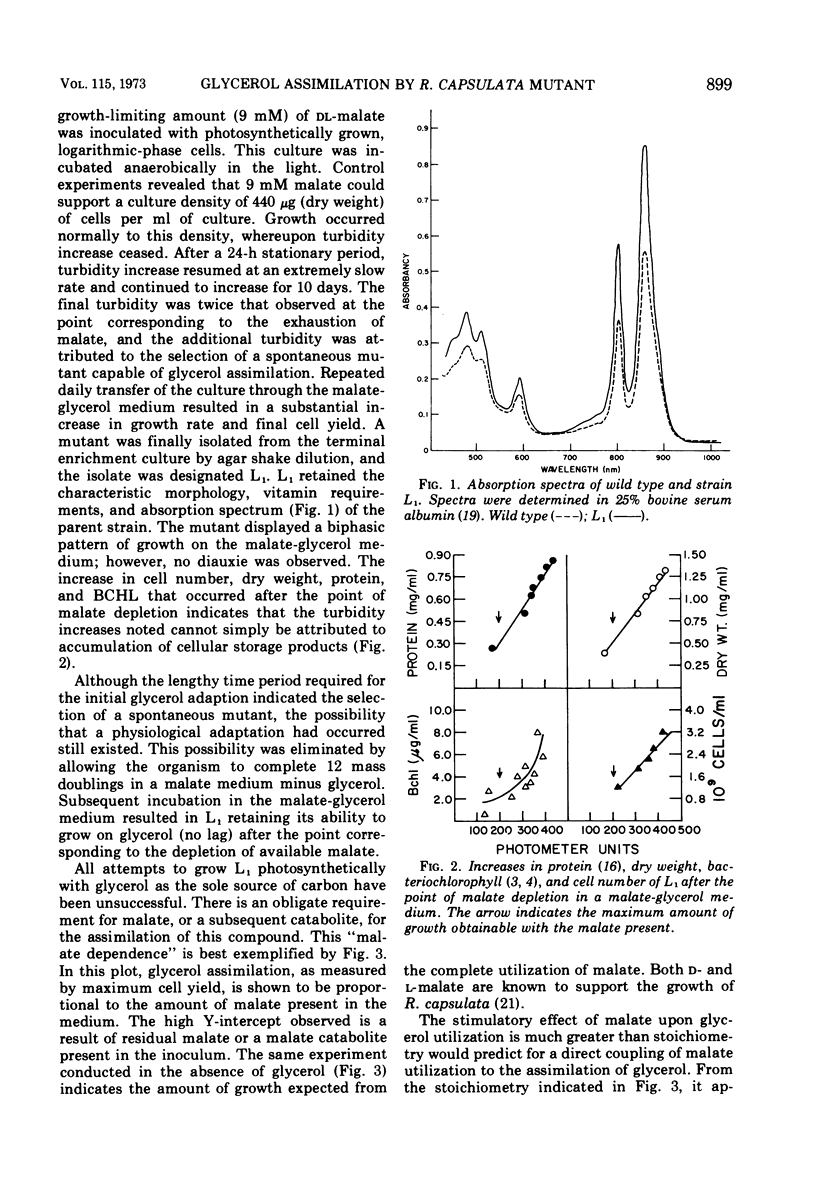
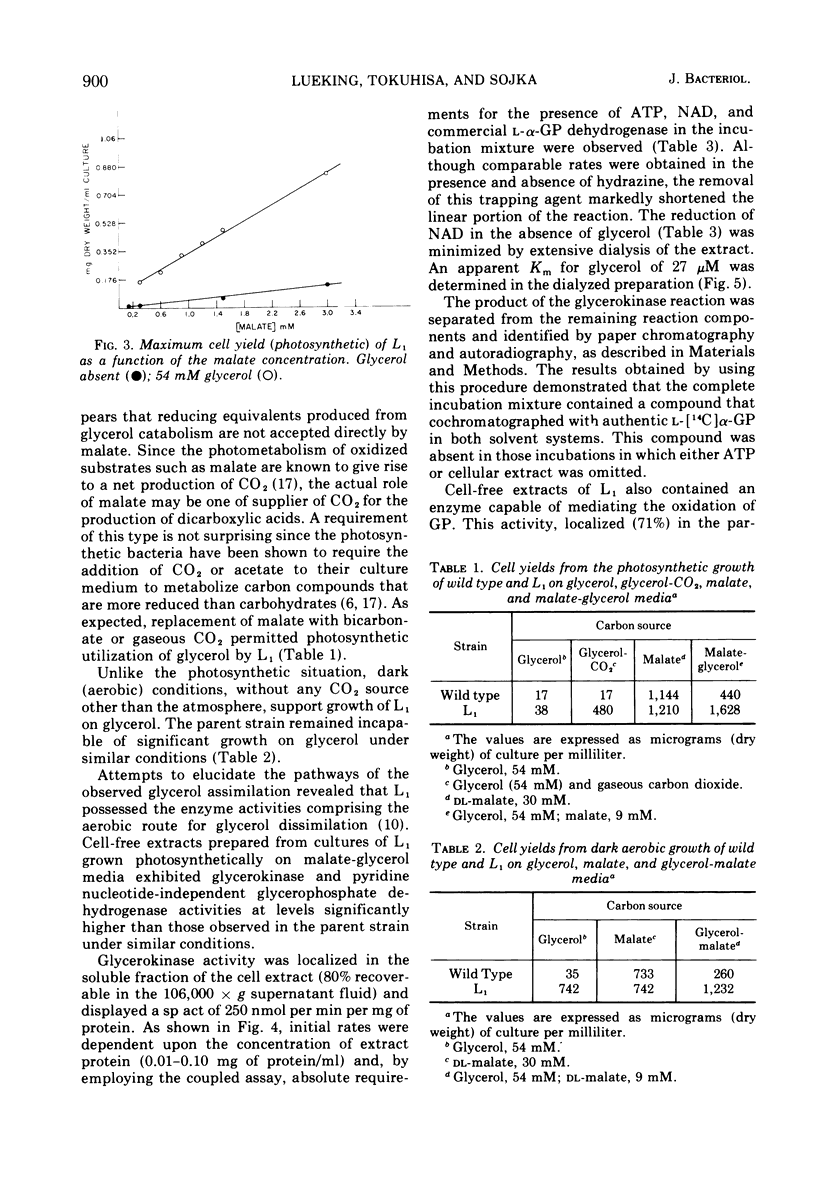
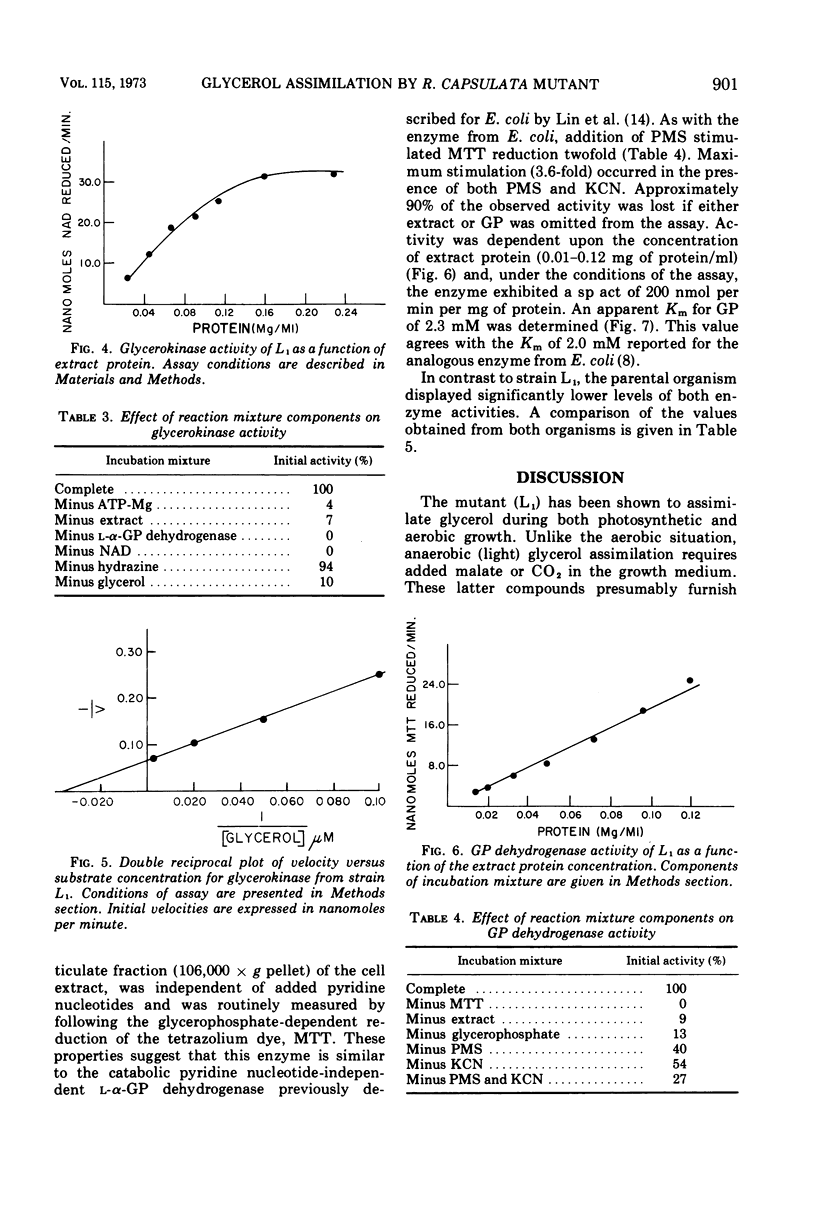
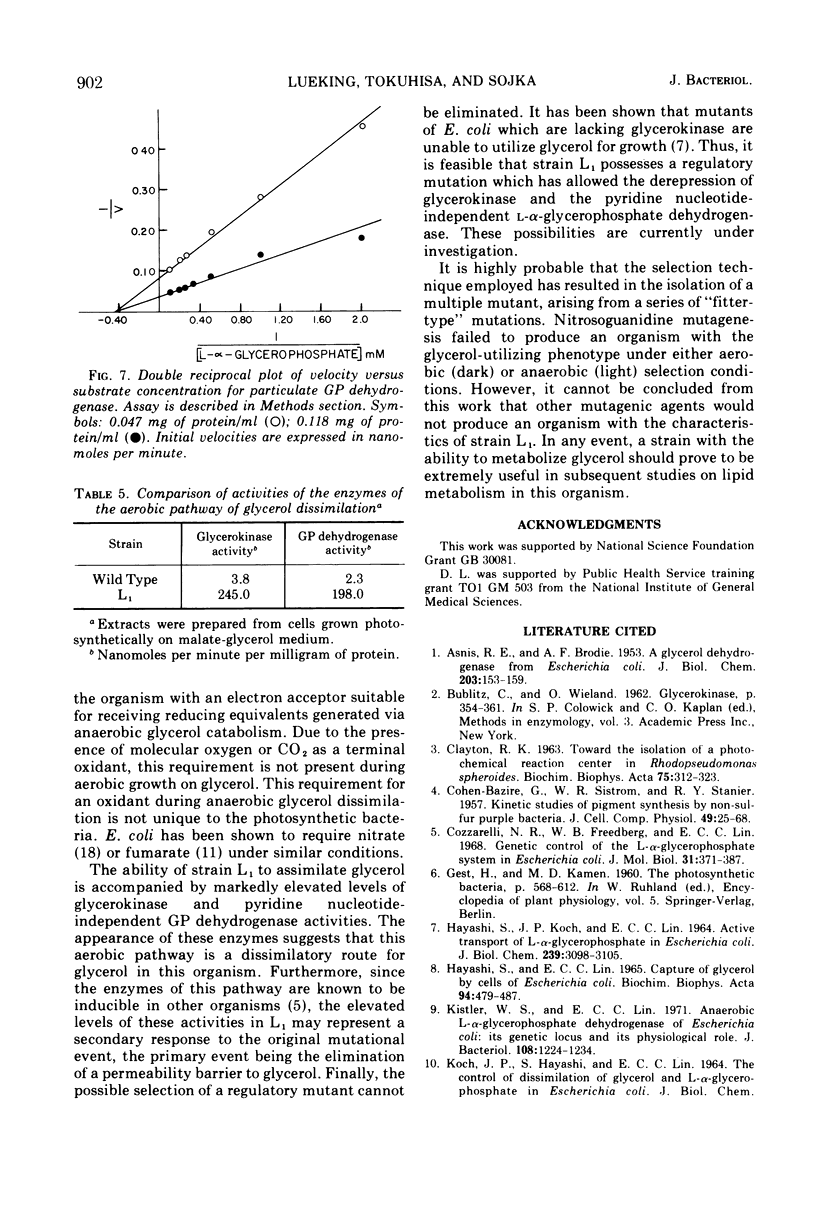
Abstract
A spontaneous mutant of Rhodopseudomonas capsulata, capable of growth on glycerol, has been isolated. The mutant requires CO2 or malate to assimilate glycerol photosynthetically. This requirement is not manifested aerobically. Glycerokinase (EC 2.7.1.30) and pyridine nucleotide-independent l-α-glycerophosphate dehydrogenase (EC 1.1.2.1) activities appear coincidently with the metabolism of glycerol, suggesting that this organism employs these enzymes for glycerol dissimilation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASNIS R. E., BRODIE A. F. A glycerol dehydrogenase from Escherichia coli. J Biol Chem. 1953 Jul;203(1):153–159. [PubMed] [Google Scholar]
- CLAYTON R. K. TOWARD THE ISOLATION OF A PHOTOCHEMICAL REACTION CENTER IN RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1963 Nov 29;75:312–323. doi: 10.1016/0006-3002(63)90618-8. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Freedberg W. B., Lin E. C. Genetic control of L-alpha-glycerophosphate system in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):371–387. doi: 10.1016/0022-2836(68)90415-4. [DOI] [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- HAYASHI S., LIN E. C. CAPTURE OF GLYCEROL BY CELLS OF ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Mar 29;94:479–487. doi: 10.1016/0926-6585(65)90056-7. [DOI] [PubMed] [Google Scholar]
- KOCH J. P., HAYASHI S., LIN E. C. THE CONTROL OF DISSIMILATION OF GLYCEROL AND L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3106–3108. [PubMed] [Google Scholar]
- Kistler W. S., Lin E. C. Anaerobic L- -glycerophosphate dehydrogenase of Escherichia coli: its genetic locus and its physiological role. J Bacteriol. 1971 Dec;108(3):1224–1234. doi: 10.1128/jb.108.3.1224-1234.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. The role of fumarate in the respiration of Bacterium coli commune. Biochem J. 1937 Nov;31(11):2095–2124. doi: 10.1042/bj0312095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C., KOCH J. P., CHUSED T. M., JORGENSEN S. E. Utilization of L-alpha-glycerophosphate by Escherichia coli without hydrolysis. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2145–2150. doi: 10.1073/pnas.48.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C., LEVIN A. P., MAGASANIK B. The effect of aerobic metabolism on the inducible glycerol dehydrogenase of Aerobacter aerogenes. J Biol Chem. 1960 Jun;235:1824–1829. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampe H. H., Drews G. Die Differenzierung des Membransystems von Rhodopseudomonas capsulata hinsichtlich seiner photosynthetischen und respiratorischen Funktionen. Arch Mikrobiol. 1972;84(1):1–19. [PubMed] [Google Scholar]
- Lampe H. H., Oelze J., Drews G. Die Fraktionierung des Membransystems von Rhodopseudomonas capsulata und seine morphogenese. Arch Mikrobiol. 1972;83(1):78–94. [PubMed] [Google Scholar]
- Quastel J. H., Stephenson M., Whetham M. D. Some Reactions of Resting Bacteria in Relation to Anaerobic Growth. Biochem J. 1925;19(2):304–317. doi: 10.1042/bj0190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sojka G. A., Freeze H. H., Gest H. Quantitative estimation of bacteriochlorophyll in situ. Arch Biochem Biophys. 1970 Feb;136(2):578–580. doi: 10.1016/0003-9861(70)90231-6. [DOI] [PubMed] [Google Scholar]
