Abstract
Tumor necrosis factor (TNF)-induced apoptosis is mediated by caspases, which are cysteine proteases related to interleukin 1β-converting enzyme. We report here that TNF-induced activation of caspases results in the cleavage and activation of cytosolic phospholipase A2 (cPLA2) and that activated cPLA2 contributes to apoptosis. Inhibition of caspases by expression of a cowpox virus-derived inhibitor, CrmA, or by a specific tetrapeptide inhibitor of CPP32/caspase-3, acetyl-Asp-Glu-Val-Asp-aldehyde (Ac-DEVD-CHO), inhibited TNF-induced activation of cPLA2 and apoptosis. TNF-induced activation of cPLA2 was accompanied by a cleavage of the 100-kDa cPLA2 to a 70-kDa proteolytic fragment. This cleavage was inhibited by Ac-DEVD-CHO in a similar manner as that of poly(ADP)ribose polymerase, a known substrate of CPP32/caspase-3. Interestingly, specific inhibition of cPLA2 enzyme activity by arachidonyl trifluoromethylketone (AACOCF3) partially inhibited TNF-induced apoptosis without inhibition of caspase activity. Thus, our results suggest a novel caspase-dependent activation pathway for cPLA2 during apoptosis and identify cPLA2 as a mediator of TNF-induced cell death acting downstream of caspases.
Keywords: apoptosis, arachidonic acid, CrmA, CPP32
Apoptosis is an active form of cell death controlled by the expression of evolutionarily highly conserved genes, which either activate or suppress the process of cell death (1). The best studied activators of apoptosis include a growing family of caspases, cysteine proteases related to interleukin 1β-converting enzyme (ICE/caspase-1), which share sequence homology with ced-3, a gene essential for apoptosis of nematode Caenorhabditis elegans (2). Another family of activators of apoptosis include proteins containing a “death domain” originally discovered in the intracellular parts of two receptors capable of mediating apoptosis, type 1 tumor necrosis factor receptor (TNF-R1) and Fas (3, 4). Recently, it was revealed that binding of receptor death domains to each other and to other intracellular proteins containing similar domains initiate the apoptotic signaling: TNF-R1-associated death domain protein (TRADD) binds to TNF-R1 (5), Fas-associated death domain protein (FADD/MORT1) binds to either Fas or TRADD (6, 7), and FLICE/MACH/caspase-8 binds to FADD (8, 9), completing the death-inducing signaling complex. Interestingly, FLICE/MACH/caspase-8 contains a caspase-related cysteine protease domain, which possibly couples the receptor crosslinking and activation of apoptotic protease cascade (8, 9). The importance of caspases in TNF- and Fas-mediated apoptosis has been clearly demonstrated by studies showing that (i) receptor activation leads to proteolytic activation of at least three family members, FLICE/MACH/caspase-8 (9), CPP32/caspase-3 (10), and ICE-LAP3/caspase-7 (11); (ii) inhibitors of caspases (cow pox virus protein CrmA, baculovirus protein p35, and specific inhibitory peptides) effectively block TNF- and Fas-mediated apoptosis (12–16); and (iii) ectopic expression of caspases in several cells results in apoptosis (2, 9, 11, 17, 18).
It is still largely unknown how TNF-induced activation of caspases is linked to the activation of second messengers, which eventually lead to the wide variety of TNF-induced effects (19). Arachidonic acid generated as a result of cPLA2 activation has been implicated in a signal transduction pathway resulting in cell death (20–25). Our new data showing that TNF induces a cleavage of cPLA2 prompted us to study the role of caspases in TNF-induced activation of cPLA2. Our results show that inhibition of caspase activity inhibits both TNF-induced cleavage and activation of cPLA2. Moreover, we show that inhibition of cPLA2 activity partially inhibits TNF-induced apoptosis. These results indicate that activation of cPLA2 requires caspases and that cPLA2 acts as a death mediator in TNF-induced apoptosis.
MATERIALS AND METHODS
Cell Culture.
