Abstract
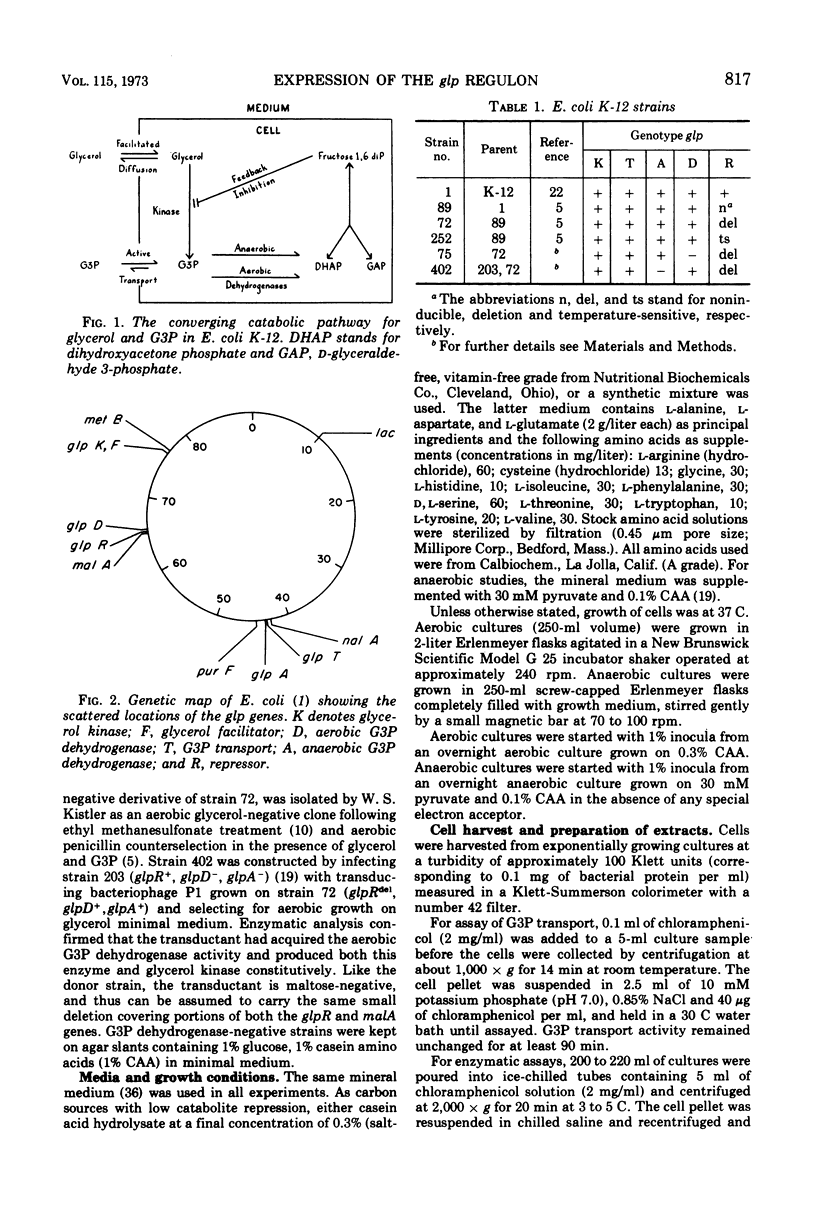
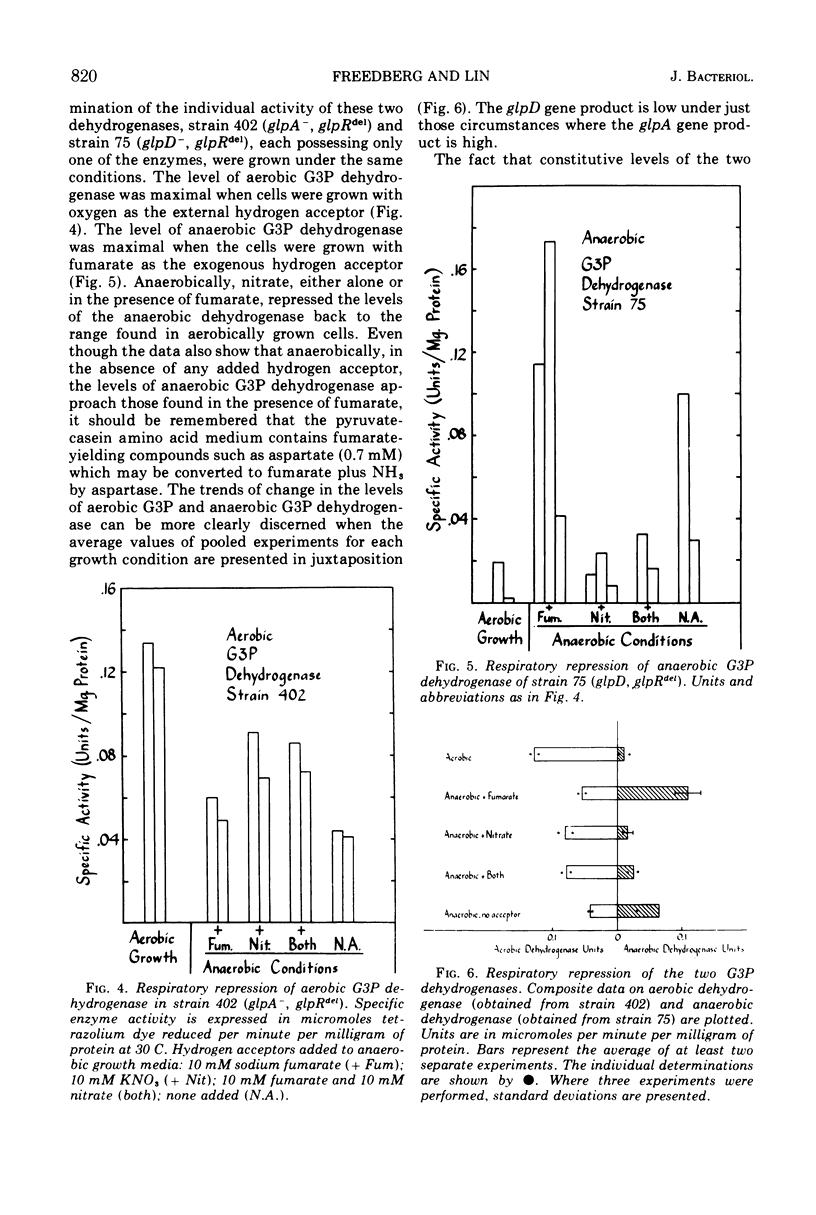
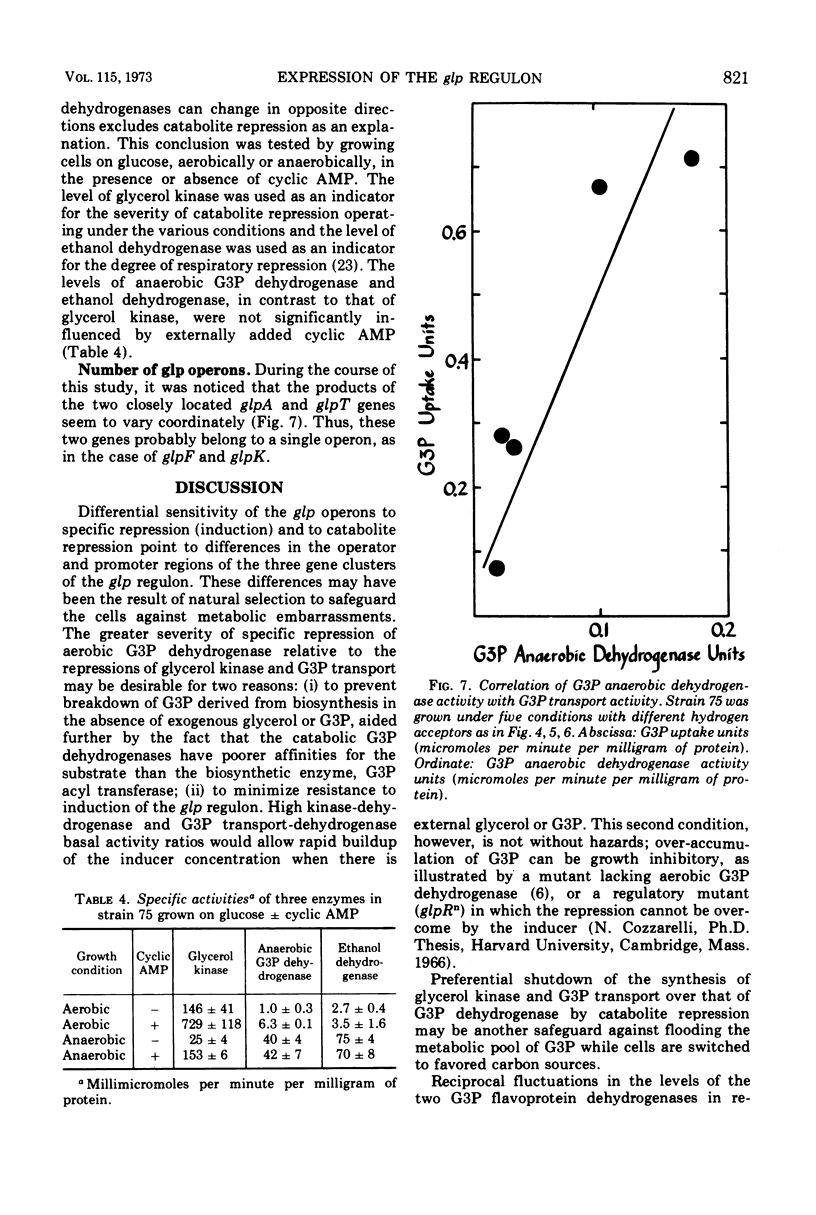
Three kinds of control mechanisms govern the expression of the members of the glp regulon for glycerol and sn-glycerol 3-phosphate (G3P) catabolism in Escherichia coli K-12: specific repression by the product of the glpR gene; catabolite repression; and respiratory repression (the effect exerted by exogenous hydrogen acceptors). The operons of the glp system show different patterns of response to each control. By growing in parallel a mutant strain with temperature-sensitive repressor (glpRts) and an isogenic control with a deletion in the regulator gene at progressively higher temperatures, it was possible to show that the synthesis of aerobic G3P dehydrogenase (glpD product) is far more sensitive to specific repression than that of either glycerol kinase (glpK product) or G3P transport (glpT product). Conversely, in the strain with a deletion in the regulator gene, the syntheses of glycerol kinase and G3P transport are more sensitive to catabolite repression than that of the aerobic G3P dehydrogenase. The levels of the two flavoprotein G3P dehydrogenases vary in opposite directions in response to changes of exogenous hydrogen acceptors. For example, the ratio of the aerobic enzyme to the anaerobic enzyme (specified by glpA) is high when molecular oxygen or nitrate serves as the hydrogen acceptor and low when fumarate plays this role. This trend is not influenced by the addition of cyclic adenosine 3′,5′-monophosphate to the growth medium. Thus, respiratory repression most likely involves a third mechanism of control, independent of specific or catabolite repression.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman-Kurtz M., Lin E. C., Richey D. P. Promoter-like mutant with increased expression of the glycerol kinase operon of Escherichia coli. J Bacteriol. 