Abstract
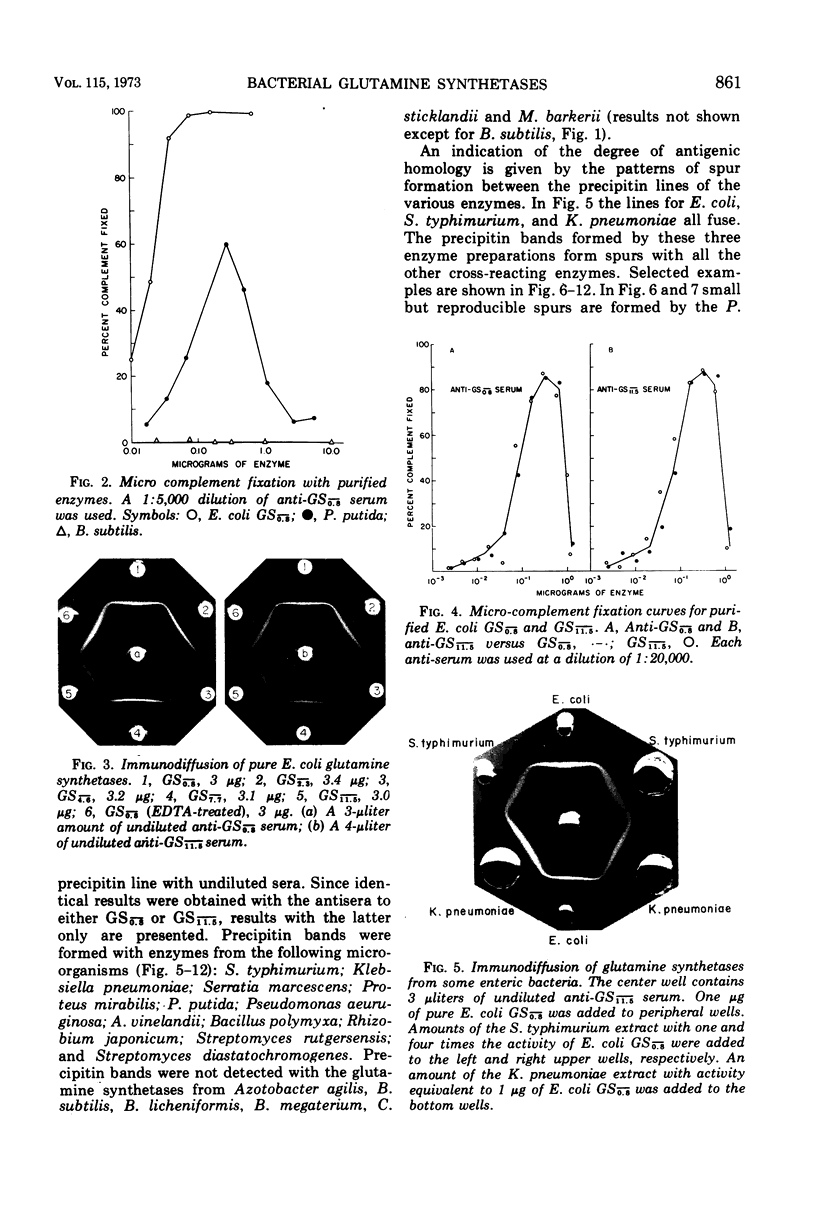
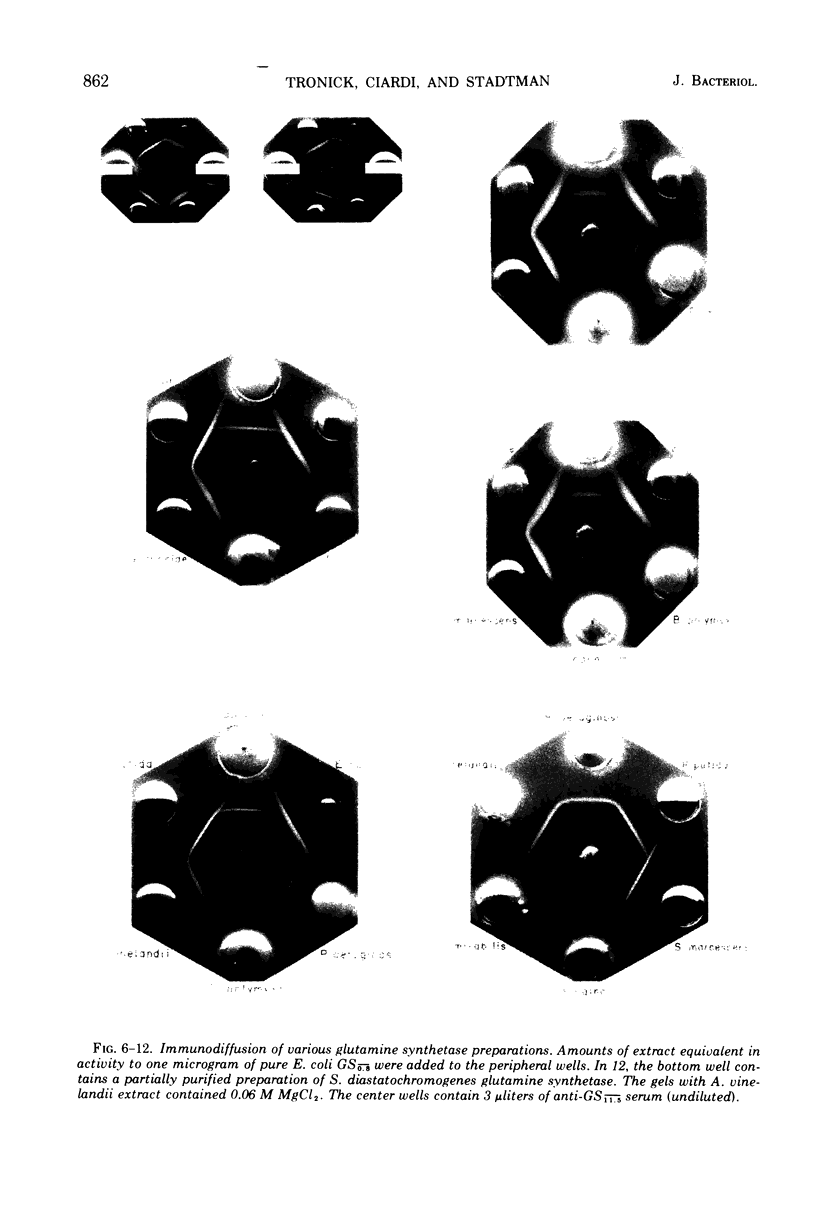
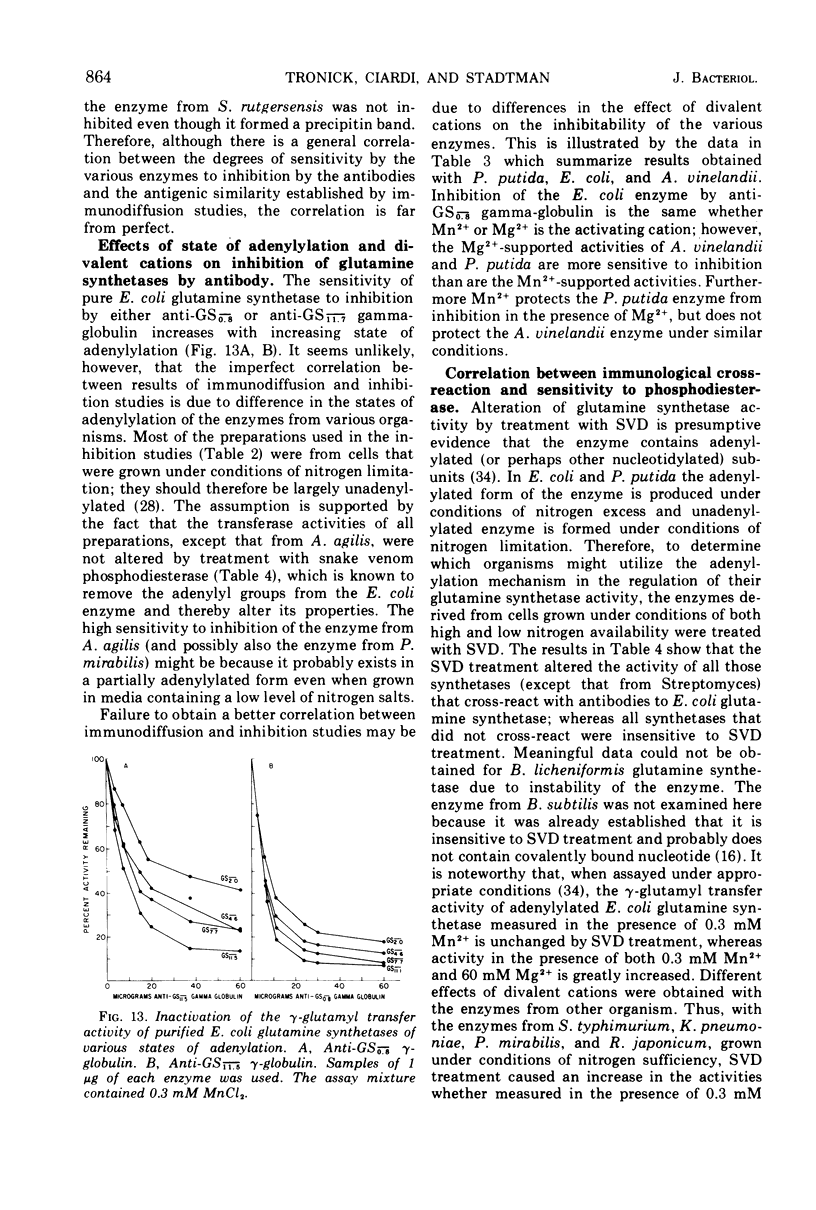
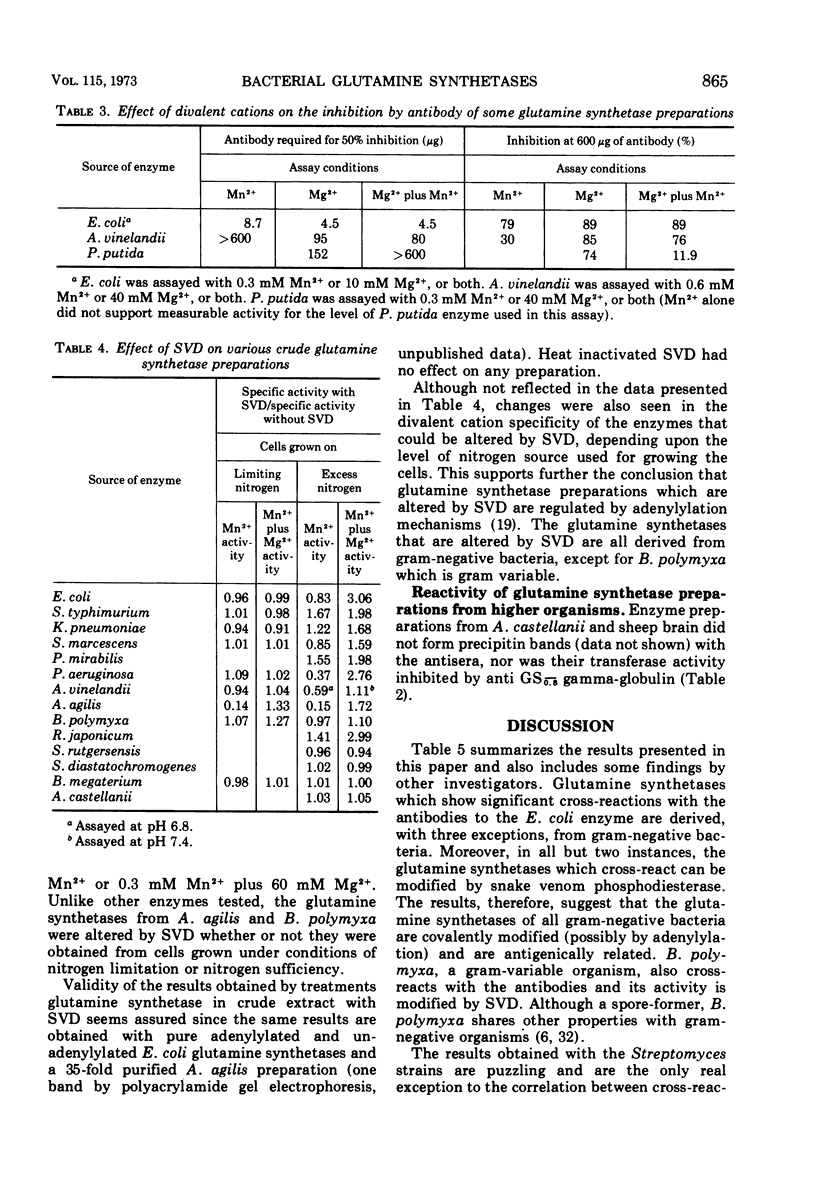
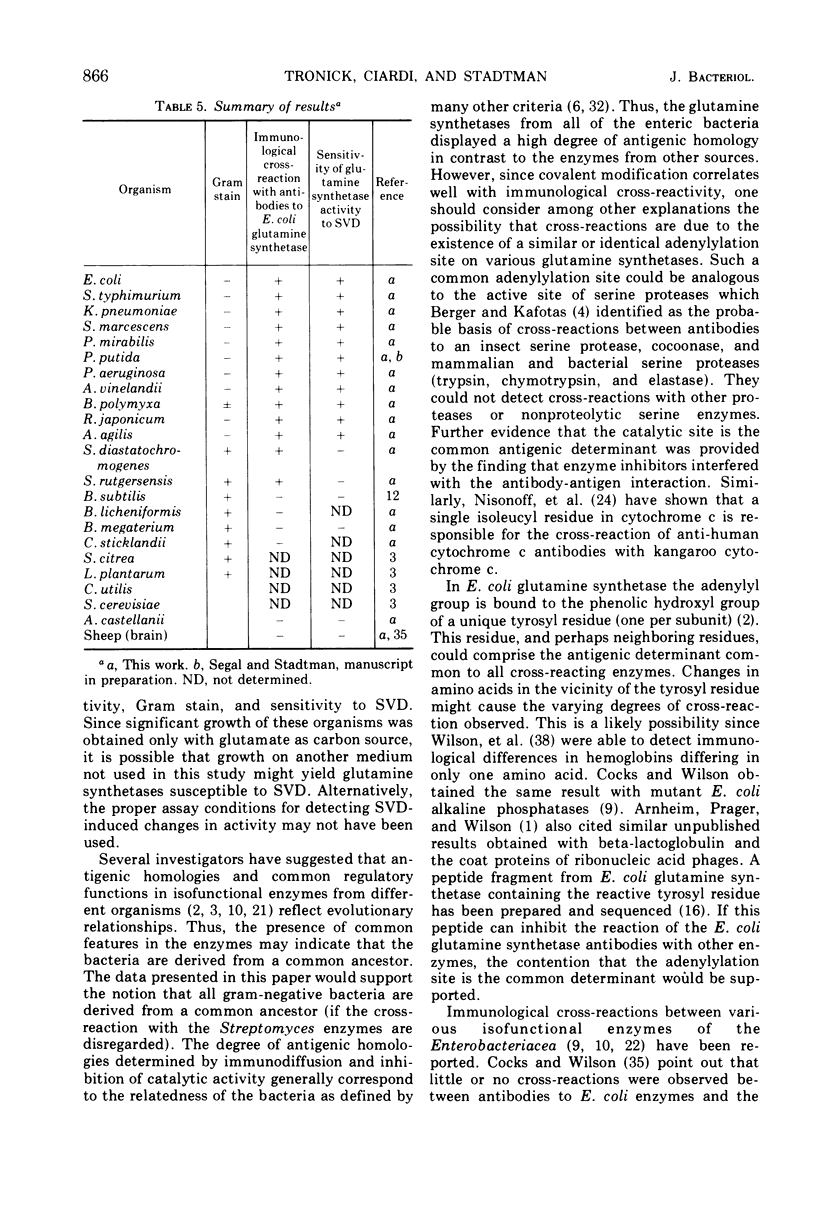
Antisera prepared against adenylylated and unadenylylated Escherichia coli glutamine synthetase cross-reacted with the glutamine synthetases from a number of gram-negative bacteria and one gram-variable species as demonstrated by immunodiffusion and inhibition of enzyme activity. In contrast, the antisera did not cross-react with the glutamine synthetases from gram-positive bacteria (with one exception) nor with the synthetases of higher organisms. Modification of the various glutamine synthetases by covalent attachment of adenosine 5′-monophosphate (or other nucleotides) was tested for by determining whether or not snake venom phosphodiesterase altered catalytic activity in a manner similar to its effect on adenylylated E. coli glutamine synthetase. Only the activity of the glutamine synthetases from gram-negative bacteria grown with specific levels of nitrogen sources could be altered by snake venom phosphodiesterase. In addition, a relative order of antigenic homology between cross-reacting enzymes was suggested based on the patterns of spur formation in the immunodiffusion assay.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheim N., Prager E. M., Wilson A. C. Immunological prediction of sequence differences among proteins. Chemical comparison of chicken, quail, and phesant lysozymes. J Biol Chem. 1969 Apr 25;244(8):2085–2094. [PubMed] [Google Scholar]