MCF-7S1 cell line is a highly TNF-sensitive subclone of MCF-7S breast carcinoma cells (26) and WEHI-S cell line is a highly TNF sensitive subclone of WEHI 164 murine fibrosarcoma cells (27). Cells were cultured at 37°C in humidified air atmosphere with 5% CO2. RPMI 1640 medium (GIBCO) supplemented with 10% heat-inactivated fetal calf serum (FCS; GIBCO) and 2 mM each of glutamine, penicillin, and streptomycin was used as a growth medium for all cell lines. Medium used for the culture of transfected cell lines was further supplemented with 0.2–0.4 mg/ml of G418 (GIBCO). All cell lines were tested and found negative for mycoplasma.
Treatments.
Recombinant human TNF with specific activity of 6 × 107 units/ml was kindly provided by G. R. Adolf (Ernst-Boehringer-Institute, Vienna). Acetyl-Tyr-Val-Ala-Asp-aldehyde (Ac-YVAD-CHO; Bachem) and acetyl-Asp-Glu-Val-Asp-aldehyde (Ac-DEVD-CHO; Neosystems, Strasbourg, France) were dissolved in water at 2 mM. Arachidonyl trifluoromethylketone (AACOCF3) was from Biomol (Plymouth Meeting, PA) and was dissolved in ethanol at 50 mM. All experiments employing tetrapeptides and AACOCF3 were performed in RPMI medium 1640 supplemented with 1% FCS and cells were preincubated with them for 3 h before the addition of TNF.
Electroporation.
pcDNA3/crmA and pcDNA3/crmA-M expression vectors encoding for cowpox virus-derived caspase inhibitor CrmA and its inactive mutant CrmA-M (point mutation at amino acid 291, Thr to Arg) were kindly provided by Vishva Dixit (University of Michigan Medical School, Ann Arbor) (28). Twenty micrograms of circular plasmid DNA was introduced into 107 cells in logarithmic growth phase by electroporation as described previously (29). After 48 h at 37°C, G418 (GIBCO) was added to a final concentration of 0.4 mg/ml, and when the drug-resistant clones had reached confluence they were individually collected and propagated.
Western Blot Analysis.
Cells were lysed in urea sample buffer (62.5 mM Tris, pH 6.8/6 M urea/10% glycerol/2% SDS/0.003% bromophenol blue/5% 2-mercaptoethanol), sonicated and incubated at 65°C for 15 min before electrophoresis through 10% SDS/PAGE (30), and blotted to a Hybond ECL filter (Amersham) using Miniblot graphite electrophoresis unit (Millipore). Filters were incubated at 25°C for 30 min in blocking buffer [5% low-fat milk powder in Tris-buffered saline (TBS)], and 2 h in diluting buffer (0.5% low-fat milk powder in TBS) containing the primary antibodies; polyclonal rabbit antiserum specific for CrmA (28) (kindly provided by Vishva Dixit) at 1:1000, mouse monoclonal antibody C2–10 recognizing poly(ADP)ribose polymerase (PARP) (31) at 1:10000, and polyclonal rat antiserum specific for cPLA2 at 1:200. After washing in TBS containing 0.08% Tween-20, filters were incubated for 25 min at 25°C in diluting buffer containing 1:10000 dilution of peroxidase-conjugated secondary antibody (Dako). After washing, chemiluminescence reaction was performed according to manufacturer’s instructions using ECL Western blotting reagents (Amersham); filters were exposed to ECL hyperfilm (Amersham) for required time.
Production of Antiserum Recognizing cPLA2.
To obtain an antiserum recognizing human and mouse cPLA2, Wistar rats were immunized subcutaneously with Freund’s complete adjuvant containing 20–40 nmol of a Diphtheria toxoid-coupled synthetic peptide CPDPYVELFISTTPDSRK (Chiron Mimotopes), sequence except for the N-terminal cysteine corresponding to amino acids 43–58 in both mouse and human cPLA2 (32). Immunization was repeated after 2 and 5 weeks using Freund’s incomplete adjuvant. Plasma was collected 1 week after the last immunization.
Functional Assays.
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) tetrazolium assay was used to measure the viability and 51Cr-release assay was used to measure the death of cells as described previously (26, 33). Activation of cPLA2 was analyzed as described previously (23) by an assay based on labeling of cells with [3H]arachidonic acid [5,6,8,9,11,12,14,15-3H(N)] (80 Ci/mmol, 0.1 mCi/ml; 1 Ci = 37 GBq; NEN) and measurement of released radioactivity from the supernatant after treatment.