1971 Jun;106(3):724–731. doi: 10.1128/jb.106.3.724-731.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. A., Wimpenny J. W. Metabolic pathways for nitrate reduction in Escherichia coli. Biochim Biophys Acta. 1968 Jul 16;162(1):39–48. doi: 10.1016/0005-2728(68)90212-0. [DOI] [PubMed] [Google Scholar]
- Cooper R. A., Anderson A. The formation and catabolism of methylglyoxal during glycolysis in Escherichia coli. FEBS Lett. 1970 Dec 11;11(4):273–276. doi: 10.1016/0014-5793(70)80546-4. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Freedberg W. B., Lin E. C. Genetic control of L-alpha-glycerophosphate system in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):371–387. doi: 10.1016/0022-2836(68)90415-4. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Koch J. P., Hayashi S., Lin E. C. Growth stasis by accumulated L-alpha-glycerophosphate in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1325–1329. doi: 10.1128/jb.90.5.1325-1329.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Lin E. C. Chromosomal location of the structural gene for glycerol kinase in Escherichia coli. J Bacteriol. 1966 May;91(5):1763–1766. doi: 10.1128/jb.91.5.1763-1766.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D. The teleonomic significance of biosynthetic control mechanisms. Cold Spring Harb Symp Quant Biol. 1961;26:1–10. doi: 10.1101/sqb.1961.026.01.005. [DOI] [PubMed] [Google Scholar]
- Freedberg W. B., Kistler W. S., Lin E. C. Lethal synthesis of methylglyoxal by Escherichia coli during unregulated glycerol metabolism. J Bacteriol. 1971 Oct;108(1):137–144. doi: 10.1128/jb.108.1.137-144.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- HAYASHI S., LIN E. C. CAPTURE OF GLYCEROL BY CELLS OF ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Mar 29;94:479–487. doi: 10.1016/0926-6585(65)90056-7. [DOI] [PubMed] [Google Scholar]
