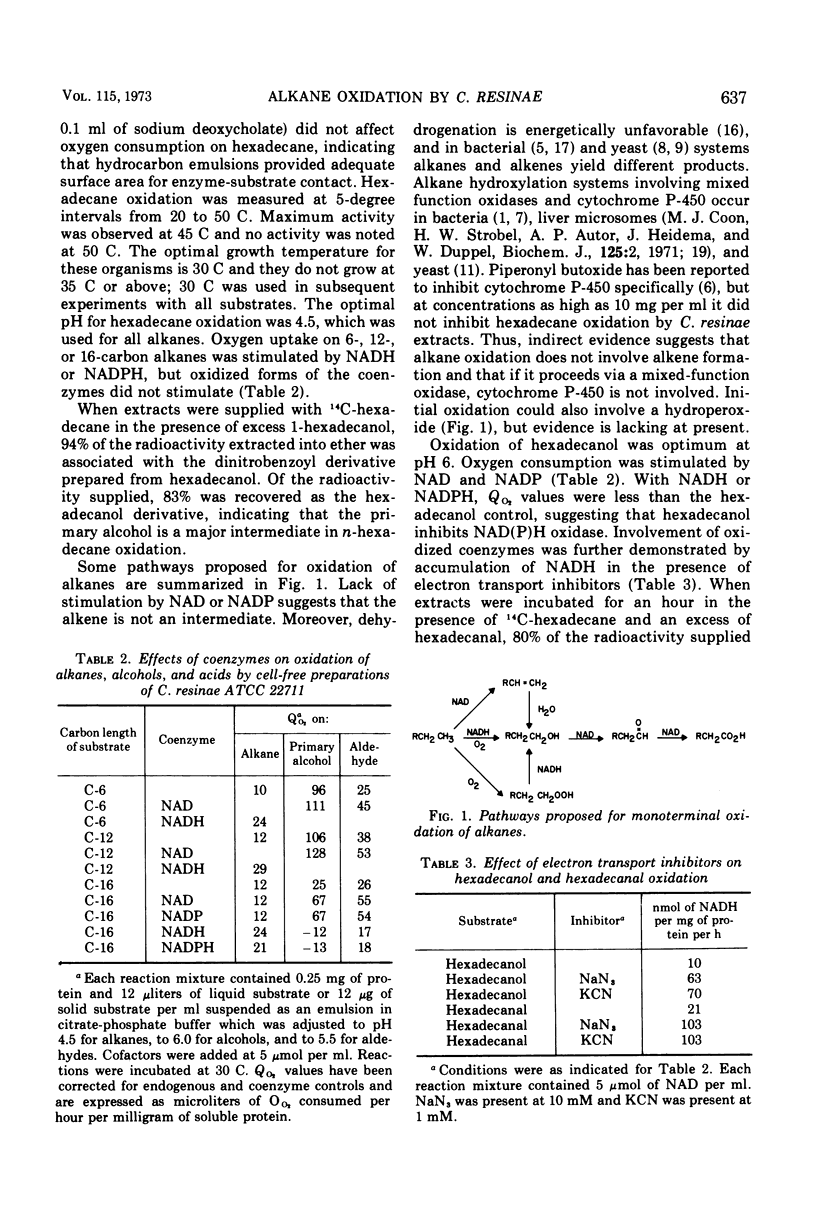
Pathways of initial oxidation of n-alkanes were examined in two strains of Cladosporium resinae. Cells grow on dodecane and hexadecane and their primary alcohol and monoic acid derivatives. The homologous aldehydes do not support growth but are oxidized by intact cells and by cell-free preparations. Hexane and its derivatives support little or no growth, but cell extracts oxidize hexane, hexanol, and hexanal. Alkane oxidation by extracts is stimulated by reduced nicotinamide adenine dinucleotide (phosphate). Alcohol and aldehyde oxidation are stimulated by nicotinamide adenine dinucleotide (phosphate), and reduced coenzymes accumulate in the presence of cyanide or azide. Extracts supplied with 14C-hexadecane convert it to the alcohol, aldehyde, and acid. Therefore, the major pathway for initial oxidation of n-alkanes is via the primary alcohol, aldehyde, and monoic acid, and the system can act on short-, intermediate-, and long-chain substrates. Thus, filamentous fungi appear to oxidize n-alkanes by pathways similar to those used by bacteria and yeasts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cardini G., Jurtshuk P. Cytochrome P-450 involvement in the oxidation of n-octane b cell-free extracts of Corynebacterium sp. strain 7E1C. J Biol Chem. 1968 Nov 25;243(22):6070–6072. [PubMed] [Google Scholar]
- Cofone L., Jr, Walker J. D., Cooney J. J. Utilization of hydrocarbons by Cladosporium resinae. J Gen Microbiol. 1973 May;76(1):243–246. doi: 10.1099/00221287-76-1-243. [DOI] [PubMed] [Google Scholar]
- Cooney J. J., Proby C. M. Fatty acid composition of Cladosporium resinae grown on glucose and on hydrocarbons. J Bacteriol. 1971 Nov;108(2):777–781. doi: 10.1128/jb.108.2.777-781.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUYBREGTSE R., VANDERLINDEN A. C. THE OXIDATION OF ALPHA-OLEFINS BY A PSEUDOMONAS. REACTIONS INVOLVING THE DOUBLE BOND. Antonie Van Leeuwenhoek. 1964;30:185–196. doi: 10.1007/BF02046725. [DOI] [PubMed] [Google Scholar]
- Jaffe H., Fujii K., Guerin H., Sengupta M., Epstein S. S. Bi-modal effect of piperonyl butoxide on the o- and p-hydroxylations of biphenyl by mouse liver microsomes. Biochem Pharmacol. 1969 May;18(5):1045–1051. doi: 10.1016/0006-2952(69)90108-7. [DOI] [PubMed] [Google Scholar]
- Katagiri M., Ganguli B. N., Gunsalus I. C. A soluble cytochrome P-450 functional in methylene hydroxylation. J Biol Chem. 1968 Jun 25;243(12):3543–3546. [PubMed] [Google Scholar]