RESULTS
Expression of CrmA Renders Cells Resistant to TNF-Mediated Cytotoxicity.
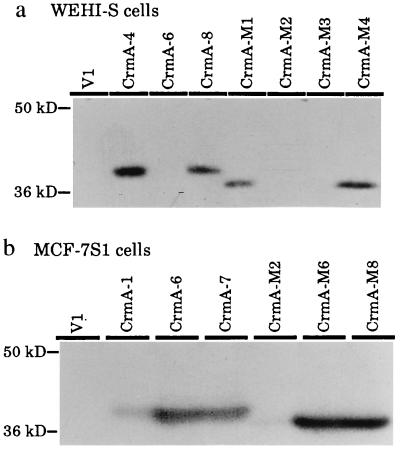
Expression plasmids encoding for a cowpox virus-derived inhibitor of caspases, CrmA, or its inactive mutant, CrmA-M, were introduced into WEHI-S mouse fibrosarcoma and MCF-7S1 human breast carcinoma cells by electroporation. We have previously demonstrated that TNF induces morphological changes characteristic of apoptosis in these cell lines (29). G418-resistant transformants were analyzed by Western blot analysis for the expression of CrmA and CrmA-M and two clones with highest expression levels from each transfection (WEHI-CrmA-4 and -8, WEHI-CrmA-M1 and -M4, MCF-CrmA-6 and -7, and MCF-CrmA-M6 and -M8) and randomly selected vector-transfected control clones (WEHI-V1 and MCF-V1) were used for further studies (Fig. 1).
Figure 1.
Expression of CrmA and mutant CrmA (CrmA-M) in stably transfected single cell clones of WEHI-S (a) and MCF-7S1 cells (b). Cells were transfected with empty pcDNA3 vector (V) or with the same vector containing cDNAs encoding for CrmA (CrmA) or for inactive mutant of CrmA (CrmA-M) (28) by electroporation as described (29). Cell lysates from approximately 2 × 105 G418-resistant single cell clones were analyzed by Western blot analysis using polyclonal antiserum against CrmA (28). The migration of molecular weight markers from NOVEX (San Diego) is indicated on the left.
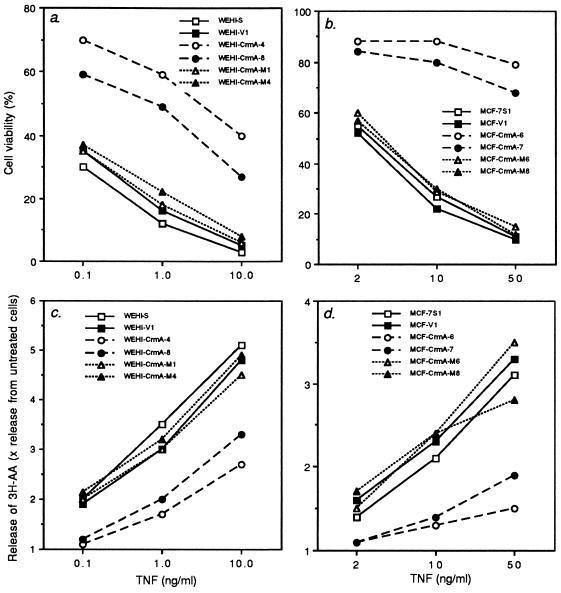
Parental cell lines and obtained transformants were treated with various concentrations of TNF, and the viability of cells was analyzed by MTT assay. Expression of CrmA, but not that of CrmA-M, conferred clear protection from TNF-induced cytotoxicity confirming that the activation of caspases is involved in TNF-mediated killing of the cells used in this study (Fig. 2 a and b).
Figure 2.
CrmA inhibits TNF-mediated cytotoxicity (a and b) and release of arachidonic acid (c and d) in WEHI-S (a and c) and MCF-7S1 (b and d) cells. (a and b) Ten thousand cells per well were plated in 96-well microtiter plates with the indicated concentrations of TNF. The percentage of surviving cells were analyzed by the MTT assay (29) after 6 h (a) or 48 h (b) incubation. (c and d) Cells (2 × 105) labeled with [3H]arachidonic acid were plated in 24-well plates. After 4 h (c) or 20 h (d) incubation with indicated concentrations of TNF, radioactivity released to the supernatant was analyzed. The release of radiolabeled arachidonic acid is reported relative to spontaneous release from cells without TNF (1.0). Cell clones are as in Fig. 1. The values represent means of a triplicate experiment. Experiments were repeated three or four times with similar results.
