Abstract
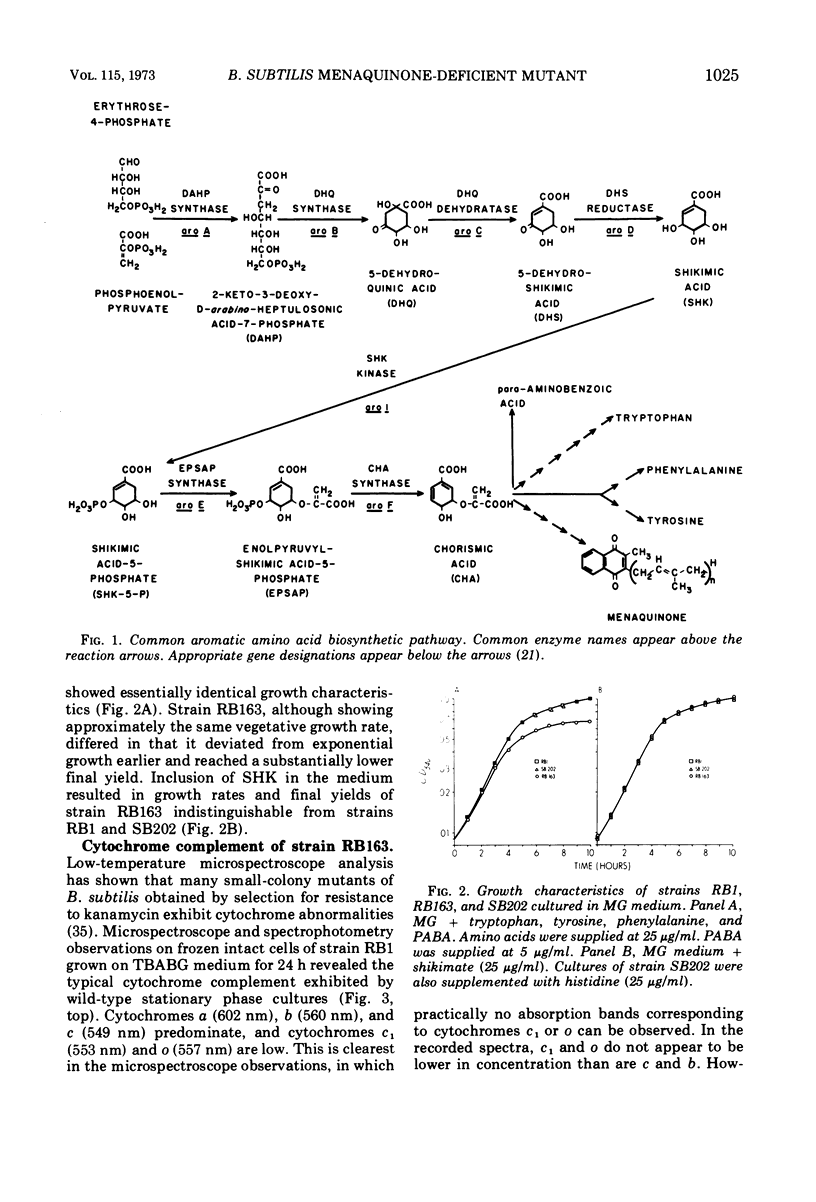
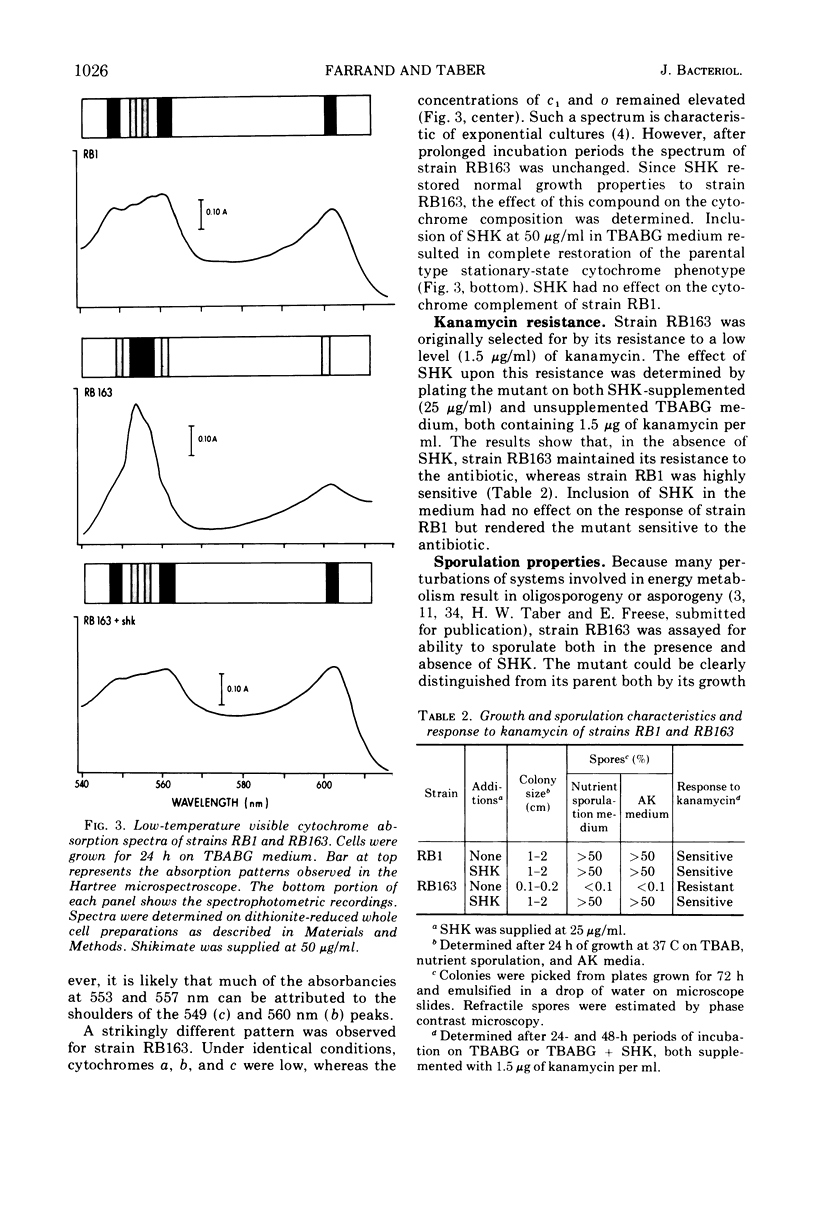
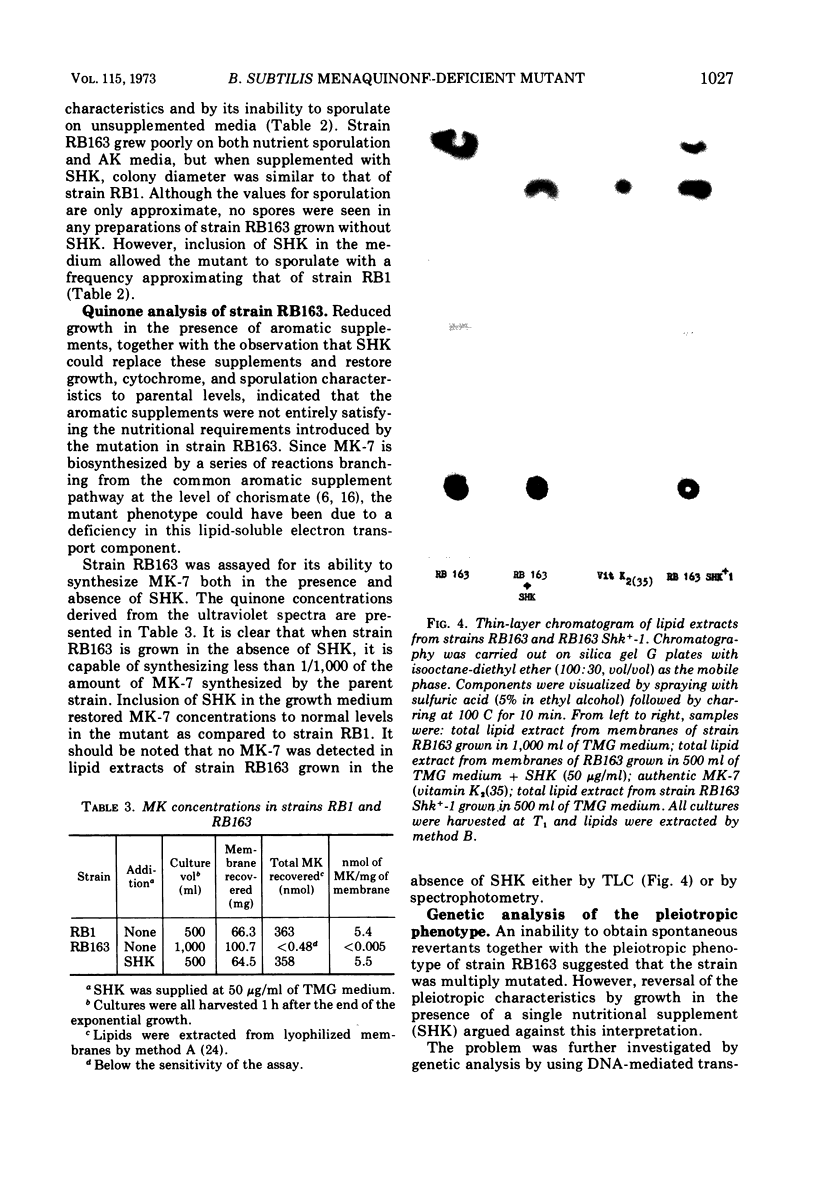
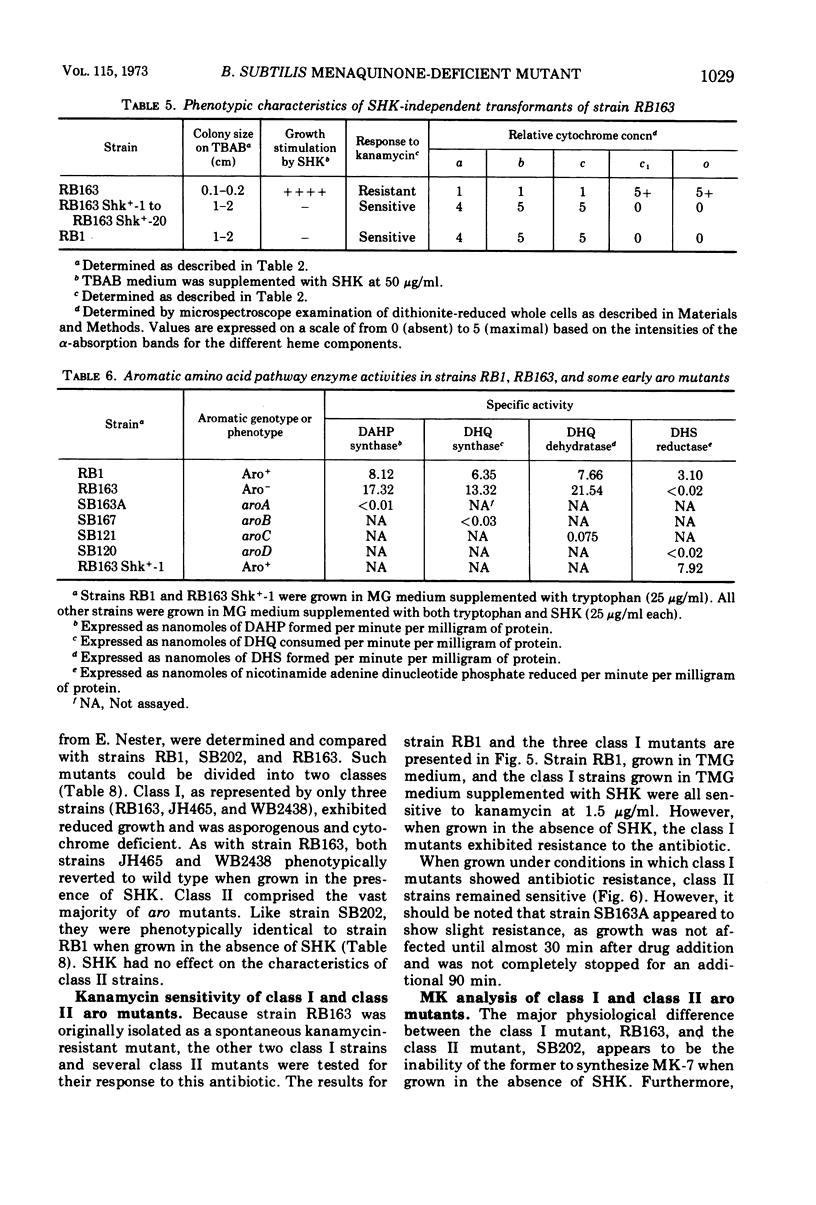
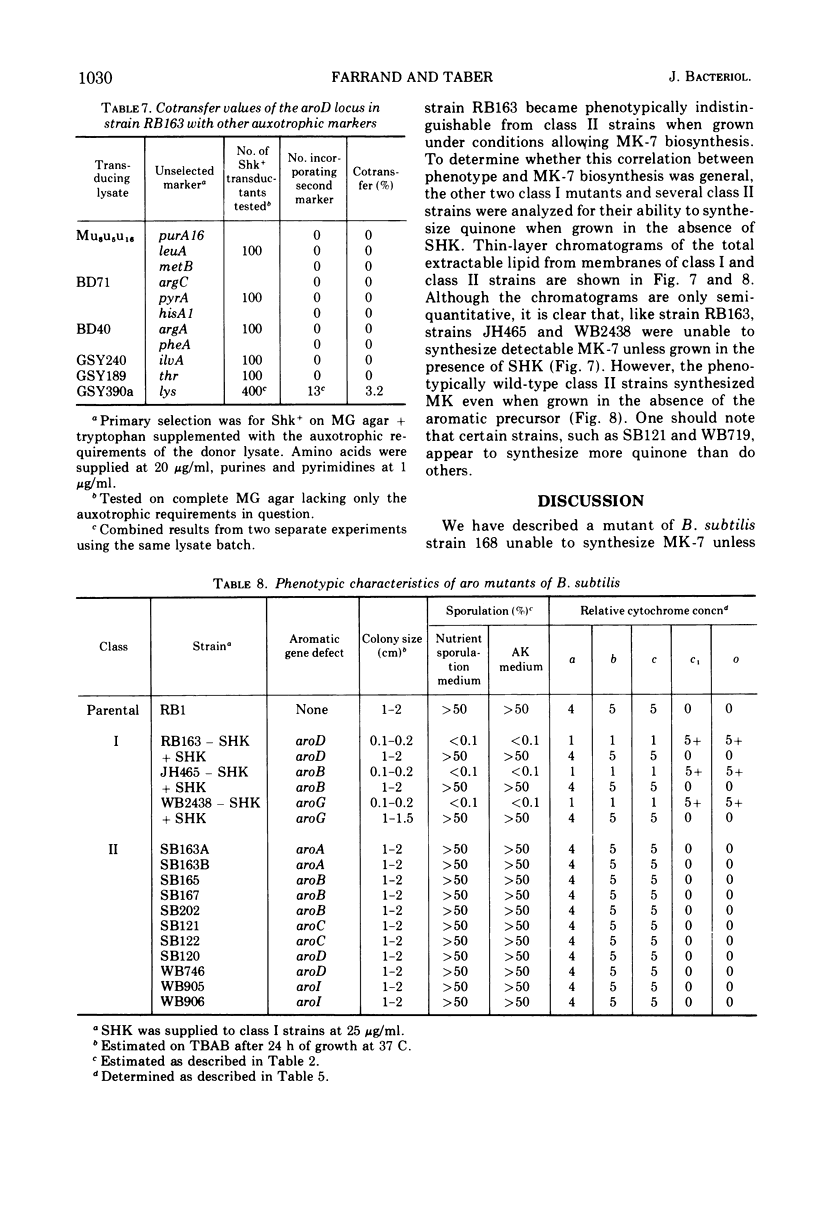
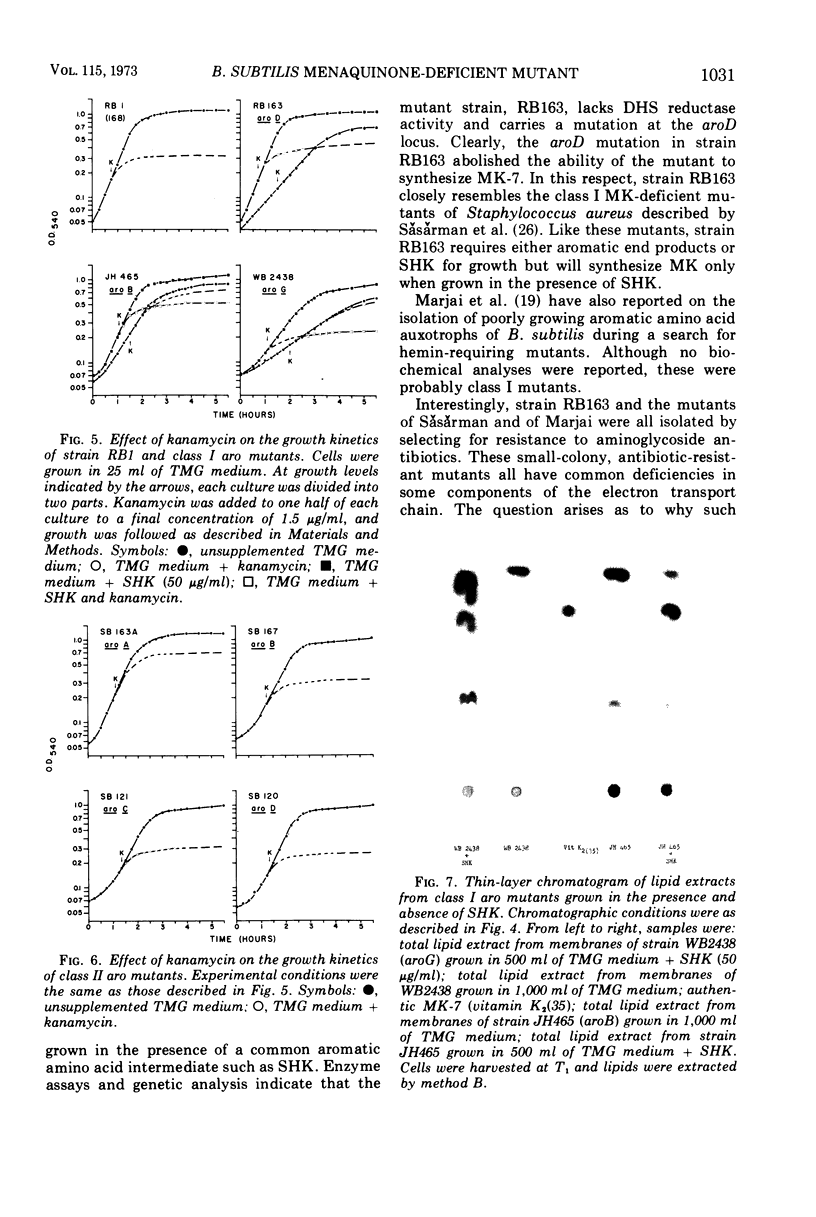
A multiple aromatic amino acid auxotroph of Bacillus subtilis 168 has been isolated which is unable to synthesize menaquinone-7 (MK-7) unless supplied with shikimic acid (SHK). The mutant, RB163, was isolated by selecting for resistance to low levels (1.5 μg/ml) of kanamycin. Enzymatic and genetic analyses show that the strain is an aroD mutant lacking 5-dehydroshikimate reductase. Under growth conditions in which its MK-7 deficiency is expressed, RB163 is deficient in cytochromes a, b, and c, exhibits low growth yields, and does not sporulate. Genetic analysis indicates that this pleiotropic phenotype is the result of a single genetic event. All phenotypic characteristics are reversible when the mutant is grown under conditions such that MK is synthesized. Comparison of strain RB163 with other aro mutants blocked before SHK (“early-aro” mutants) reveals interesting differences. Most early-aro mutants are cytochrome- and MK-sufficient, sporogenous, and sensitive to kanamycin when grown in the