- HIRSCH C. A., RASMINSKY M., DAVIS B. D., LIN E. C. A FUMARATE REDUCTASE IN ESCHERICHIA COLI DISTINCT FROM SUCCINATE DEHYDROGENASE. J Biol Chem. 1963 Nov;238:3770–3774. [PubMed] [Google Scholar]
- HIRSCHMANN H. The nature of substrate asymmetry in stereoselective reactions. J Biol Chem. 1960 Oct;235:2762–2767. [PubMed] [Google Scholar]
- Hayashi S. I., Lin E. C. Product induction of glycerol kinase in Escherichia coli. J Mol Biol. 1965 Dec;14(2):515–521. doi: 10.1016/s0022-2836(65)80200-5. [DOI] [PubMed] [Google Scholar]
- Hayashi S. I., Lin E. C. Purification and properties of glycerol kinase from Escherichia coli. J Biol Chem. 1967 Mar 10;242(5):1030–1035. [PubMed] [Google Scholar]
- JACOBS N. J., VANDEMARK P. J. Comparison of the mechanism of glycerol oxidation in aerobically and anaerobically grown Streptococcus faecalis. J Bacteriol. 1960 Apr;79:532–538. doi: 10.1128/jb.79.4.532-538.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH J. P., HAYASHI S., LIN E. C. THE CONTROL OF DISSIMILATION OF GLYCEROL AND L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3106–3108. [PubMed] [Google Scholar]
- Kistler W. S., Hirsch C. A., Cozzarelli N. R., Lin E. C. Second pyridine nucleotide-independent 1-alpha-glycerophosphate dehydrogenase in Escherichia coli K-12. J Bacteriol. 1969 Nov;100(2):1133–1135. doi: 10.1128/jb.100.2.1133-1135.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler W. S., Lin E. C. Anaerobic L- -glycerophosphate dehydrogenase of Escherichia coli: its genetic locus and its physiological role. J Bacteriol. 1971 Dec;108(3):1224–1234. doi: 10.1128/jb.108.3.1224-1234.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler W. S., Lin E. C. Purification and properties of the flavine-stimulated anaerobic L- -glycerophosphate dehydrogenase of Escherichia coli. J Bacteriol. 1972 Oct;112(1):539–547. doi: 10.1128/jb.112.1.539-547.