TNF-Induced Activation of cPLA2 Requires Caspase Activity.
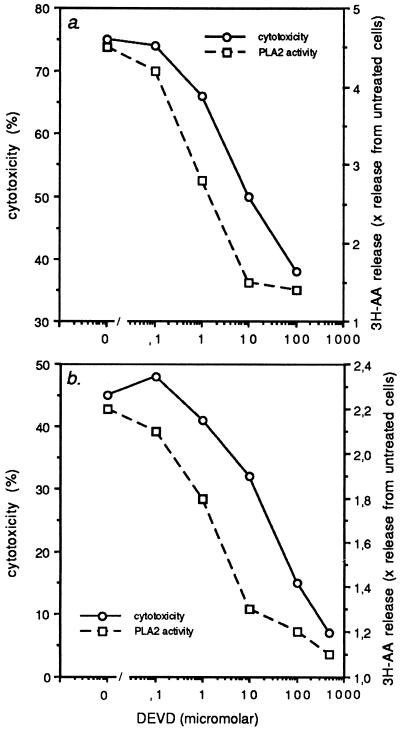
In various sensitive target cells, including WEHI-S and MCF-7S1 cells, the activation of cPLA2 by TNF has been shown to lead to the enzymatic release of arachidonic acid from membrane phospholipids (20–25). To test whether activation of cPLA2 is dependent on the activation of caspases, TNF-induced activation of cPLA2 in cells expressing CrmA or CrmA-M were analyzed by measuring the release of radioactivity from cells labeled with [3H]arachidonic acid. A 4-h exposure of WEHI-S cells to TNF at concentrations ranging from 0.1–10 ng/ml induced a dose-dependent release of radioactivity, the maximum release being approximately 5-fold higher than that from cells incubated in media alone (Fig. 2c). The release of radioactivity from transfection control cells (WEHI-V1) in response to TNF did not differ significantly from that in parental cells, indicating that transfection procedure had not affected the activation of arachidonic acid metabolism. Interestingly, TNF-induced activation of cPLA2 in clones expressing CrmA (WEHI-CrmA-4 and -8) was inhibited in a similar manner as TNF-mediated cytotoxicity (Fig. 2 a and c). Similar results were obtained with MCF-7S1 transformants (Fig. 2 b and d). CrmA-M was unable to inhibit TNF-induced activation of cPLA2, indicating that CrmA-mediated inhibition of cPLA2 was due to inhibition of caspases and not to other putative activities of CrmA. This was further confirmed by results showing that a specific tetrapeptide inhibitor of CPP32/caspase-3, Ac-DEVD-CHO (34), at concentrations that inhibited TNF-induced apoptosis also inhibited arachidonic acid release (Fig. 3). An ICE/caspase-1-specific tetrapeptide inhibitor, Ac-YVAD-CHO (34), had a similar effect, but approximately 10 times higher concentrations were needed for the inhibition (data not shown). Inhibition of the release of arachidonic acid was not due to the better survival of cells as the assay was performed at a time point when cell membranes were not yet damaged as analyzed by trypan blue exclusion and chromium release assays (data not shown). Moreover, killing the cells by freezing and thawing did not result in the release of arachidonic acid.
Figure 3.
Inhibition of CPP32/caspase-3 inhibits TNF-induced cytotoxicity and activation of cPLA2 in WEHI-S (a) and MCF-7S1 (b) cells. Cytotoxicity induced by TNF was analyzed by 6 h (a) or 24 h (b) chromium release assay and the cPLA2 activity was analyzed by measuring the release of arachidonic acid following 4 h (a) or 18 h (b) incubation with TNF. Cells were incubated for 3 h with indicated concentrations of Ac-DEVD-CHO before the addition of 10 ng/ml of TNF. The values represent means of a triplicate experiment. Experiments were repeated twice with similar results.
