Abstract
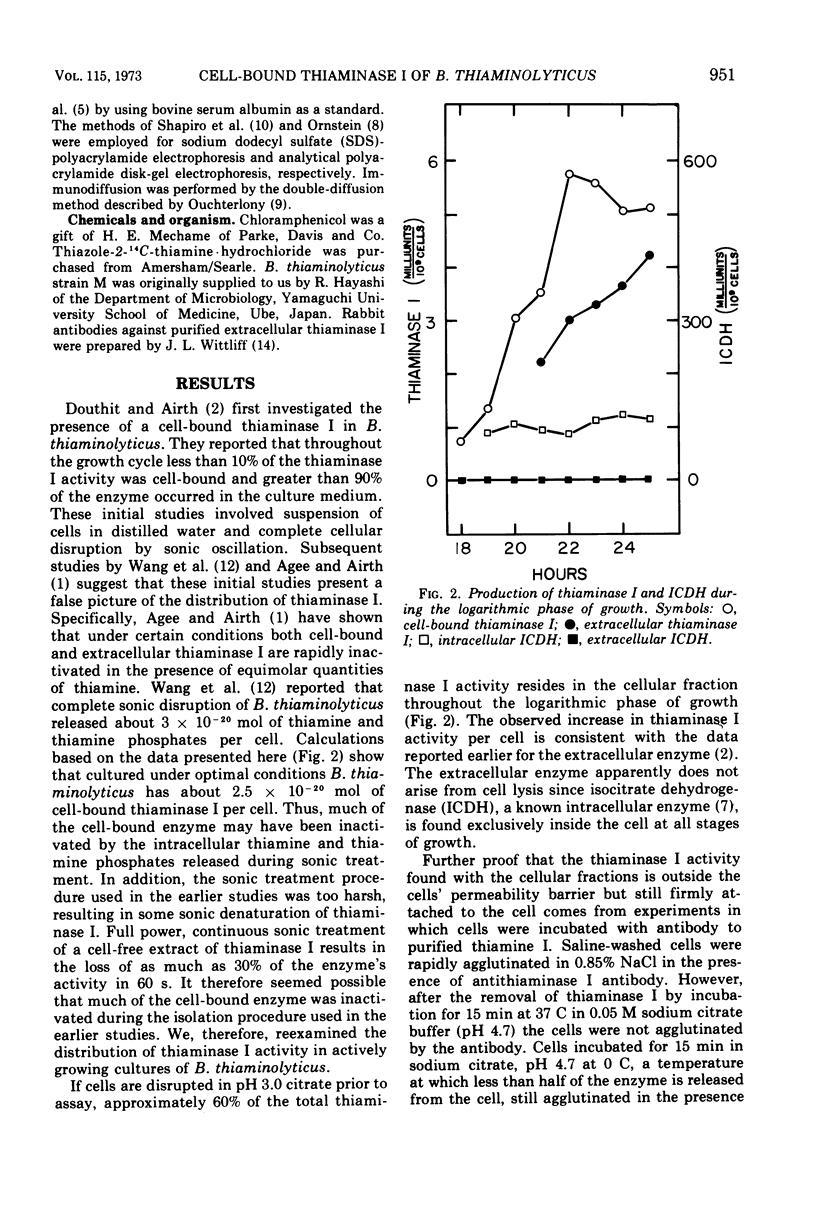
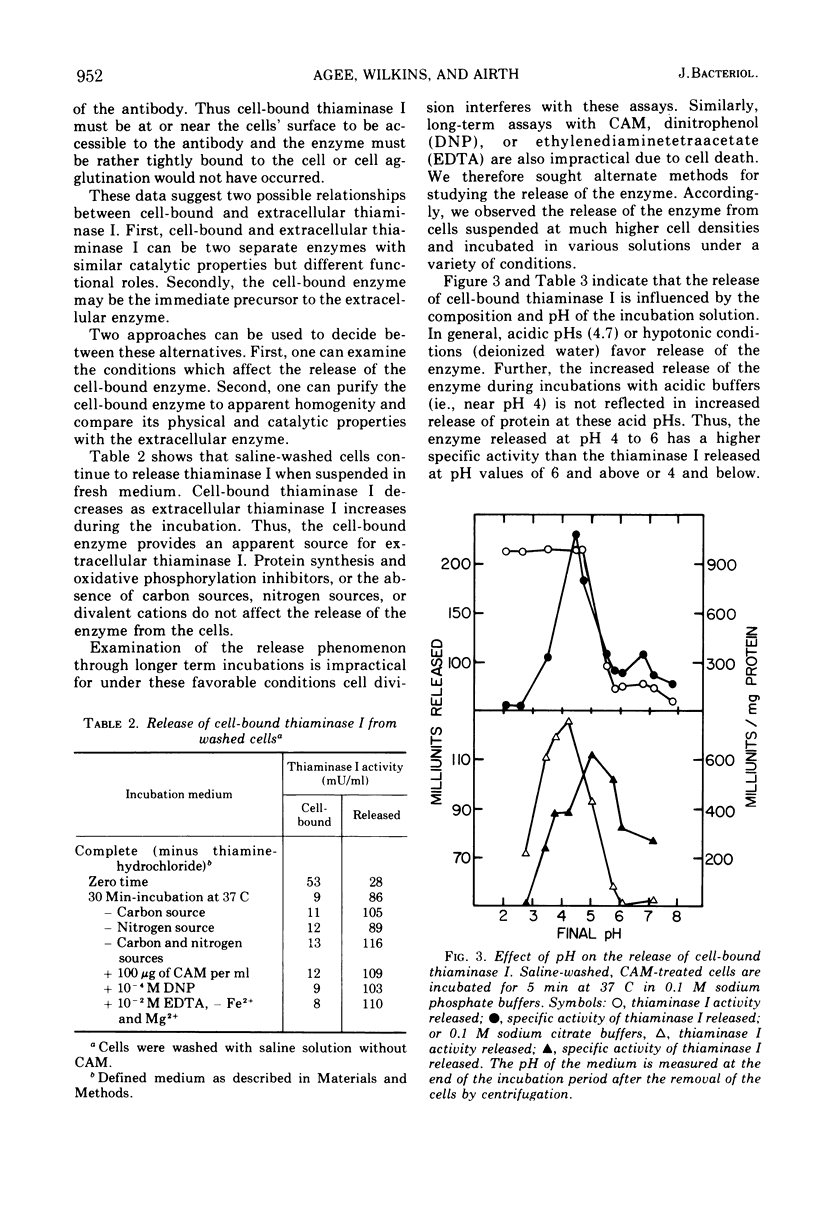
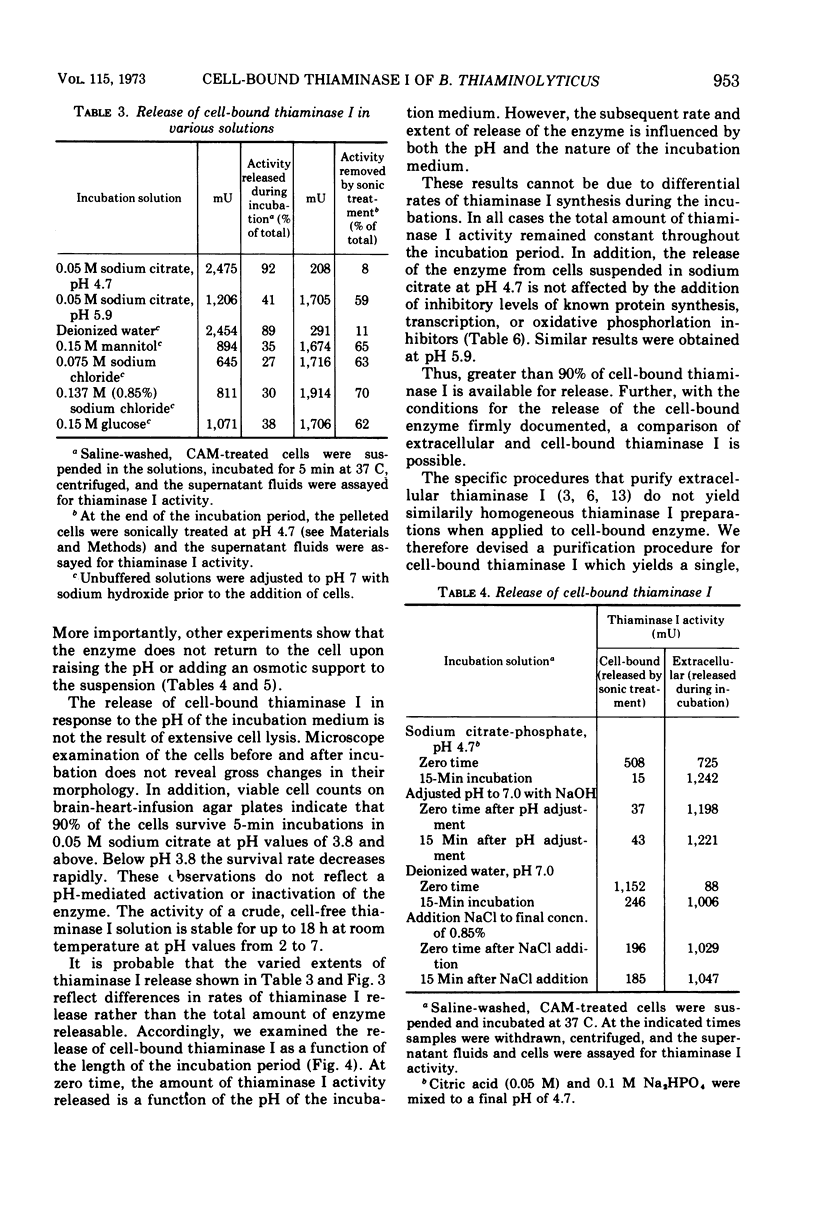
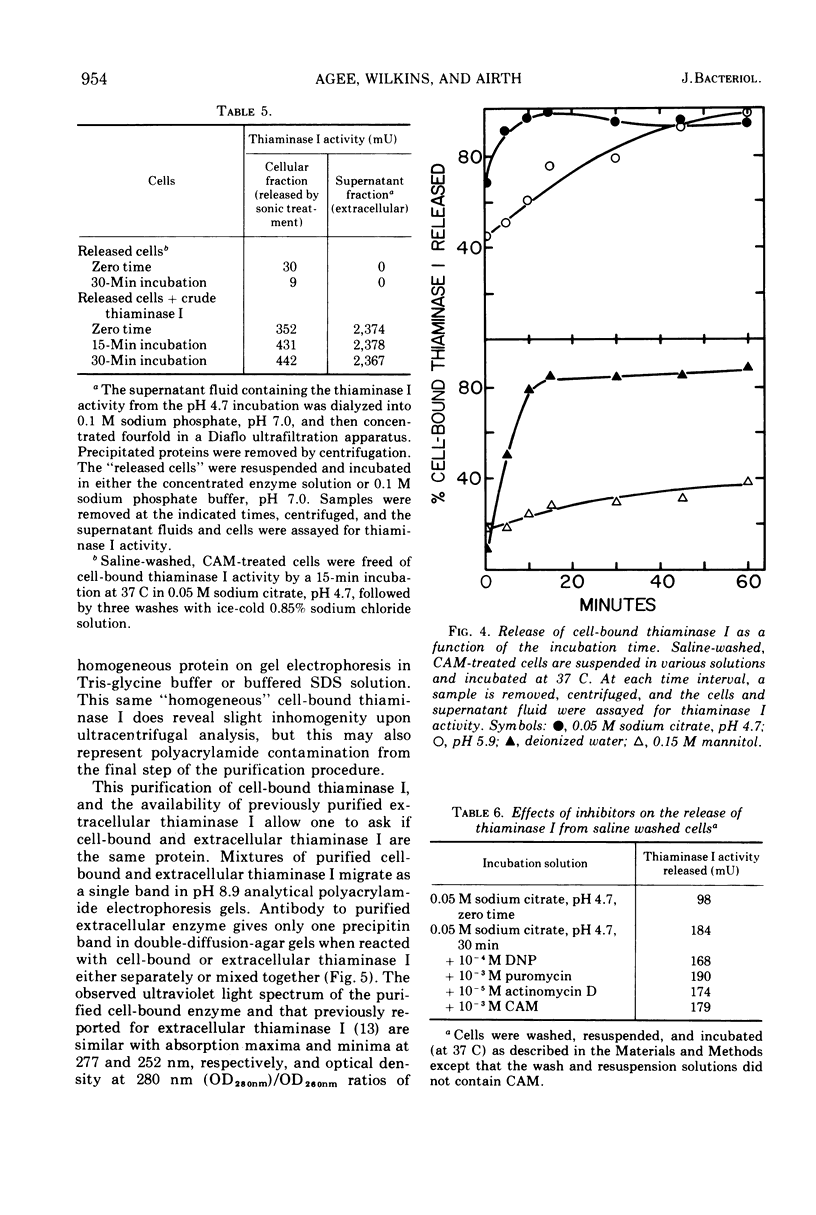
The distribution of the extracellular enzyme, thiaminase I, was determined for logarithmically growing cultures of Bacillus thiaminolyticus. About 60% of the enzyme is associated with the cells throughout the growth cycle. The remainder of the enzyme is in the culture medium. The release of the cell-bound thiaminase I is examined under a variety of conditions. The rate and extent of release is dependent on