absence of SHK. However, in addition to strain RB163, two other aro mutants were found to show the pleiotropic phenotype. These three mutants have in common, and differ from other early-aro strains in, the inability to synthesize MK. It is suggested that the phenotypically wild-type aro mutants are bradytrophic, allowing enough substrate flow through the common aromatic pathway to satisfy the MK requirement. The pleiotropic mutants are thought to be completely blocked in the common pathway, thus accounting for their inability to synthesize MK.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan R. J., Mendelson N. H., Brooks D., Young F. E. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J Bacteriol. 1972 Apr;110(1):281–290. doi: 10.1128/jb.110.1.281-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAIX P., PETIT J. F. Etude de différents spectres cytochromiques de Bacillus subtilis. Biochim Biophys Acta. 1956 Oct;22(1):66–71. doi: 10.1016/0006-3002(56)90224-4. [DOI] [PubMed] [Google Scholar]
- Carls R. A., Hanson R. S. Isolation and characterization of tricarboxylic acid cycle mutants of Bacillus subtilis. J Bacteriol. 1971 Jun;106(3):848–855. doi: 10.1128/jb.106.3.848-855.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Young I. G., McCann L. M., Gibson F. Biosynthesis of ubiquinone in Escherichia coli K-12: location of genes affecting the metabolism of 3-octaprenyl-4-hydroxybenzoic acid and 2-octaprenylphenol. J Bacteriol. 1969 Aug;99(2):450–458. doi: 10.1128/jb.99.2.450-458.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOWNEY R. J. VITAMIN K-MEDIATED ELECTRON TRANSFER IN BACILLUS SUBTILIS. J Bacteriol. 1964 Oct;88:904–911. doi: 10.1128/jb.88.4.904-911.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBIN D. T., HANCOCK R., DAVIS B. D. THE SEQUENCE OF SOME EFFECTS OF STREPTOMYCIN IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Aug 13;74:476–489. doi: 10.1016/0006-3002(63)91390-8. [DOI] [PubMed] [Google Scholar]
- Dansette P., Azerad R. A new intermediate in naphthoquinone and menaquinone biosynthesis. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1090–1095. doi: 10.1016/0006-291x(70)90906-x. [DOI] [PubMed] [Google Scholar]
- Dunphy P. J., Gutnick D. L., Phillips P. G., Brodie A. F. A new natural naphthoquinone in Mycobacterium phlei. Cis-dihydromenaquinone-9, structure and function. J Biol Chem. 1968 Jan 25;243(2):398–407. [PubMed] [Google Scholar]
