Abstract
Eleven succinate-accumulating mutants of Bacillus subtilis have been mapped by transformation and transduction crosses and characterized with respect to activities of citric acid cycle enzymes. These mutants could be divided into three genetic groups. Nine of the mutants were found to map between argA and leu in the citF locus. A second group was located between lys-1 and trpC2 and the third group could not be located on the B. subtilis chromosome in extensive transduction crosses. All of the citF mutants lack detectable succinate dehydrogenase activity, whereas both of the other groups show a reduced level of this enzyme. In addition, most of the mutants in the citF locus lack cytochrome a, whereas the level of this cytochrome is normal in the other two groups. A procedure has been devised for the solubilization of the succinate dehydrogenase from the membrane of B. subtilis with the non-ionic detergent Brij 58. Some properties of the soluble and bound forms of succinate dehydrogenase are described.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsell D. C., Cota-Robles E. H. Electron transport particles released upon lysis of spheroplasts of Escherichia coli B by Brij 58. Biochim Biophys Acta. 1970 Sep 1;216(2):250–261. doi: 10.1016/0005-2728(70)90216-1. [DOI] [PubMed] [Google Scholar]
- Bishop D. G., Rutberg L., Samuelsson B. The chemical composition of the cytoplasmic membrane of Bacillus subtilis. Eur J Biochem. 1967 Nov;2(4):448–453. doi: 10.1111/j.1432-1033.1967.tb00158.x. [DOI] [PubMed] [Google Scholar]
- Bishop D. G., Rutberg L., Samuelsson B. The solubilization of the cytoplasmic membrane of Bacillus subtilis by sodium dodecyl sulphate. Eur J Biochem. 1967 Nov;2(4):454–459. doi: 10.1111/j.1432-1033.1967.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Boll M. Action of sodium dodecyl sulfate on electron transport enzymes of Rhodospirillum rubrum. Experientia. 1970 Sep 26;26(9):956–957. doi: 10.1007/BF02114127. [DOI] [PubMed] [Google Scholar]
