Abstract
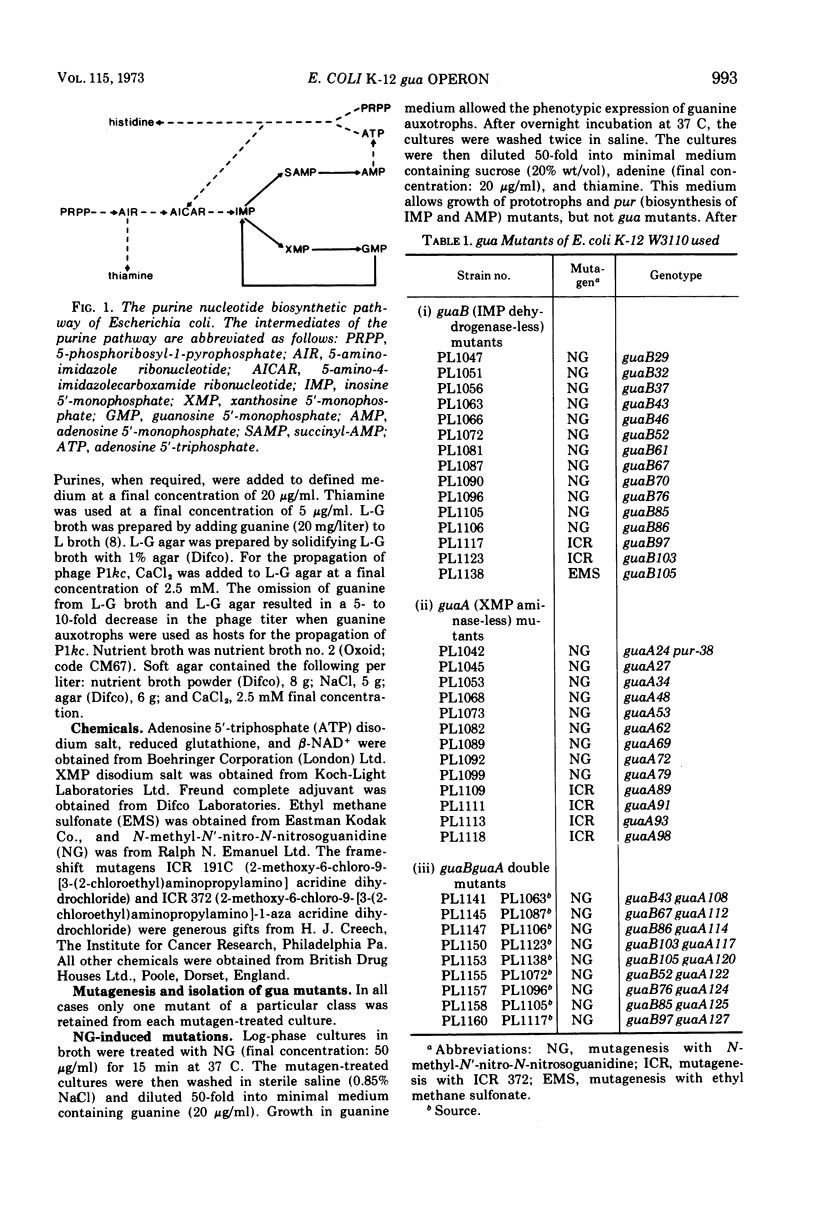
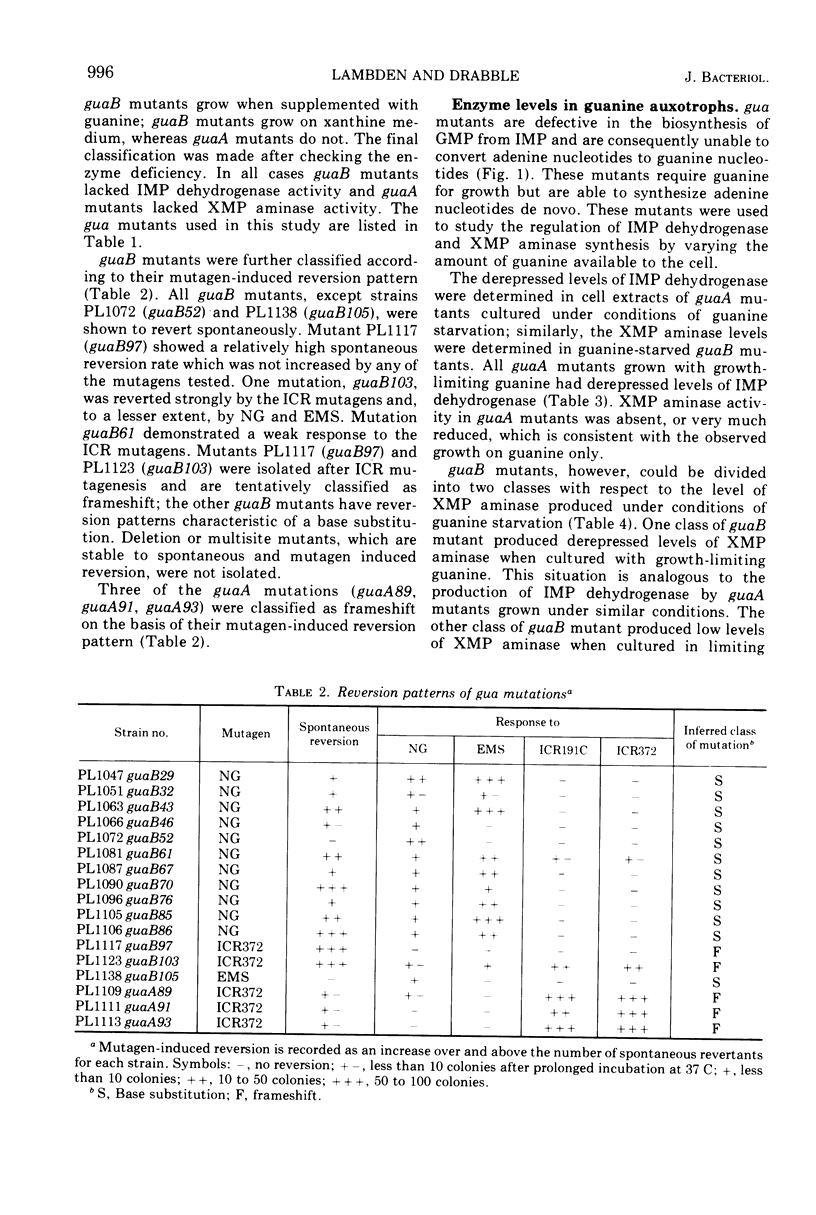
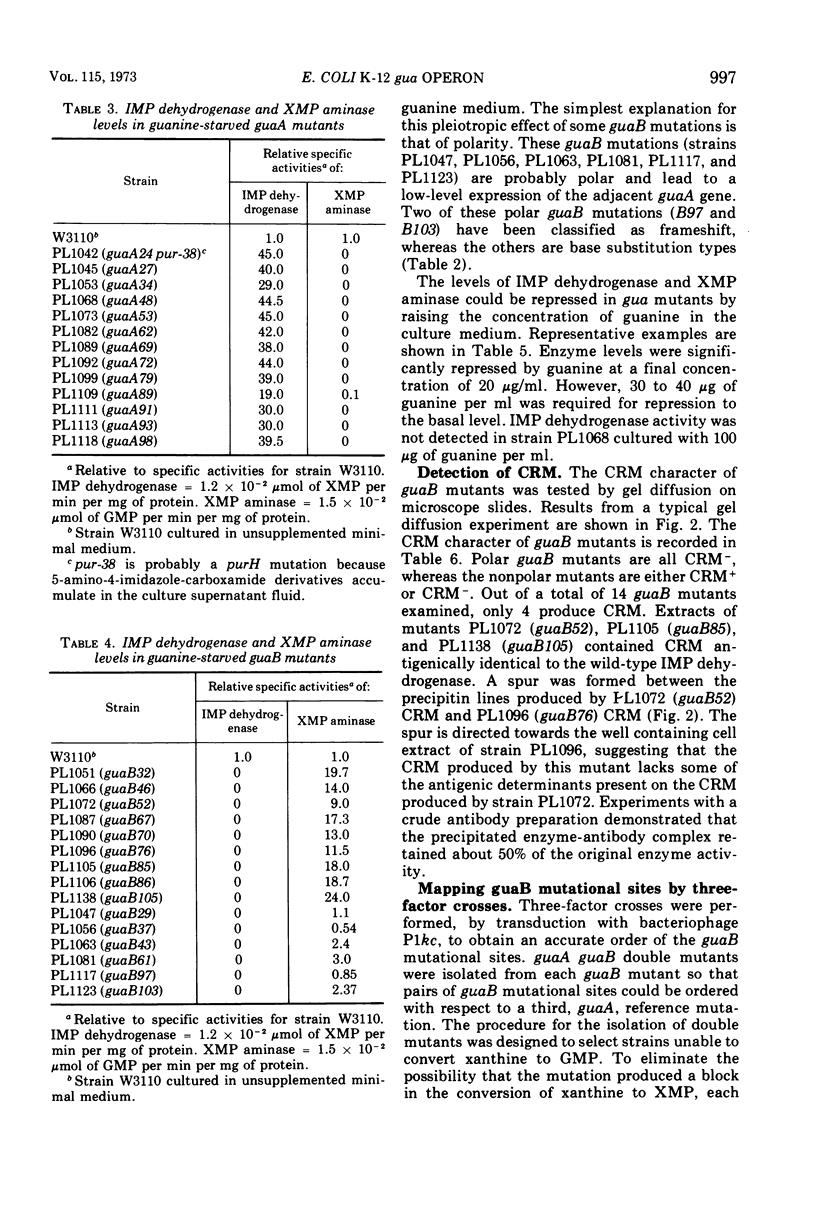
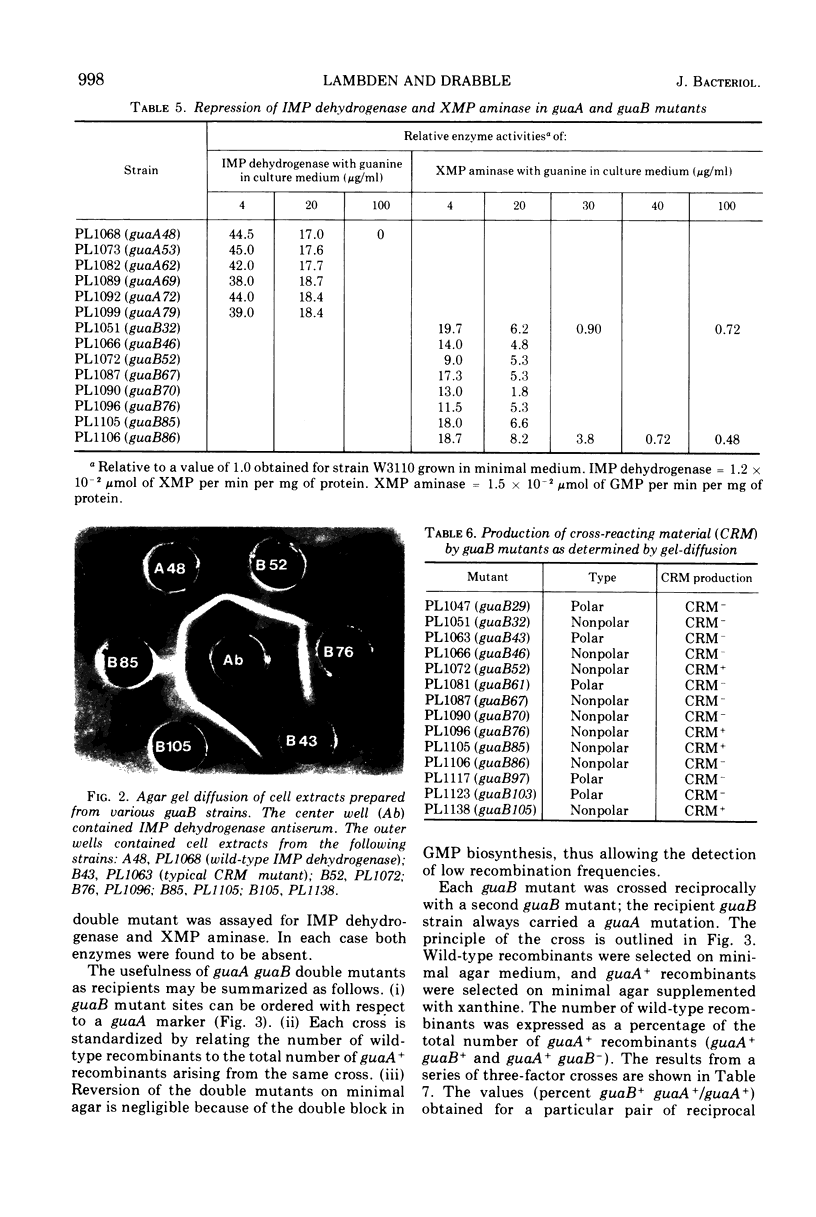
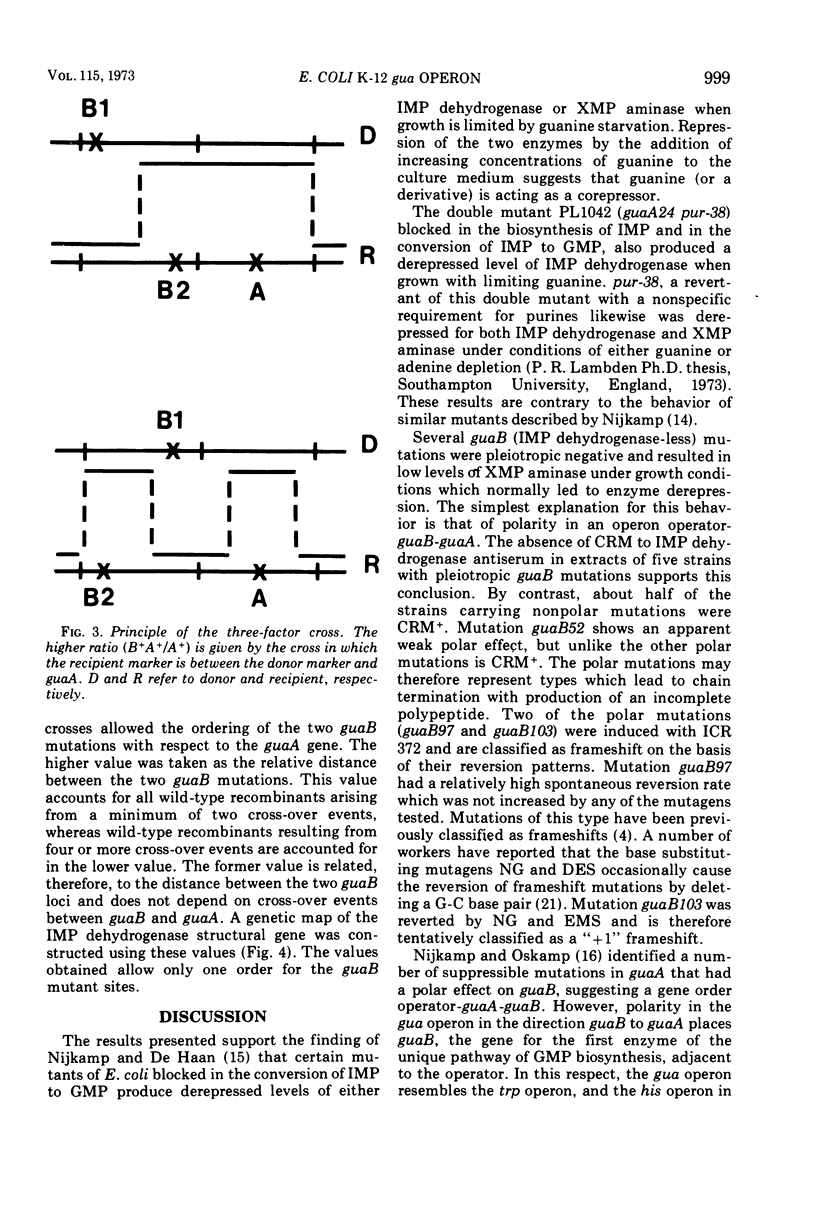
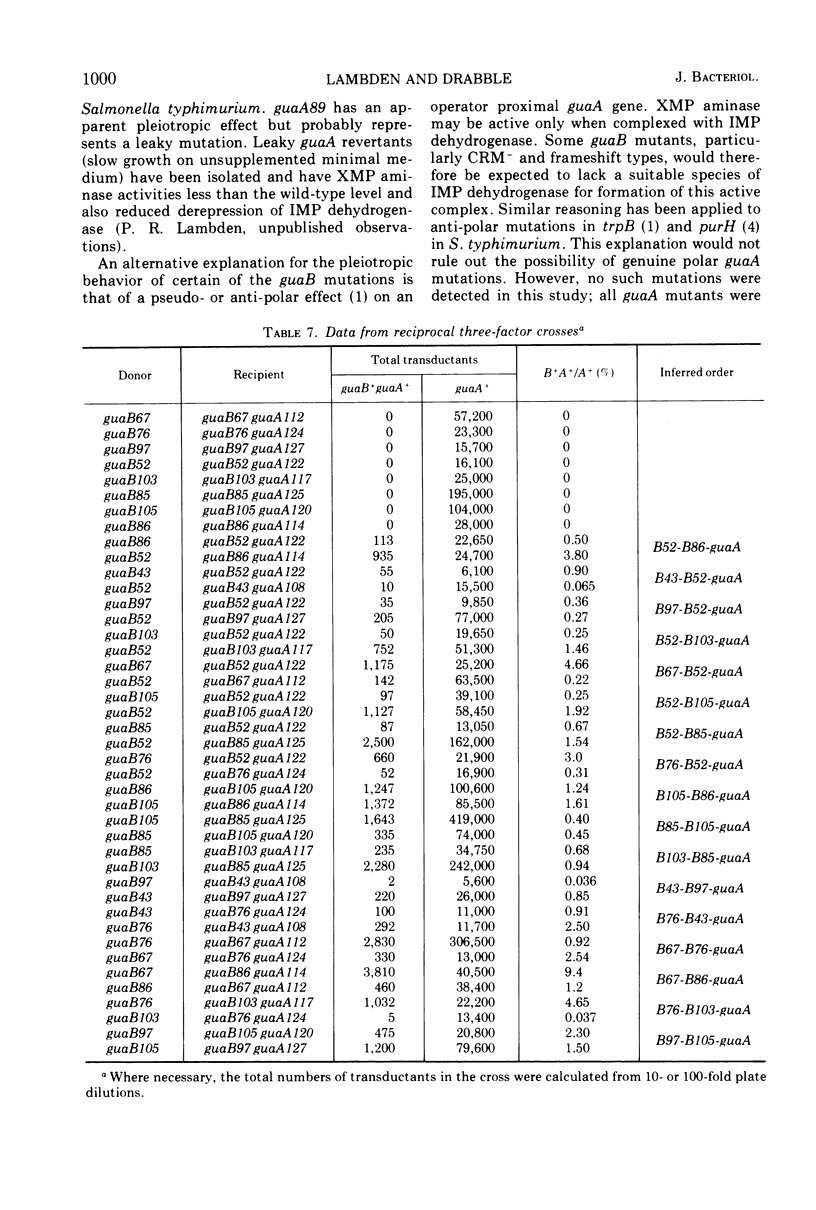
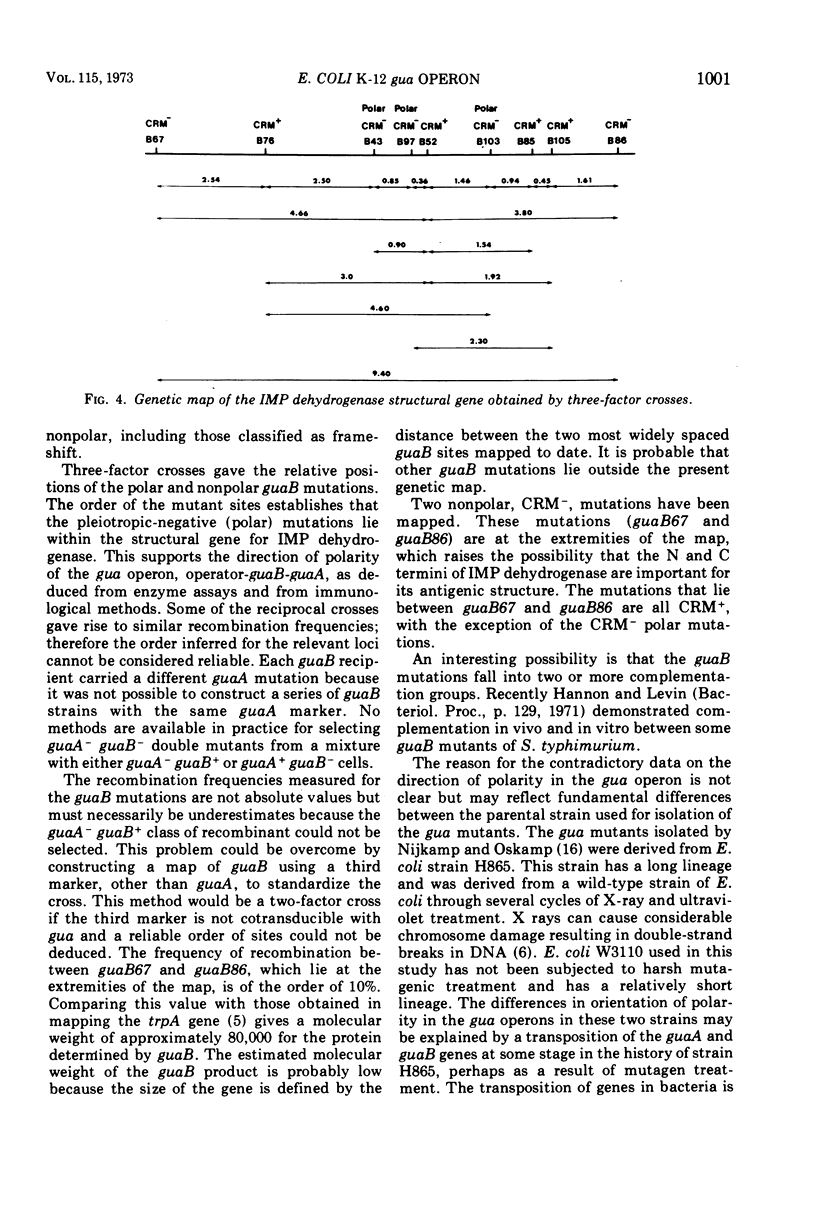
Guanine auxotrophs of Escherichia coli K-12 were isolated after mutagenesis with N-methyl-N′-nitro-N-nitrosoguanidine, ethyl methane sulfonate, or the acridine mustard ICR 372. guaA (xanthosine 5′-monophosphate [XMP] aminase-less) mutants were distinguished from guaB (inosine 5′-monophosphate [IMP] dehydrogenase-less) mutants by their growth response to xanthine and by enzyme assay. Mutations were