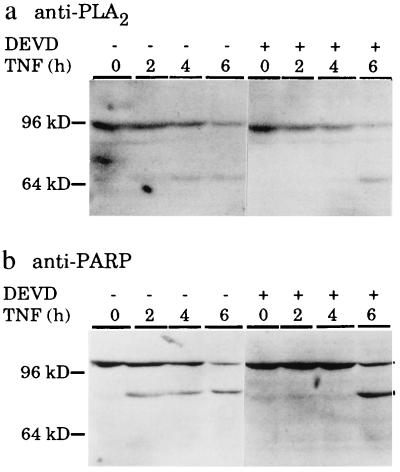
TNF Induces a Caspase-Dependent Cleavage of cPLA2.
Inspection of the published protein sequence of human cPLA2 (35) revealed a sequence DELD, identical to that recognized by CPP32/caspase-3 in D4-GDI protein (36) and very similar to the CPP32/caspase-3 recognition sequence DEVD in PARP (28). The DELD sequence is conserved in mouse, rat, and chicken cPLA2 (32). We therefore studied the possible cleavage of cPLA2 following TNF-treatment by Western blot analysis using a rat antiserum raised against a Diphtheria toxoid-coupled synthetic peptide corresponding to amino acids 43–58 in both mouse and human cPLA2. The obtained antiserum recognized a single specific band of the expected size, approximately 100 kDa (35), in Western blot analysis of proteins from non-treated WEHI-S cells (Fig. 4a and 5b). TNF-treatment of WEHI-S cells resulted in a dose- and time-dependent cleavage of the 100-kDa cPLA2 to an approximately 70-kDa proteolytic fragment recognized by the antiserum. The putative CPP32/caspase-3 cleavage site is at the amino acid 522 of 749 amino acids and as the antibody was raised against a peptide in the amino terminus of the protein, the 70-kDa fragment seen could present the first 522 amino acids. The appearance of the 70-kDa fragment occurred at similar doses of TNF (Fig. 5 b and c) and with similar kinetics (Fig. 4) as that of the 85-kDa apoptotic fragment of PARP. Moreover, TNF-induced cleavage of cPLA2 was inhibited by Ac-DEVD-CHO in a similar manner as that of PARP (Fig. 4).
Figure 4.
Western blot demonstrating TNF-induced and Ac-DEVD-CHO-inhibited cleavage of cPLA2 (a) and PARP (b). WEHI-S cells were incubated for 3 h with (+) or without (−) 100 μM of Ac-DEVD-CHO and were then treated with 50 ng/ml of TNF for indicated times. Cell lysates from approximately 2 × 105 cells were prepared and analyzed by Western blotting as described (31) using 1:200 dilution of polyclonal rat antiserum against cPLA2 (a) or 1:10000 dilution of monoclonal mouse anti-PARP antibody (31) (b) and ECL reagents from Amersham. The migration of molecular weight markers from NOVEX are indicated on the left.
Figure 5.
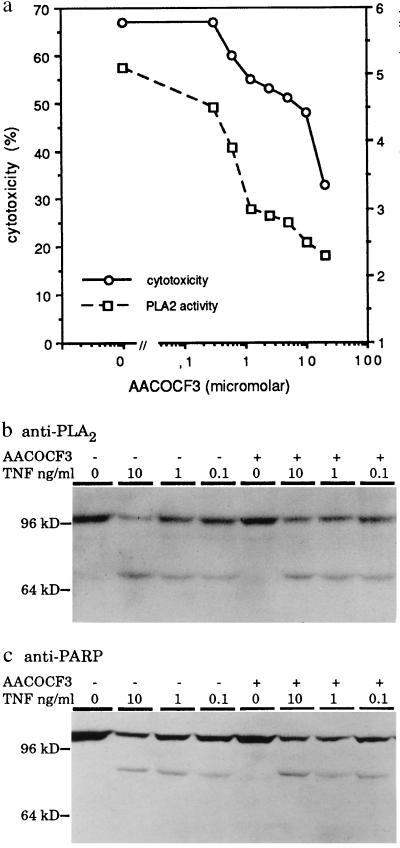
A specific cPLA2 inhibitor, AACOCF3, inhibits TNF-induced cytotoxicity and release of arachidonic acid (a) without affecting ICE-like protease activity (b and c). (a) WEHI-S cells were preincubated for 3 h with indicated concentrations of AACOCF3. Cytotoxicity percentage was determined by chromium release assay and the cPLA2 activity by measuring the release of arachidonic acid after 6 h and 4 h incubation with 10 ng/ml of TNF, respectively. (b and c) Cells were incubated for 3 h with (+) or without (−) 10 μM AACOCF3 and then treated with indicated concentrations of TNF for 6 h. Western blot analysis was as described in the legend for Fig. 4.