- Sojka G. A., Gest H. Integration of energy conversion and biosynthesis in the photosynthetic bacterium Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1486–1493. doi: 10.1073/pnas.61.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl C. L., Sojka G. A. Growth of Rhodopseudomonas capsulata on L- and D-malic acid. Biochim Biophys Acta. 1973 Feb 28;297(2):241–245. doi: 10.1016/0304-4165(73)90070-6. [DOI] [PubMed] [Google Scholar]
- Steiner S., Sejka G. A., Conti S. F., Gest H., Lester R. L. Modification of membrane composition in growing photosynthetic bacteria. Biochim Biophys Acta. 1970 Jun 2;203(3):571–574. doi: 10.1016/0005-2736(70)90194-x. [DOI] [PubMed] [Google Scholar]
- Zilinsky J. W., Sojka G. A., Gest H. Energy charge regulation in photosynthetic bacteria. Biochem Biophys Res Commun. 1971 Mar 5;42(5):955–961. doi: 10.1016/0006-291x(71)90523-7. [DOI] [PubMed] [Google Scholar]
- van Niel C. B. THE CULTURE, GENERAL PHYSIOLOGY, MORPHOLOGY, AND CLASSIFICATION OF THE NON-SULFUR PURPLE AND BROWN BACTERIA. Bacteriol Rev. 1944 Mar;8(1):1–118. doi: 10.1128/br.8.1.1-118.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]