- Fortnagel P. The regulation of aconitase and isocitrate dehydrogenase in sporulation mutants of Bacillus subtilis. Biochim Biophys Acta. 1970 Nov 24;222(2):290–298. doi: 10.1016/0304-4165(70)90116-9. [DOI] [PubMed] [Google Scholar]
- HANCOCK R. EARLY EFFECTS OF STREPTOMYCIN ON BACILLUS MEGATERIUM. J Bacteriol. 1964 Sep;88:633–639. doi: 10.1128/jb.88.3.633-639.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON H. M., WITOSLAWSKI J. J., CAMPBELL E. H. REVERSIBLE DISRUPTION OF A WAVELENGTH DISCRIMINATION IN PIGEONS FOLLOWING ADMINISTRATION OF PHENIPRAZINE. Toxicol Appl Pharmacol. 1964 Nov;6:690–695. doi: 10.1016/0041-008x(64)90119-x. [DOI] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. BIOCHEMISTRY OF SPORULATION. II. ENZYMATIC CHANGES DURING SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1963 Jul;86:45–50. doi: 10.1128/jb.86.1.45-50.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner B. I., Gutnick D. L. Use of neomycin in the isolation of mutants blocked in energy conservation in Escherichia coli. J Bacteriol. 1972 Jul;111(1):287–289. doi: 10.1128/jb.111.1.287-289.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leistner E., Schmitt J. H., Zenk M. H. Alpha-naphthol: a precursor of vitamin K2. Biochem Biophys Res Commun. 1967 Sep 27;28(6):845–850. doi: 10.1016/0006-291x(67)90054-x. [DOI] [PubMed] [Google Scholar]