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C., KOCH J. P., CHUSED T. M., JORGENSEN S. E. Utilization of L-alpha-glycerophosphate by Escherichia coli without hydrolysis. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2145–2150. doi: 10.1073/pnas.48.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhedran P., Sommer B., Lin E. C. CONTROL OF ETHANOL DEHYDROGENASE LEVELS IN AEROBACTER AEROGENES. J Bacteriol. 1961 Jun;81(6):852–857. doi: 10.1128/jb.81.6.852-857.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICHINOTY F., COUDERT G. [The repressive action of oxygen on the biosynthesis of fumaric reductase of Aerobacter aerogenes]. Experientia. 1962 Jun 15;18:257–259. doi: 10.1007/BF02148218. [DOI] [PubMed] [Google Scholar]
- PICHINOTY F., D'ORNANO L. Inhibition by oxygen of biosynthesis and activity of nitrate-reductase in Aerobacter aerogenes. Nature. 1961 Aug 26;191:879–881. doi: 10.1038/191879a0. [DOI] [PubMed] [Google Scholar]
- PICHINOTY F., D'ORNANO L. [Research on the reduction of nitrous oxide by Micrococcus denitrificans]. Ann Inst Pasteur (Paris) 1961 Sep;101:418–426. [PubMed] [Google Scholar]
- PICHINOTY F., d' ORNANO [Influence of the culture conditions on the formation of nitrate reductase of Aerobacter aerogenes]. Biochim Biophys Acta. 1961 Mar 18;48:218–220. doi: 10.1016/0006-3002(61)90783-1. [DOI] [PubMed] [Google Scholar]
- PICHINOTY F., d' ORNANO [Inhibition by oxygen of the biosynthesis of nitrate-reductase in denitrifying bacteria]. C R Hebd Seances Acad Sci. 1961 Apr 10;252:2294–2296. [PubMed] [Google Scholar]
- PICHINOTY F., d' ORNANO [On the mechanism of the inhibition of bacterial denitrification by oxygen]. Biochim Biophys Acta. 1961 Sep 16;52:386–389. doi: 10.1016/0006-3002(61)90692-8. [DOI] [PubMed] [Google Scholar]
