Abstract
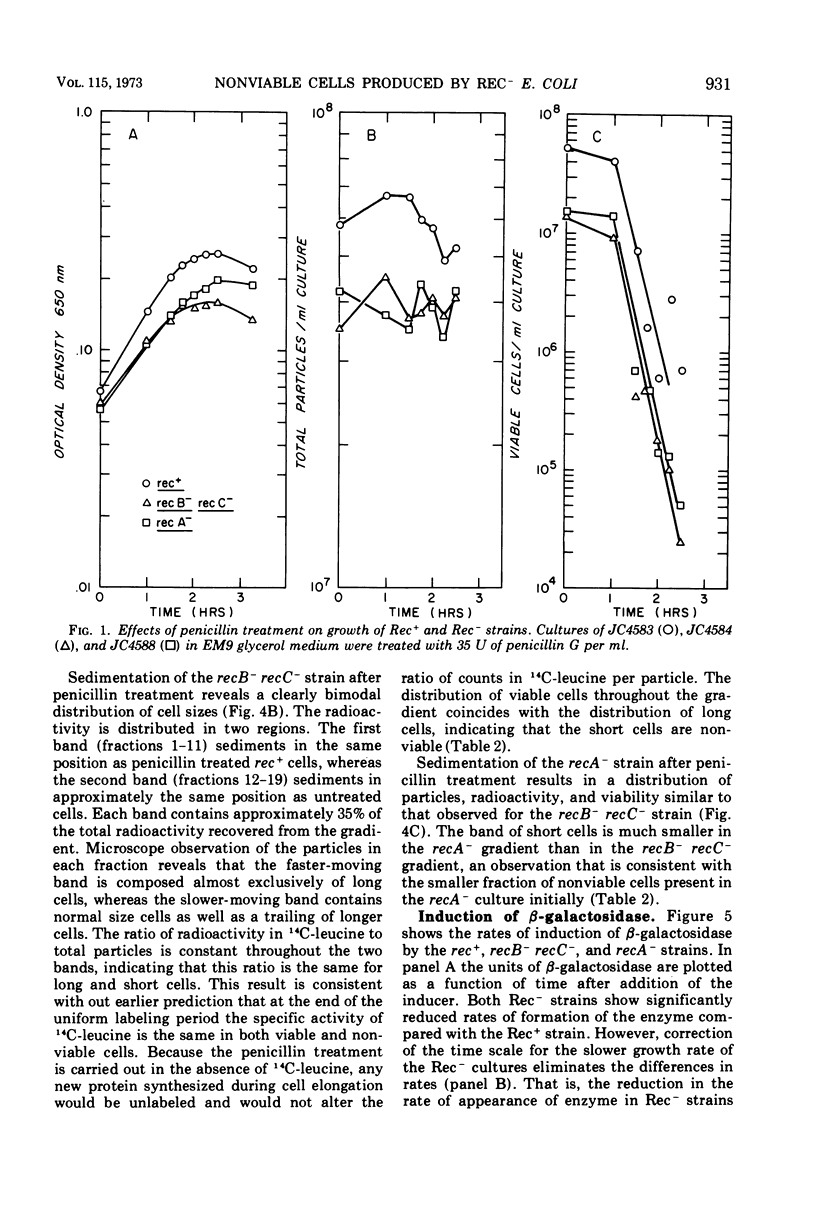
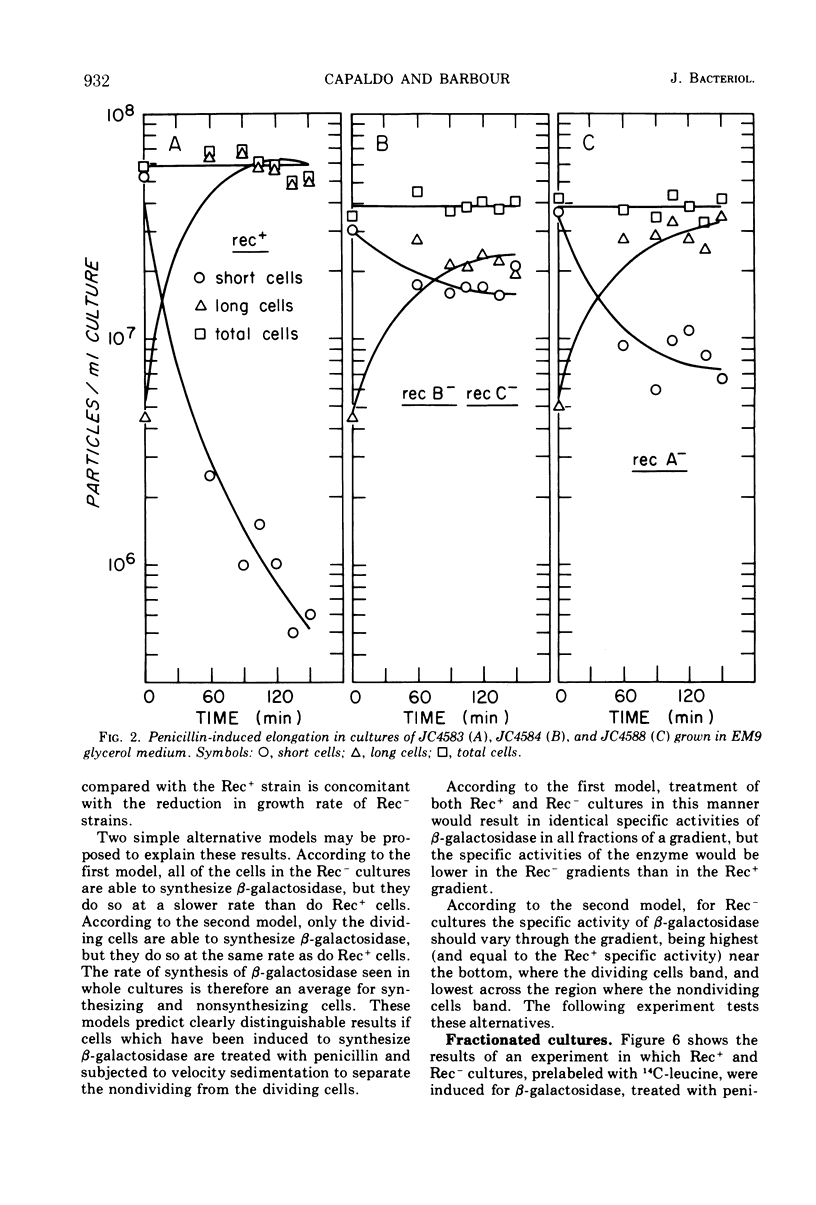
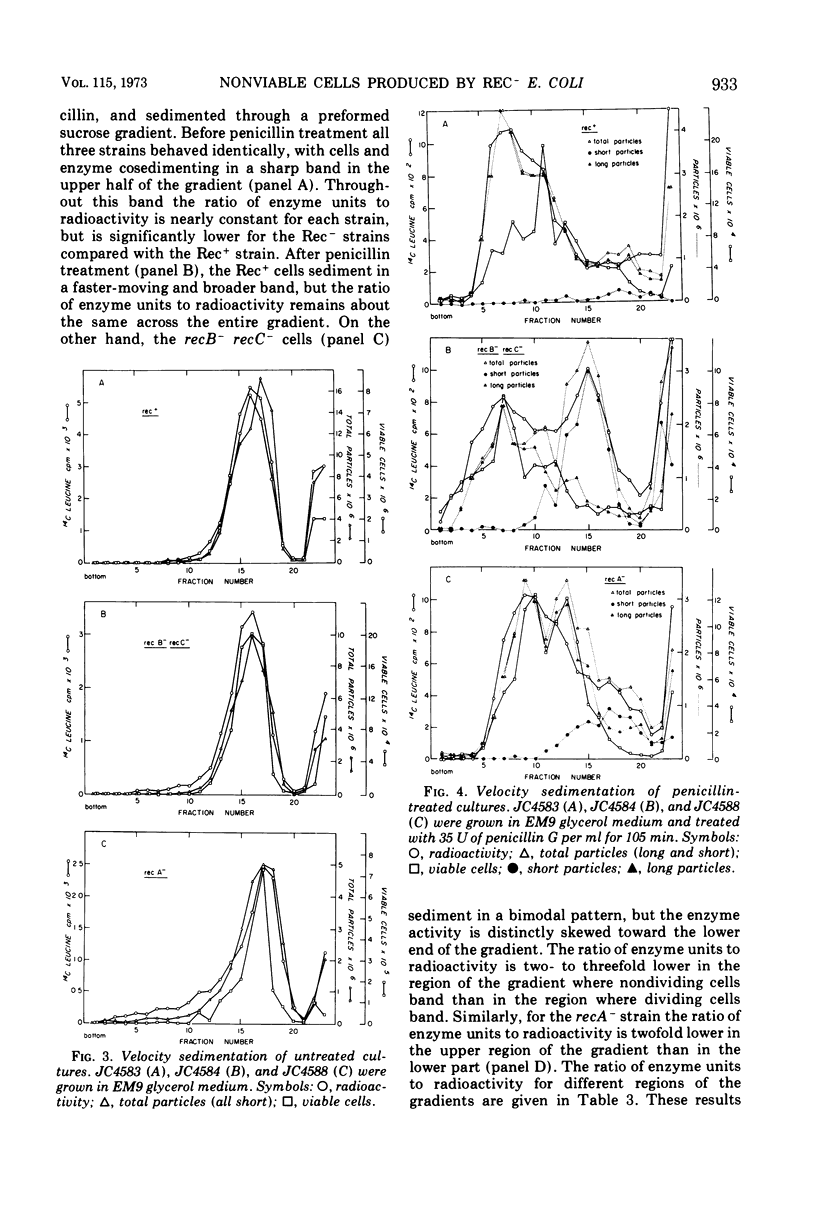
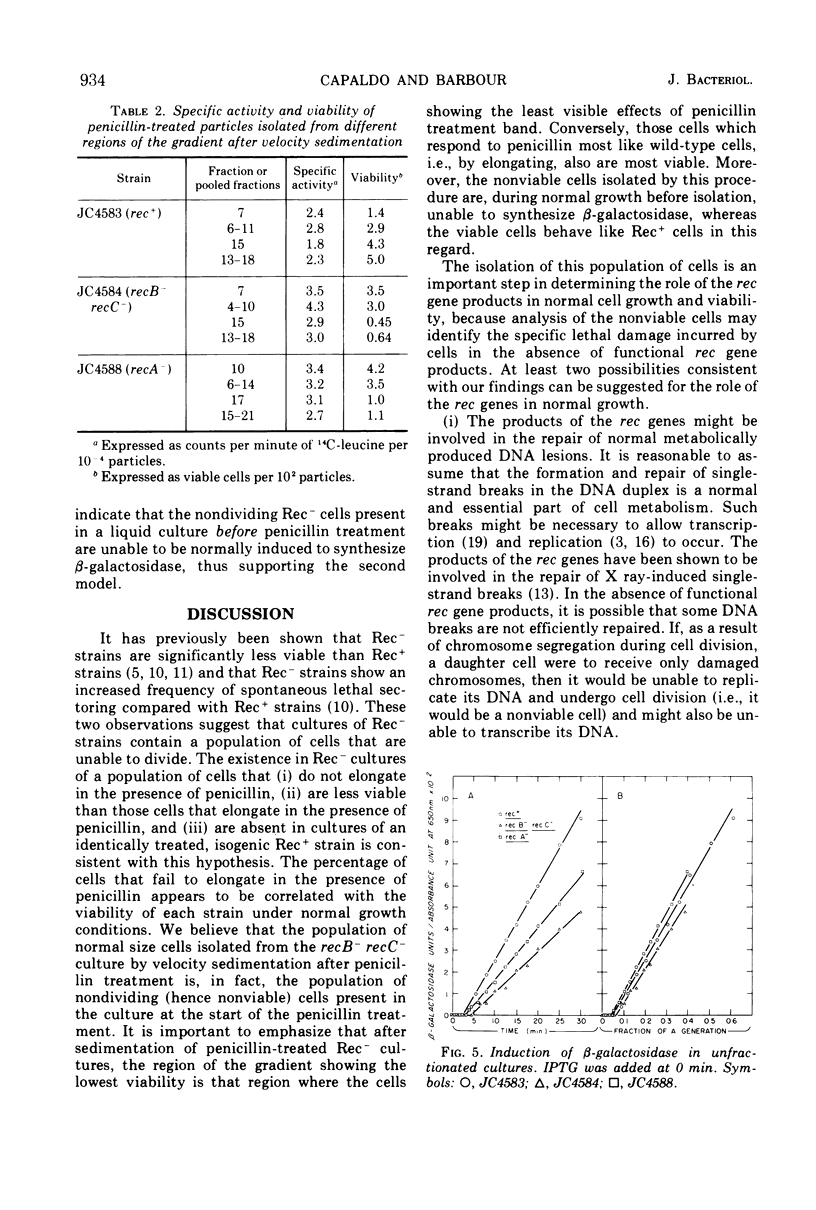
During normal growth, cultures of recombination-deficient (Rec−) strains contain a population of cells that do not form colonies. Such cells are not present in a culture of an isogenic Rec+ strain. We present a procedure for isolating and studying this defective population of cells. Exposure of a growing Rec− or Rec+ culture to low levels of penicillin causes the dividing cells to elongate. The size of the nonviable cells present in the Rec− cultures is unaffected. The nonviable cells are then separated from the elongated cells by velocity sedimentation. This isolation technique provides a convenient way of analyzing the composition, biosynthetic capacity, and enzymatic function of the nonviable cells before isolation. In this paper we present data showing that before fractionation the nonviable cells in the Rec− culture are defective in their ability to synthesize β-galactosidase, whereas the Rec− viable cells behave like the Rec+ cells in this regard. This observation confirms the existence of at least two classes of cells in liquid cultures of Rec− strains grown under normal conditions. That