- Lorence J. H., Nester E. W. Multiple molecular forms of chorismate mutase in Bacillus subtillis. Biochemistry. 1967 May;6(5):1541–1553. doi: 10.1021/bi00857a041. [DOI] [PubMed] [Google Scholar]
- MITSUHASHI S., DAVIS B. D. Aromatic biosynthesis. XII. Conversion of 5-dehydroquinic acid to 5-dehydroshikimic acid dy 5-dehydroquinase. Biochim Biophys Acta. 1954 Sep;15(1):54–61. doi: 10.1016/0006-3002(54)90093-1. [DOI] [PubMed] [Google Scholar]
- Marjai E., Kiss I., Ivánovics G. Auxotrophic mutation associated with low streptomycin resistance and slow growth in Bacillus subtilis. Acta Microbiol Acad Sci Hung. 1970;17(2):133–145. [PubMed] [Google Scholar]
- Nasser D., Nester E. W. Aromatic amino acid biosynthesis: gene-enzyme relationships in Bacillus subtilis. J Bacteriol. 1967 Nov;94(5):1706–1714. doi: 10.1128/jb.94.5.1706-1714.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg B., Hoch J. A. Citric acid cycle: gene-enzyme relationships in Bacillus subtilis. J Bacteriol. 1970 Nov;104(2):826–833. doi: 10.1128/jb.104.2.826-833.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- SHERMAN F., SLONIMSKI P. P. RESPIRATION-DEFICIENT MUTANTS OF YEAST. II. BIOCHEMISTRY. Biochim Biophys Acta. 1964 Jul 15;90:1–15. doi: 10.1016/0304-4165(64)90113-8. [DOI] [PubMed] [Google Scholar]