- Bott K. F., Wilson G. A. Development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1967 Sep;94(3):562–570. doi: 10.1128/jb.94.3.562-570.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
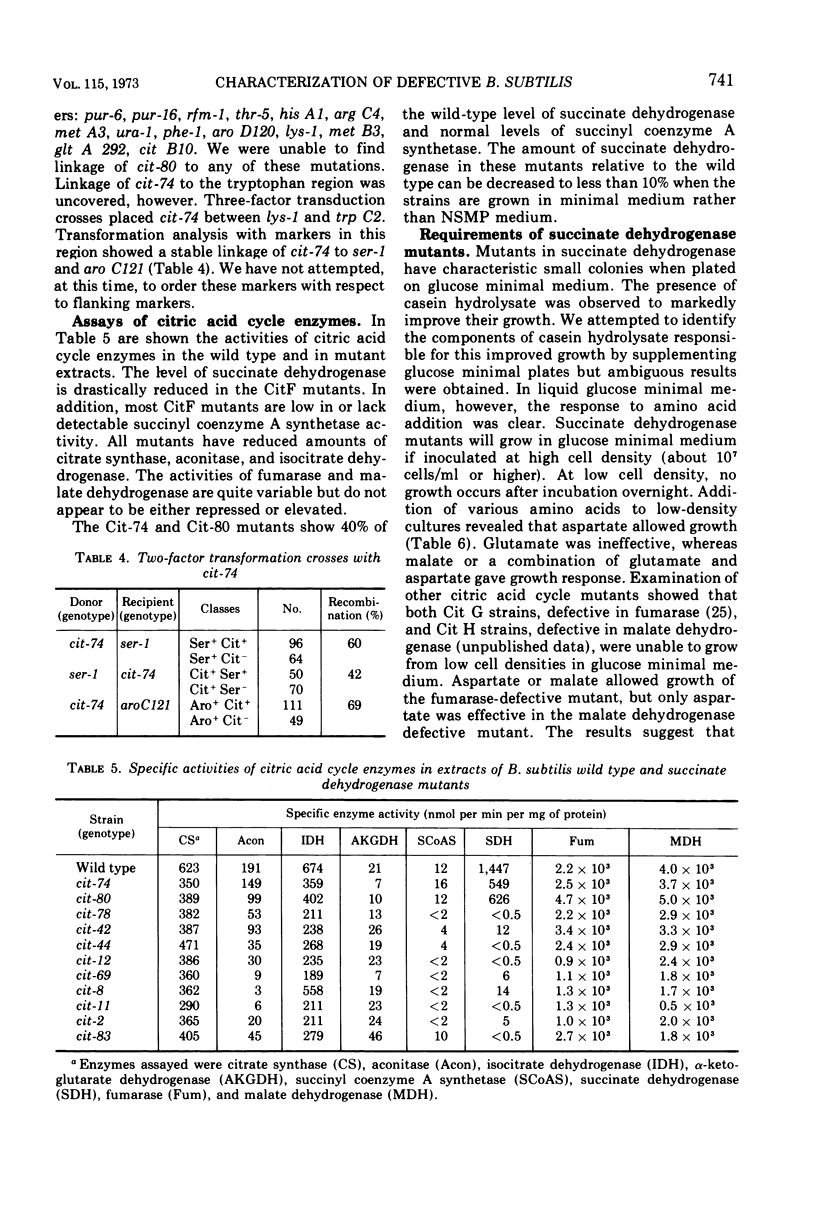
- Carls R. A., Hanson R. S. Isolation and characterization of tricarboxylic acid cycle mutants of Bacillus subtilis. J Bacteriol. 1971 Jun;106(3):848–855. doi: 10.1128/jb.106.3.848-855.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton B. C. Fine-structure mapping by transformation in the tryptophan region of Bacillus subtilis. J Bacteriol. 1966 May;91(5):1795–1803. doi: 10.1128/jb.91.5.1795-1803.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLS H. A. A colorimetric method for the assay of soluble succinic dehydrogenase and pyridinenucleotide-linked dehydrogenases. Arch Biochem Biophys. 1959 Dec;85:561–562. doi: 10.1016/0003-9861(59)90527-2. [DOI] [PubMed] [Google Scholar]
- Flechtner V. R., Hanson R. S. Coarse and fine control of citrate synthase from Bacillus subtilis. Biochim Biophys Acta. 1969 Jul 30;184(2):252–262. doi: 10.1016/0304-4165(69)90027-0. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnagel P. The regulation of aconitase and isocitrate dehydrogenase in sporulation mutants of Bacillus subtilis. Biochim Biophys Acta. 1970 Nov 24;222(2):290–298. doi: 10.1016/0304-4165(70)90116-9. [DOI] [PubMed] [Google Scholar]
- Hanson R. S., Cox D. P. Effect of different nutritional conditions on the synthesis of tricarboxylic acid cycle enzymes. J Bacteriol. 1967 Jun;93(6):1777–1787. doi: 10.1128/jb.93.6.1777-1787.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler R. W., Burgess A. H. Respiration and protein synthesis in Escherichia coli membrane-envelope fragments. VI. Solubilization and characterization of the electron transport chain. J Cell Biol. 1972 Nov;55(2):266–281. doi: 10.1083/jcb.55.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Barat M., Anagnostopoulos C. Transformation and transduction in recombination-defective mutants of Bacillus subtilis. J Bacteriol. 1967 Jun;93(6):1925–1937. doi: 10.1128/jb.93.6.1925-1937.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