classified as base substitutions or frameshift on the basis of mutagen-induced reversion patterns. All guaA strains, including three frameshift mutants, produced derepressed levels of IMP dehydrogenase when cultured with a growth-limiting concentration of guanine. The guaB strains were of two types: (i) those producing derepressed levels of XMP aminase, and (ii) those producing basal levels of XMP aminase when grown under conditions of guanine starvation. In the guaB strains of the second type, the expression of the adjacent guaA gene is reduced. It is proposed that this pleiotropic effect of some guaB mutations is a result of polarity. The orientation of polarity suggests the gene order “operator”-guaB-guaA. Gel diffusion studies with IMP dehydrogenase antiserum showed that strains carrying polar guaB mutations do not produce cross-reacting material (CRM). The remaining guaB mutants were either CRM+ or CRM−. Mapping the mutations by three-factor crosses showed that polar and nonpolar guaB sites are clustered in a small genetic region cotransducible with guaA. The relative positions of the guaB mutational sites established that the polar mutations lie within the structural gene for IMP dehydrogenase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauerle R. H., Margolin P. A multifunctional enzyme complex in the tryptophan pathway of Salmonella typhimurium: comparison of polarity and pseudopolarity mutations. Cold Spring Harb Symp Quant Biol. 1966;31:203–214. doi: 10.1101/sqb.1966.031.01.028. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gots J. S., Dalal F. R., Shumas S. R. Genetic eparation of the inosinic acid cyclohydrolase-transformylase complex of Salmonella typhimurium. J Bacteriol. 1969 Aug;99(2):441–449. doi: 10.1128/jb.99.2.441-449.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LERNER P., YANOFSKY C. An immunological study of mutants of Escherichia coli lacking the enzyme tryptophan synthetase. J Bacteriol. 1957 Oct;74(4):494–501. doi: 10.1128/jb.74.4.494-501.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVELESS A., HOWARTH S. Mutation of bacteria at high levels of survival by ethyl methane sulphonate. Nature. 