The expression level of cPLA2 in MCF-7S1 cells was under the detection limit of the assay. To test whether cleavage of cPLA2 also occurs in human cells, we transfected MCF-7S1 cells with an expression construct encoding for human cPLA2. In the resulting MCF-cPLA2 cells, a 100-kDa cPLA2 band was clearly detectable and it was cleaved to the 70-kDa fragment following 18 h TNF treatment (data not shown).
Inhibition of cPLA2 Activity Partially Inhibits TNF-Mediated Cytotoxicity.
We next studied whether a specific inhibition of TNF-induced activation of cPLA2 inhibits TNF-mediated apoptosis. Remarkably, inhibition of TNF-induced release of arachidonic acid by a specific cPLA2 inhibitor, a trifluoromethylketone analog of arachidonic acid (AACOCF3) (37), was accompanied by a dose-dependent inhibition of TNF-induced cytotoxicity indicating that activation of cPLA2 contributes to TNF-mediated cytotoxicity (Fig. 5a). Inhibition of TNF-induced cell death by AACOCF3 had no effect on the activity of caspases as analyzed by the appearance of the apoptotic fragments of cPLA2 and PARP (Fig. 5 b and c).
DISCUSSION
The results presented above demonstrate a novel activation pathway for cPLA2. The classical cPLA2 activation pathway involves an agonist-induced phosphorylation of cPLA2 at Ser-505 by mitogen-activated protein kinase and calcium-dependent translocation of cPLA2 from cytosol to membranes where the substrate is localized (35, 38, 39). TNF-mediated activation of cPLA2 in WEHI-S cells did not result in its phosphorylation as judged by the lack of phosphorylation-associated electrophoretic mobility-shift of cPLA2 protein (Figs. 4a and 5b; ref. 39). The ability of caspase inhibitors, CrmA (Fig. 2) and Ac-DEVD-CHO (Fig. 3), to inhibit TNF-induced arachidonic acid release in tumor cells indicates that cPLA2 is activated by a pathway involving caspases. This is consistent with earlier results showing that inhibition of TNF-mediated apoptosis by various protective proteins acting upstream from caspases, heat shock protein 70 (23), A20 (29), Bcl-2 and Bcl-x (26), and adenovirus protein E3 14.7-kDa (40) is always accompanied by inhibition of TNF-induced activation of cPLA2.
Interestingly, TNF-treatment resulted in the cleavage of the 100-kDa cPLA2 into an approximately 70-kDa fragment. As cPLA2 contains a CPP32/caspase-3 recognition sequence and as Ac-DEVD-CHO inhibited cPLA2 cleavage in a similar manner as it inhibited the cleavage of a CPP32/caspase-3 substrate, PARP, cPLA2 is likely to be cleaved by CPP32/caspase-3 or a closely related protease. Whether TNF-induced activation of cPLA2 in apoptosis is a result of its proteolysis and one of its proteolytic fragments is functioning as an active enzyme remains to be studied. Alternatively, a putative cPLA2-inhibitor (41) could be inactivated by caspases or cPLA2 could be activated by other caspase-activated enzymes, like protein kinase C δ (42, 43), or by other yet unknown substrates.
Activation of cPLA2 has been suggested to be essential for TNF-mediated cytotoxicity in L929 cells as TNF-resistant L929 mutants that had lost the expression of cPLA2 could be rendered TNF sensitive by exogenous expression of cPLA2 (24). Moreover, inhibition of cPLA2 expression by antisense oliconucleotides has been shown to render melanoma cells resistant to TNF-mediated cytotoxicity (25). Present data showing that a specific cPLA2 inhibitor, AACOCF3, inhibits TNF-mediated cell death, further supports the hypothesis that cPLA2 is an essential part of TNF-induced death pathway. The mechanism by which cPLA2 mediates apoptosis is still unclear. As it is activated by caspases it must act in the final execution step of apoptosis. cPLA2-mediated cleavage of arachidonic acid from membranes may disrupt the integrity of various cellular membranes. Alternatively, the released arachidonic acid may act as a second messenger and activate other apoptosis associated enzymes like sphingomyelinase (44).