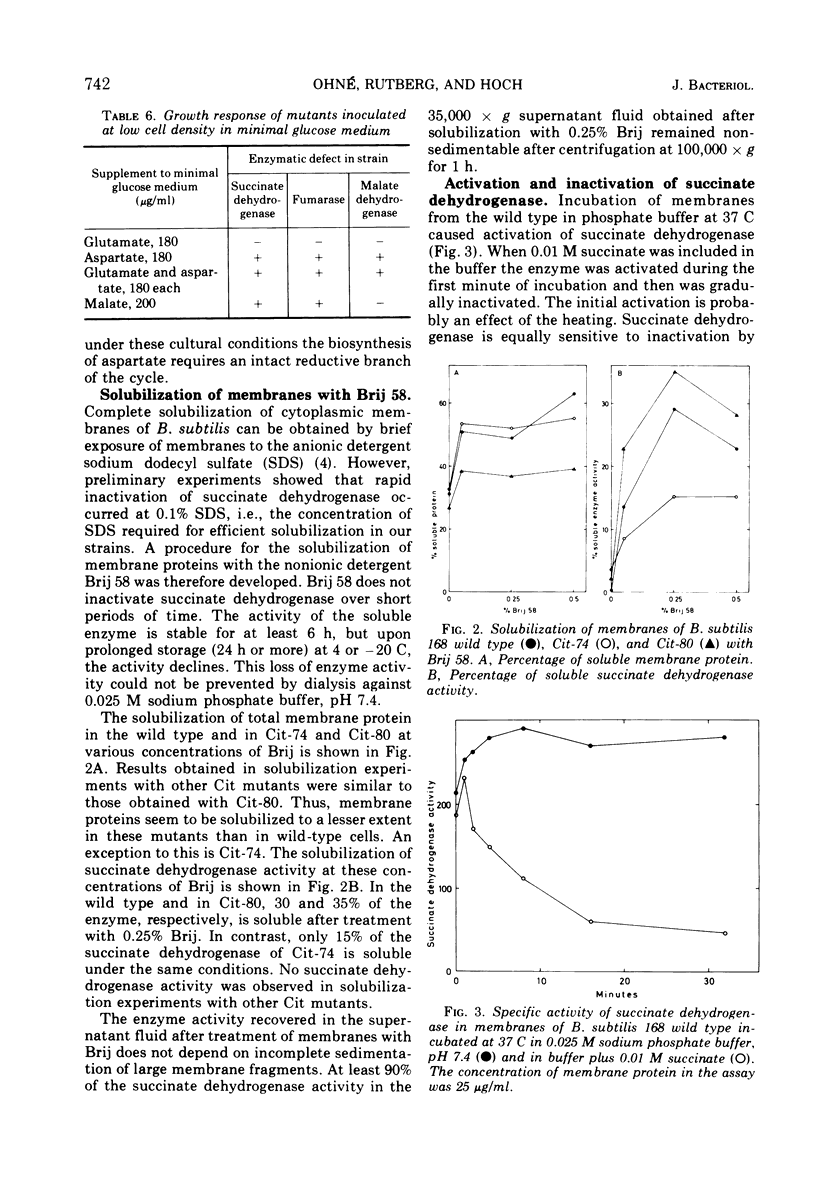
- Kim I. C., Bragg P. D. Some properties of the succinate dehydrogenase of Escherichia coli. Can J Biochem. 1971 Oct;49(10):1098–1104. doi: 10.1139/o71-159. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Henning U. Limiting availability of binding sites for dehydrogenases on the cell membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Apr;69(4):925–929. doi: 10.1073/pnas.69.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lang D. R., Felix J., Lundgren D. G. Development of a membrane-bound resiratory system prior to and during sporulation in Bacillus cereus and its relationship to membrane structure. J Bacteriol. 1972 Jun;110(3):968–977. doi: 10.1128/jb.110.3.968-977.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen P., Freer J. H. Factors influencing the activity of succinate dehydrogenase in membrane preparations from Micrococcus lysodeikticus. Biochem J. 1970 Nov;120(2):237–243. doi: 10.1042/bj1200237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock J. J., Linder R., Salton M. R. Characterization of the membrane-bound succinic dehydrogenase of Micrococcus lysodeikticus. J Bacteriol. 1971 Jul;107(1):230–238. doi: 10.1128/jb.107.1.230-238.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg B., Hoch J. A. Citric acid cycle: gene-enzyme relationships in Bacillus subtilis. J Bacteriol. 1970 Nov;104(2):826–833. doi: 10.1128/jb.104.2.826-833.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg L. Mapping of a temperate bacteriophage active on Bacillus subtilis. J Virol. 1969 Jan;3(1):38–44. doi: 10.1128/jvi.3.1.38-44.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]