- SRINIVASAN P. R., ROTHSCHILD J., SPRINSON D. B. THE ENZYMIC CONVERSION OF 3-DEOXY-D-ARABINO-HEPTULOSONIC ACID 7-PHOSPHATE TO 5-DEHYDROQUINATE. J Biol Chem. 1963 Oct;238:3176–3182. [PubMed] [Google Scholar]
- SRINIVASAN P. R., SPRINSON D. B. 2-Keto-3-deoxy-D-arabo-heptonic acid 7-phosphate synthetase. J Biol Chem. 1959 Apr;234(4):716–722. [PubMed] [Google Scholar]
- Salton M. R., Schmitt M. D. Effects of diphenylamine on carotenoids and menaquinones in bacterial membranes. Biochim Biophys Acta. 1967 May 2;135(2):196–207. doi: 10.1016/0005-2736(67)90114-9. [DOI] [PubMed] [Google Scholar]
- Sasarman A., Surdeanu M., Portelance V., Dobardzic R., Sonea S. Vitamin K-deficient mutants of bacteria. Nature. 1969 Oct 18;224(5216):272–272. doi: 10.1038/224272a0. [DOI] [PubMed] [Google Scholar]
- Sasarman A., Surdeanu M., Szabados J., Greceanu V., Horodniceanu T. Menaphthone-requiring mutants of Staphylococcus aureus. Rev Can Biol. 1968 Dec;27(4):333–339. [PubMed] [Google Scholar]
- Staal S. P., Hoch J. A. Conditional dihydrostreptomycin resistance in Bacillus subtilis. J Bacteriol. 1972 Apr;110(1):202–207. doi: 10.1128/jb.110.1.202-207.