Acknowledgments
We thank Dr. Vishva Dixit (University of Michigan, Ann Arbor) for pcDNA-CrmA and pcDNA-CrmA-M plasmids and rabbit anti-CrmA antiserum and Dr. G. R. Adolf (Ernst-Boehringer-Institute, Vienna) for recombinant human tumor necrosis factor α. This work was supported by grants from the Danish Cancer Society, Jens and Edith Sørensen Foundation, Bernhard and Meta Rasmussen Foundation, Jeppe and Ovita Juhl Foundation, and Astrid Thaysen Foundation.
ABBREVIATIONS
- AACOCF3
arachidonyl trifluoromethylketone
- Ac-DEVD-CHO
acetyl-Asp-Glu-Val-Asp-aldehyde
- cPLA2
cytosolic phospholipase A2
- ICE
interleukin 1β-converting enzyme
- PARP
poly(ADP)ribose polymerase
- TNF
tumor necrosis factor
References
- 1.White E. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Yuan J-Y, Shaham S, Ledoux S, Ellis H M, Horvitz H R. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 3.Tartaglia L A, Ayers T M, Wong G H W, Goeddel D V. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 4.Itoh N, Nagata S. J Biol Chem. 1993;268:10932–10937. [PubMed] [Google Scholar]
- 5.Hsu H, Xiong J, Goeddel D V. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 6.Chinnaiyan A M, O’Rourke K, Tewari M, Dixit V M. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 7.Hsu H, Shu H-B, Pan M-G, Goeddel D V. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 8.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 9.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 10.Sclegel J, Peters I, Orrenius S, Miller D K, Thornberry N A, Yamin T-T, Nicholson D W. J Biol Chem. 1996;271:1841–1844. doi: 10.1074/jbc.271.4.1841. [DOI] [PubMed] [Google Scholar]
- 11.Duan H, Chinnaiyan A M, Hudson P L, Wing J P, He W, Dixit V M. J Biol Chem. 1996;271:1621–1625. doi: 10.1074/jbc.271.3.1621. [DOI] [PubMed] [Google Scholar]
- 12.Los M, Van de Craen M, Penning L C, Schenk H, Westendorp M, Bauerle P A, Dröge W, Krammer P H, Fiers W, Scultze-Osthoff K. Nature (London) 1995;375:81–83. doi: 10.1038/375081a0. [DOI] [PubMed] [Google Scholar]
- 13.Enari M, Hug H, Nagata S. Nature (London) 1995;375:78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- 14.Tewari M, Dixit V M. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 15.Beidler D R, Tewari M, Friesen P D, Poirier G, Dixit V M. J Biol Chem. 1995;270:16526–16528. doi: 10.1074/jbc.270.28.16526. [DOI] [PubMed] [Google Scholar]
- 16.Miura M, Friedlander R M, Yuan J. Proc Natl Acad Sci USA. 1995;92:8318–8322. doi: 10.1073/pnas.92.18.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miura M, Zhu H, Rotello R, Hartwieg E A, Yuan J. Cell. 1993;75:653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Miura M, Bergeron L, Zhu H, Yuan J. Cell. 1994;78:739–750. doi: 10.1016/s0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- 19.Jäättelä M. Lab Invest. 1991;64:724–742. [PubMed] [Google Scholar]
- 20.Clark M A, Chen M-J, Crooke S T, Bomalaski J S. Biochem J. 1988;250:125–132. doi: 10.1042/bj2500125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knauer M F, Longmuir K J, Yamamoto R S, Fitzgerald T P, Granfer G A. J Cell Physiol. 1990;142:469–479. doi: 10.1002/jcp.1041420305. [DOI] [PubMed] [Google Scholar]