1959 Dec 5;184:1780–1782. doi: 10.1038/1841780a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGASANIK B., MOYED H. S., GEHRING L. B. Enzymes essential for the biosynthesis of nucleic acid guanine; inosine 5'-phosphate dehydrogenase of Aerobacter aerogenes. J Biol Chem. 1957 May;226(1):339–350. [PubMed] [Google Scholar]
- MOYED H. S., MAGASANIK B. Enzymes essential for the biosynthesis of nucleic acid guanine; xanthosine 5'-phosphate aminase of Aerobacter aerogenes. J Biol Chem. 1957 May;226(1):351–363. [PubMed] [Google Scholar]
- Nijkamp H. J., De Haan P. G. Genetic and biochemical studies of the guanosine 5'-monophosphate pathway in Escherichia coli. Biochim Biophys Acta. 1967 Aug 22;145(1):31–40. doi: 10.1016/0005-2787(67)90651-x. [DOI] [PubMed] [Google Scholar]
- Nijkamp H. J., Oskamp A. A. Regulation of the biosynthesis of guanosine 5'-monophosphate: evidence for one operon. J Mol Biol. 1968 Jul 14;35(1):103–109. doi: 10.1016/s0022-2836(68)80040-3. [DOI] [PubMed] [Google Scholar]
- Nijkamp H. J. Regulatory role of adenine nucleotides in the biosynthesis of guanosine 5'-monophosphate. J Bacteriol. 1969 Nov;100(2):585–593. doi: 10.1128/jb.100.2.585-593.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeschger N. S., Hartman P. E. ICR-induced frameshift mutations in the histidine operon of Salmonella. J Bacteriol. 1970 Feb;101(2):490–504. doi: 10.1128/jb.101.2.490-504.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell G. Rajagopalan KV, Handler P: Purification and properties of inosinic acid dehydrogenase from Escherichia coli. J Biol Chem. 1969 Sep 10;244(17):4793–4797. [PubMed] [Google Scholar]
- UDAKA S., MOYED H. S. INHIBITION OF PARENTAL AND MUTANT XANTHOSINE 5'-PHOSPHATE AMINASES BY PSICOFURANINE. J Biol Chem. 1963 Aug;238:2797–2803. [PubMed] [Google Scholar]
- Yourno J., Heath S. Nature of the hisD3018 frameshift mutation in Salmonella typhimurium. J Bacteriol. 1969 Oct;100(1):460–468. doi: 10.1128/jb.100.1.460-468.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]