- Arnon R. Antibodies to enzymes--a tool in the study of antigenic specificity determinants. Curr Top Microbiol Immunol. 1971;54:47–93. doi: 10.1007/978-3-642-65123-6_3. [DOI] [PubMed] [Google Scholar]
- Arnon R., Neurath H. An immunological approach to the study of evolution of trypsins. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1323–1328. doi: 10.1073/pnas.64.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E., Kafatos F. C. Immunochemistry of an insect protease, cocoonase, and its zymogen. Immunochemistry. 1971 May;8(5):391–403. doi: 10.1016/0019-2791(71)90502-7. [DOI] [PubMed] [Google Scholar]
- Biggins D. R., Kelly M., Postgate J. R. Resolution of nitrogenase of Mycobacterium flavum 30l into two components and cross reaction with nitrogenase components from other bacteria. Eur J Biochem. 1971 May 11;20(1):140–143. doi: 10.1111/j.1432-1033.1971.tb01371.x. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Segal A., Stadtman E. R. Modulation of glutamine synthetase adenylylation and deadenylylation is mediated by metabolic transformation of the P II -regulatory protein. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2949–2953. doi: 10.1073/pnas.68.12.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks G. T., Wilson A. C. Enzyme evolution in the Enterobacteriaceae. J Bacteriol. 1972 Jun;110(3):793–802. doi: 10.1128/jb.110.3.793-802.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks G. T., Wilson A. C. Immunological detection of single amino acid substitutions in alkaline phosphatase. Science. 1969 Apr 11;164(3876):188–189. doi: 10.1126/science.164.3876.188. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Ginsburg A., Yeh J., Shelton E., Stadtman E. R. Bacillus subtilis glutamine synthetase. Purification and physical characterization. J Biol Chem. 1970 Oct 25;245(20):5195–5205. [PubMed] [Google Scholar]
- Deuel T. F., Stadtman E. R. Some kinetic properties of Bacillus subtilis glutamine synthetase. J Biol Chem. 1970 Oct 25;245(20):5206–5213. [PubMed] [Google Scholar]
- Gancedo C., Holzer H. Enzymatic inactivation of glutamine synthetase in Enterobacteriaceae. Eur J Biochem. 1968 Apr 3;4(2):190–192. doi: 10.1111/j.1432-1033.1968.tb00192.x. [DOI] [PubMed] [Google Scholar]
- Gasser F., Gasser C. Immunological relationships among lactic dehydrogenases in the genera Lactobacillus and Leuconostoc. J Bacteriol. 1971 Apr;106(1):113–125. doi: 10.1128/jb.106.1.113-125.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg A., Yeh J., Hennig S. B., Denton M. D. Some effects of adenylylation on the biosynthetic properties of the glutamine synthetase from Escherichia coli. Biochemistry. 1970 Feb 3;9(3):633–649. doi: 10.1021/bi00805a025. [DOI] [PubMed] [Google Scholar]