the pH and the nature of the incubation solution. The release process appears to be relatively independent of de novo protein synthesis, energy derived from oxidative phosphorylation, or divalent metal ions. The absence of carbon or nitrogen sources has little effect on the release of the enzyme. Cell-bound thiaminase I probably is the immediate precursor for extracellular thiaminase I found in the culture medium. Washed cells continue to release thiaminase I at the expense of cell-bound enzyme. In addition, purified cell-bound thiaminase I is indistinguishable from purified extracellular thiaminase I by a number of physical and kinetic criteria.
Full text
PDF

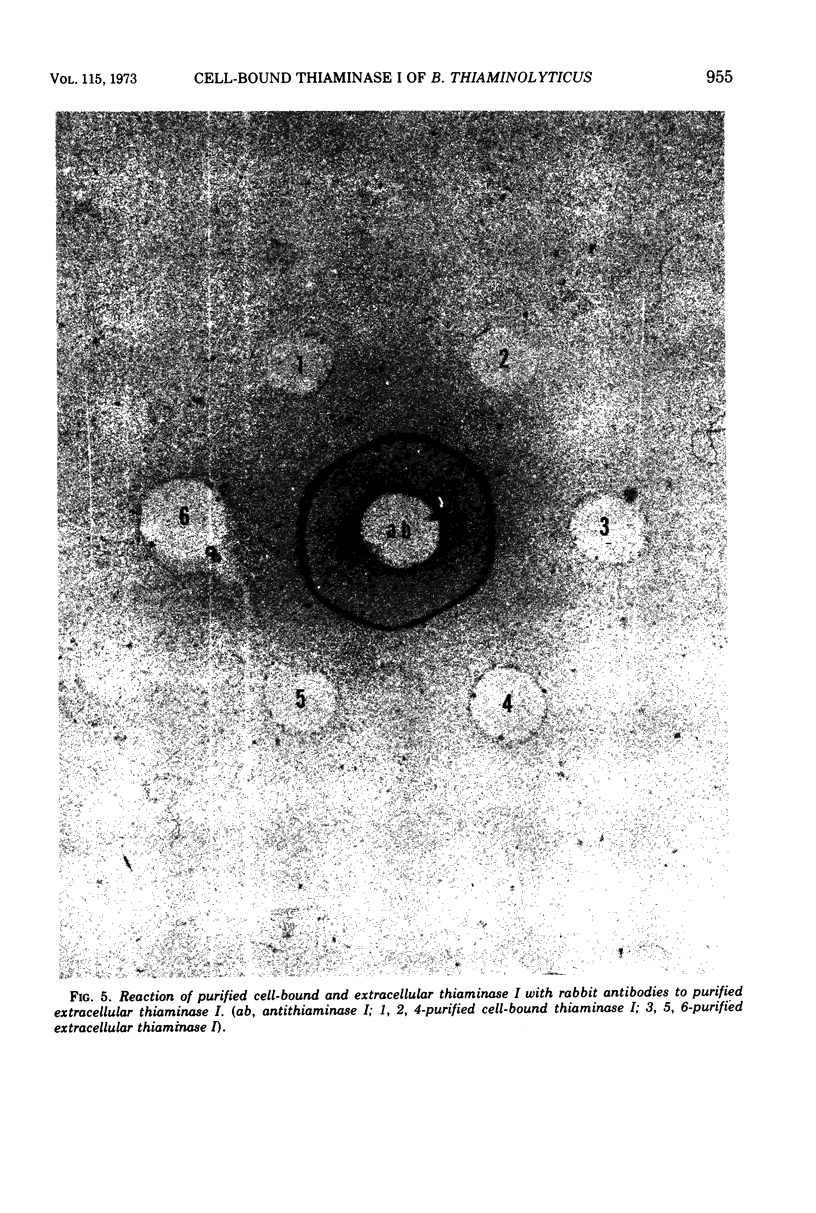

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agee C. C., Airth R. L. Reversible inactivation of thiaminase I of Bacillus thiaminolyticus by its primary substrate, thiamine. J Bacteriol. 1973 Sep;115(3):957–965. doi: 10.1128/jb.115.3.957-965.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douthit H. A., Airth R. L. Thiaminase I of Bacillus thiaminolyticus. Arch Biochem Biophys. 1966 Feb;113(2):331–337. doi: 10.1016/0003-9861(66)90194-9. [DOI] [PubMed] [Google Scholar]