- 22.Neale M L, Fiera A, Matthews N. Immunology. 1988;64:81–85. [PMC free article] [PubMed] [Google Scholar]
- 23.Jäättelä M. J Immunol. 1993;151:4286–4294. [PubMed] [Google Scholar]
- 24.Hayakawa M, Ishida N, Takeuchi K, Shibamoto S, Hori T, Oku N, Ito F, Tsujimoto M. J Biol Chem. 1993;268:11290–11295. [PubMed] [Google Scholar]
- 25.Voelkel-Johnson C, Thorne T E, Laster S M. J Immunol. 1996;156:201–207. [PubMed] [Google Scholar]
- 26.Jäättelä M, Benedict M, Tewari M, Shayman J A, Dixit V M. Oncogene. 1995;10:2297–2305. [PubMed] [Google Scholar]
- 27.Jäättelä M, Pinola M, Saksela E. Lymphokine Cytokine Res. 1991;10:119–125. [PubMed] [Google Scholar]
- 28.Tewari M, Quan L T, O’Rourke K, Desnoyers S, Zeng Z, Beidler D R, Poirier G G, Salvesen G S, Dixit V M. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 29.Jäättelä M, Mouritzen H, Elling F, Bastholm L. J Immunol. 1996;156:1166–1173. [PubMed] [Google Scholar]
- 30.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Kauffmann S H, Desnoyers S, Ottaviano Y, Davidson N E, Poirier G G. Cancer Res. 1993;53:3976–3985. [PubMed] [Google Scholar]
- 32.Nalefski E A, Sultzman L A, Martin D M, Kriz R W, Towler P S, Knopf J L, Clark J D. J Biol Chem. 1994;269:18239–18249. [PubMed] [Google Scholar]
- 33.Jäättelä M, Saksela K, Saksela E. Eur J Immunol. 1989;19:1413–1417. doi: 10.1002/eji.1830190810. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A, Munday N A, Raju S M, Smulson M E, Yamin T T, Yu V L, Miller D K. Nature (London) 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 35.Sharp J D, White D L, Chiou G, Goodson T, Gamboa G C, McClure D, Burgett S, Hoskins J, Skatrud P L, Sportsman J R, Becker G W, Kang L H, Roberts E F, Kramer R M. J Biol Chem. 1991;266:14850–14853. [PubMed] [Google Scholar]
- 36.Na S, Chuang T-S, Cunningham A, Turi T G, Hanke J H, Bokoch G M, Danley D E. J Biol Chem. 1996;271:11209–11213. doi: 10.1074/jbc.271.19.11209. [DOI] [PubMed] [Google Scholar]
- 37.Street I, Lin H K, Lalibert F, Ghomashchi F, Wang Z, Perrier H, Tremblay N M, Huang Z, Weech P K, Gelb M H. Biochemistry. 1993;32:5935–5940. doi: 10.1021/bi00074a003. [DOI] [PubMed] [Google Scholar]
- 38.Channon J Y, Leslie C C. J Biol Chem. 1990;265:5409–5413. [PubMed] [Google Scholar]
- 39.Lin L-L, Wartmann M, Lin A Y, Knopf J L, Seth A, Davis R J. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 40.Thorne T E, Voelkel-Johanson C, Casey W M, Parks L W, Laster S M. J Virol. 1996;70:8502–8507. doi: 10.1128/jvi.70.12.8502-8507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mutch D G, Powell C B, Kao M-S, Collins J L. Cancer Res. 1992;52:866–872. [PubMed] [Google Scholar]
- 42.Emoto Y, Manome Y, Meinhard G, Kisaki H, Kharbanda S, Robertson M, Ghayur T, Wong W W, Kamen R, Weichselbaum R, Kufe D. EMBO J. 1995;14:6148–6156. doi: 10.1002/j.1460-2075.1995.tb00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghayur T, Huguin M, Talanian R V, Ratnofsky S, Quinlan C, Emoto Y, Pandey P, Datta R, Huang Y, Kharbanda S, Allen H, Kamen R, Wong W, Kufe D. J Exp Med. 1996;184:2399–2404. doi: 10.1084/jem.184.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jayadev S, Linardic C M, Hannun Y A. J Biol Chem. 1994;269:5757–5763. [PubMed] [Google Scholar]