- Heinrikson R. L., Kingdon H. S. The amino acid sequence in the vicinity of the covalently bound adenylic acid in glutamine synthetase from Escherichia coli. J Biol Chem. 1970 Jan 10;245(1):138–142. [PubMed] [Google Scholar]
- Holzer H. Regulation of enzymes by enzyme-catalyzed chemical modification. Adv Enzymol Relat Areas Mol Biol. 1969;32:297–326. doi: 10.1002/9780470122778.ch7. [DOI] [PubMed] [Google Scholar]
- Hubbard J. S., Stadtman E. R. Regulation of glutamine synthetase. V. Partial purification and properties of glutamine synthetase from Bacillus licheniformis. J Bacteriol. 1967 Oct;94(4):1007–1015. doi: 10.1128/jb.94.4.1007-1015.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdon H. S., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. 8. ATP: glutamine synthetase adenylyltransferase, an enzyme that catalyzes alterations in the regulatory properties of glutamine synthetase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1703–1710. doi: 10.1073/pnas.58.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Meyer E. Y., Kulczyk S. Comparative biochemical and immunological study of malic enzyme from two species of lactic acid bacteria: evolutionary implications. J Bacteriol. 1971 Apr;106(1):126–137. doi: 10.1128/jb.106.1.126-137.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. M., Mills S. E. Immunochemical and enzymatic comparisons of the tryptophan synthase alpha subunits from five species of Enterobacteriaceae. J Bacteriol. 1969 Mar;97(3):1310–1320. doi: 10.1128/jb.97.3.1310-1320.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON J. W., WILSON P. W., BURRIS R. H. Direct demonstration of ammonia as an intermediate in nitrogen fixation by Azotobacter. J Biol Chem. 1953 Sep;204(1):445–451. [PubMed] [Google Scholar]
- Nisonoff A., Reichlin M., Margoliash E. Immunological activity of cytochrome c. II. Localization of a major antigenic determinant of human cytochrome c. J Biol Chem. 1970 Mar 10;245(5):940–946. [PubMed] [Google Scholar]
- OUCHTERLONY O. Antigen-antibody reactions in gels. IV. Types of reactions in coordinated systems of diffusion. Acta Pathol Microbiol Scand. 1953;32(2):230–240. [PubMed] [Google Scholar]
- Shapiro B. M., Ginsburg A. Effects of specific divalent cations on some physical and chemical properties of glutamine synthetase from Escherichia coli. Taut and relaxed enzyme forms. Biochemistry. 1968 Jun;7(6):2153–2167. doi: 10.1021/bi00846a018. [DOI] [PubMed] [Google Scholar]
- Shapiro B. M., Kingdon H. S., Stadtman E. R. Regulation of glutamine synthetase. VII. Adenylyl glutamine synthetase: a new form of the enzyme with altered regulatory and kinetic properties. Proc Natl Acad Sci U S A. 1967 Aug;58(2):642–649. doi: 10.1073/pnas.58.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro B. M., Stadtman E. R. 5'-adenylyl-O-tyrosine. The novel phosphodiester residue of adenylylated glutamine synthetase from Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3769–3771. [PubMed] [Google Scholar]