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroobant P., Young I. G., Gibson F. Mutants of Escherichia coli K-12 blocked in the final reaction of ubiquinone biosynthesis: characterization and genetic analysis. J Bacteriol. 1972 Jan;109(1):134–139. doi: 10.1128/jb.109.1.134-139.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Portelance V., Dobardzic R., Sonea S. Classification of vitamin K-deficient mutants of Staphylococcus aureus. J Gen Microbiol. 1971 Feb;65(2):125–130. doi: 10.1099/00221287-65-2-125. [DOI] [PubMed] [Google Scholar]
- Tochikubo K. Changes in terminal respiratory pathways of Bacillus subtilis during germination, outgrowth and vegetative growth. J Bacteriol. 1971 Nov;108(2):652–661. doi: 10.1128/jb.108.2.652-661.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANIV H., GILVARG C. Aromatic biosynthesis. XIV. 5-Dehydroshikimic reductase. J Biol Chem. 1955 Apr;213(2):787–795. [PubMed] [Google Scholar]
- Young I. G., McCann L. M., Stroobant P., Gibson F. Characterization and genetic analysis of mutant strains of Escherichia coli K-12 accumulating the biquinone precursors 2-octaprenyl-6-methoxy-1,4-benzoquinone and 2-octaprenyl-3-methyl-6-methoxy-1,4-benzoquinone. J Bacteriol. 1971 Mar;105(3):769–778. doi: 10.1128/jb.105.3.769-778.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]