- EBATA J., MURATA K. The purification of thiaminase I produced by Bacillus thiaminolyticus. J Vitaminol (Kyoto) 1961 Jun 10;7:115–121. doi: 10.5925/jnsv1954.7.115. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Wang L., Airth R. L. Repression of thiaminase I in Bacillus thiaminolyticus. Biochem Biophys Res Commun. 1967 May 5;27(3):325–330. doi: 10.1016/s0006-291x(67)80101-3. [DOI] [PubMed] [Google Scholar]
- Wang L., Wilkins J. H., Airth R. L. Repression of thiaminase I by thiamine and related compounds in Bacillus thiaminolyticus. Can J Microbiol. 1968 Oct;14(10):1143–1147. doi: 10.1139/m68-191. [DOI] [PubMed] [Google Scholar]
- Wittliff J. L., Airth R. L. The extracellular thiaminase I of Bacillus thiaminolyticus. I. Purification and physicochemical properties. Biochemistry. 1968 Feb;7(2):736–744. doi: 10.1021/bi00842a032. [DOI] [PubMed] [Google Scholar]
- Wittliff J. L., Mandy W. J., Airth R. L. The extracellular thiaminase I of Bacillus thiaminolyticus. II. Preparation of the antisera and serological properties. Biochemistry. 1968 Jun;7(6):2380–2384. doi: 10.1021/bi00846a047. [DOI] [PubMed] [Google Scholar]