- Richey D. P., Lin E. C. Importance of facilitated diffusion for effective utilization of glycerol by Escherichia coli. J Bacteriol. 1972 Nov;112(2):784–790. doi: 10.1128/jb.112.2.784-790.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanno Y., Wilson T. H., Lin E. C. Control of permeation to glycerol in cells of Escherichia coli. Biochem Biophys Res Commun. 1968 Jul 26;32(2):344–349. doi: 10.1016/0006-291x(68)90392-6. [DOI] [PubMed] [Google Scholar]
- Showe M. K., DeMoss J. A. Localization and regulation of synthesis of nitrate reductase in Escherichia coli. J Bacteriol. 1968 Apr;95(4):1305–1313. doi: 10.1128/jb.95.4.1305-1313.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair P. R., White D. C. Effect of nitrate, fumarate, and oxygen on the formation of the membrane-bound electron transport system of Haemophilus parainfluenzae. J Bacteriol. 1970 Feb;101(2):365–372. doi: 10.1128/jb.101.2.365-372.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorner J. W., Paulus H. Composition and subunit structure of glycerol kinase from Escherichia coli. J Biol Chem. 1971 Jun 25;246(12):3885–3894. [PubMed] [Google Scholar]
- Weiner J. H., Heppel L. A. Purification of the membrane-bound and pyridine nucleotide-independent L-glycerol 3-phosphate dehydrogenase from Escherichia coli. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1360–1365. doi: 10.1016/0006-291x(72)90222-7. [DOI] [PubMed] [Google Scholar]
- Wimpenny J. W., Cole J. A. The regulation of metabolism in facultative bacteria. 3. The effect of nitrate. Biochim Biophys Acta. 1967 Oct 9;148(1):233–242. doi: 10.1016/0304-4165(67)90298-x. [DOI] [PubMed] [Google Scholar]
- Zwaig N., Kistler W. S., Lin E. C. Glycerol kinase, the pacemaker for the dissimilation of glycerol in Escherichia coli. J Bacteriol. 1970 Jun;102(3):753–759. doi: 10.1128/jb.102.3.753-759.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaig N., Lin E. C. Feedback inhibition of glycerol kinase, a catabolic enzyme in Escherichia coli. Science. 1966 Aug 12;153(3737):755–757. doi: 10.1126/science.153.3737.755. [DOI] [PubMed] [Google Scholar]