- Klug M. J., Markovetz A. J. Degradation of hydrocarbons by members of the genus Candida 3. Oxidative intermediates from 1-hexadecene and 1-heptadecene by Candida lipolytica. J Bacteriol. 1968 Oct;96(4):1115–1123. doi: 10.1128/jb.96.4.1115-1123.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug M. J., Markovetz A. J. Degradation of hydrocarbons by members of the genus Candida. II. Oxidation of n-alkanes and l-alkenes by Candida lipolytica. J Bacteriol. 1967 Jun;93(6):1847–1852. doi: 10.1128/jb.93.6.1847-1852.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug M. J., Markovetz A. J. Utilization of aliphatic hydrocarbons by micro-organisms. Adv Microb Physiol. 1971;5:1–43. doi: 10.1016/s0065-2911(08)60404-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lebeault J. M., Lode E. T., Coon M. J. Fatty acid and hydrocarbon hydroxylation in yeast: role of cytochrome P-450 in Candida tropicalis. Biochem Biophys Res Commun. 1971 Feb 5;42(3):413–419. doi: 10.1016/0006-291x(71)90386-x. [DOI] [PubMed] [Google Scholar]
- Lebeault J. M., Meyer F., Roche B., Azoulay E. Oxydation des alcools supérieurs chez Candida tropicalis cultivé sur hydrocarbures. Biochim Biophys Acta. 1970 Dec 16;220(3):386–395. doi: 10.1016/0005-2744(70)90270-6. [DOI] [PubMed] [Google Scholar]
- Lebeault J. M., Roche B., Duvnjak Z., Azoulay E. Alcool-et aldéhyde-déshydrogénases particulaires de Candida tropicalis cultivé sur hydrocarbures. Biochim Biophys Acta. 1970 Dec 16;220(3):373–385. doi: 10.1016/0005-2744(70)90269-x. [DOI] [PubMed] [Google Scholar]
- Lebeault J. M., Roche B., Duvnjak Z., Azoulay E. Isolation and study of the enzymes involved in the metabolism of hydrocarbons by Candida tropicalis. Arch Mikrobiol. 1970;72(2):140–153. doi: 10.1007/BF00409520. [DOI] [PubMed] [Google Scholar]
- Markovetz A. J., Klug M. J., Forney F. W. Oxidation of 1-tetradecene by Pseudomonas aeruginosa. J Bacteriol. 1967 Apr;93(4):1289–1293. doi: 10.1128/jb.93.4.1289-1293.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna E. J., Kallio R. E. The biology of hydrocarbons. Annu Rev Microbiol. 1965;19:183–208. doi: 10.1146/annurev.mi.19.100165.001151. [DOI] [PubMed] [Google Scholar]
- van der Linden A. C., v Ravenswaay Claasen J. C. Hydrophobic enzymes in hydrocarbon degradation. Lipids. 1971 Jul;6(7):437–443. doi: 10.1007/BF02531225. [DOI] [PubMed] [Google Scholar]