- Shapiro B. M., Stadtman E. R. The regulation of glutamine synthesis in microorganisms. Annu Rev Microbiol. 1970;24:501–524. doi: 10.1146/annurev.mi.24.100170.002441. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Ginsburg A., Ciardi J. E., Yeh J., Hennig S. B., Shapiro B. M. Multiple molecular forms of glutamine synthetase produced by enzyme catalyzed adenylation and deadenylylation reactions. Adv Enzyme Regul. 1970;8:99–118. doi: 10.1016/0065-2571(70)90011-7. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Wachter D., Gasser C., Wilson A. C. Comparative immunological studies of two Pseudomonas enzymes. J Bacteriol. 1970 May;102(2):351–362. doi: 10.1128/jb.102.2.351-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate S. S., Meister A. Regulation of rat liver glutamine synthetase: activation by alpha-ketoglutarate and inhibition by glycine, alanine, and carbamyl phosphate. Proc Natl Acad Sci U S A. 1971 Apr;68(4):781–785. doi: 10.1073/pnas.68.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]
- WILSON A. C., KAPLAN N. O., LEVINE L., PESCE A., REICHLIN M., ALLISON W. S. EVOLUTION OF LACTIC DEHYDROGENASES. Fed Proc. 1964 Nov-Dec;23:1258–1266. [PubMed] [Google Scholar]