class of cells which is unable to synthesize β-galactosidase is the same class that cannot form colonies when plated on solid medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour S. D. Biochemical and genetic studies of recombination proficiency in Escherichia coli K12. IV. Analysis of recombinants formed by a recombination deficient (recB21 recC22) strain. Mol Gen Genet. 1972;117(4):303–309. doi: 10.1007/BF00333024. [DOI] [PubMed] [Google Scholar]
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo-Kimball F., Barbour S. D. Involvement of recombination genes in growth and viability of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):204–212. doi: 10.1128/jb.106.1.204-212.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J. The beginning of a genetic analysis of recombination proficiency. J Cell Physiol. 1967 Oct;70(2 Suppl):165–180. doi: 10.1002/jcp.1040700412. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson P. T. Recombination deficient mutants of Escherichia coli K12 that map between thy A and argA. Genetics. 1968 Sep;60(1):19–30. doi: 10.1093/genetics/60.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefner K. Spontaneous lethal sectoring, a further feature of Escherichia coli strains deficient in the function of rec and uvr genes. J Bacteriol. 1968 Sep;96(3):652–659. doi: 10.1128/jb.96.3.652-659.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertman I. M. Survival, DNA-breakdown and induction of prophage lambda in a Escherichia coli K 12 recA uvrB double mutant. Genet Res. 1969 Dec;14(3):291–307. doi: 10.1017/s0016672300002111. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. Pleiotropic effect of the rec A gene of Escherichia coli: uncoupling of cell division from deoxyribonucleic acid replication. J Bacteriol. 1971 May;106(2):539–542. doi: 10.1128/jb.106.2.539-542.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp D. S., Smith K. C. Repair of radiation-induced damage in Escherichia coli. II. Effect of rec and uvr mutations on radiosensitivity, and repair of x-ray-induced single-strand breaks in deoxyribonucleic acid. J Bacteriol. 1970 Jul;103(1):49–54. doi: 10.1128/jb.103.1.49-54.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk M., Kinross J. Conditional lethality of recA and recB derivatives of a strain of Escherichia coli K-12 with a temperature-sensitive deoxyribonucleic acid polymerase I. J Bacteriol. 1972 Mar;109(3):971–978. doi: 10.1128/jb.109.3.971-978.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Tomizawa J. I., Fuke M. Replication of bacteriophage DNA, II. Structure of replicating DNA of phage lambda. Proc Natl Acad Sci U S A. 1968 Jul;60(3):861–865. doi: 10.1073/pnas.60.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M. An ATP-dependent deoxyribonuclease from Escherichia coli with a possible role in genetic recombination. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1292–1299. doi: 10.1073/pnas.64.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling C., Hanawalt P. Nonconservative DNA replication in bacteria after thymine starvation. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1728–1735. doi: 10.1073/pnas.54.6.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa H. Structural genes of ATP-dependent deoxyribonuclease of Escherichia coli. Nat New Biol. 1972 Sep 6;239(88):14–16. doi: 10.1038/newbio239014a0. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol. 1969 Oct;100(1):231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J., Low B. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J Bacteriol. 1969 Jan;97(1):244–249. doi: 10.1128/jb.97.1.244-249.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Mount D. W. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J Bacteriol. 1969 Nov;100(2):923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



