Abstract
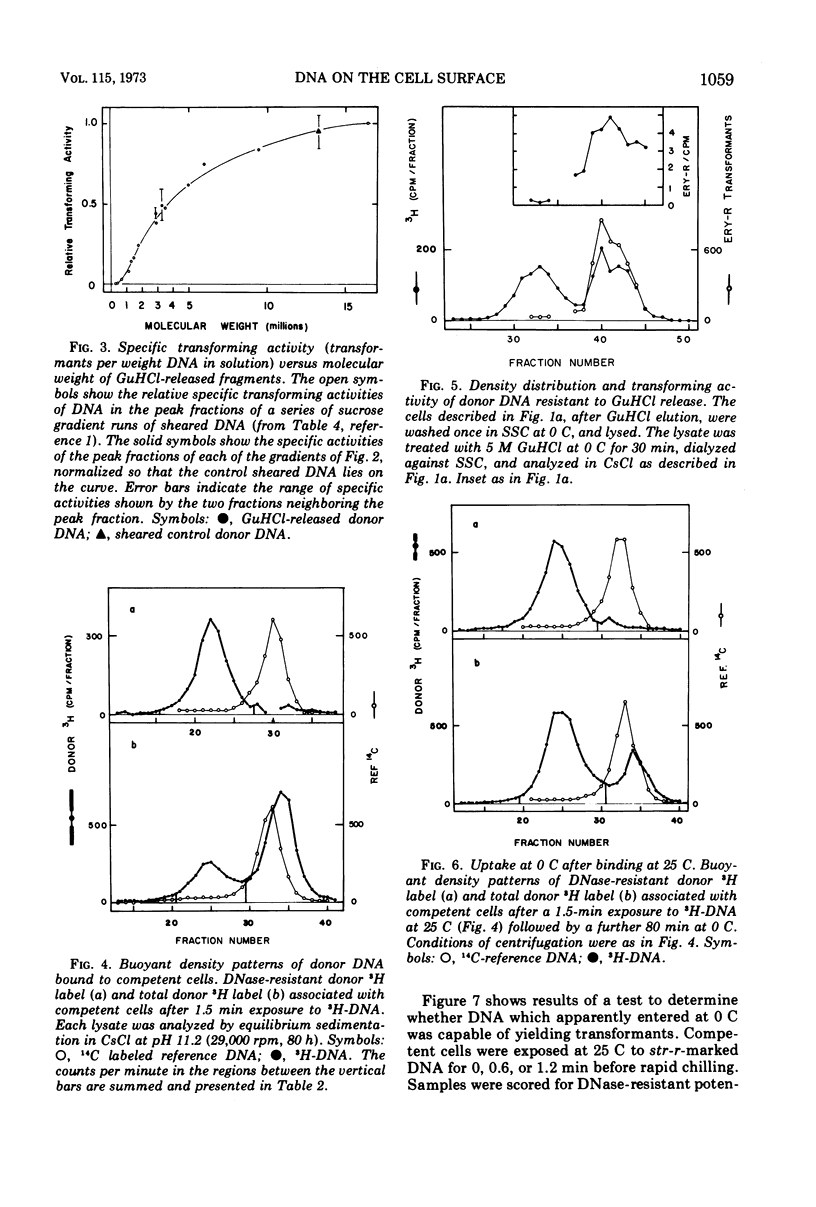
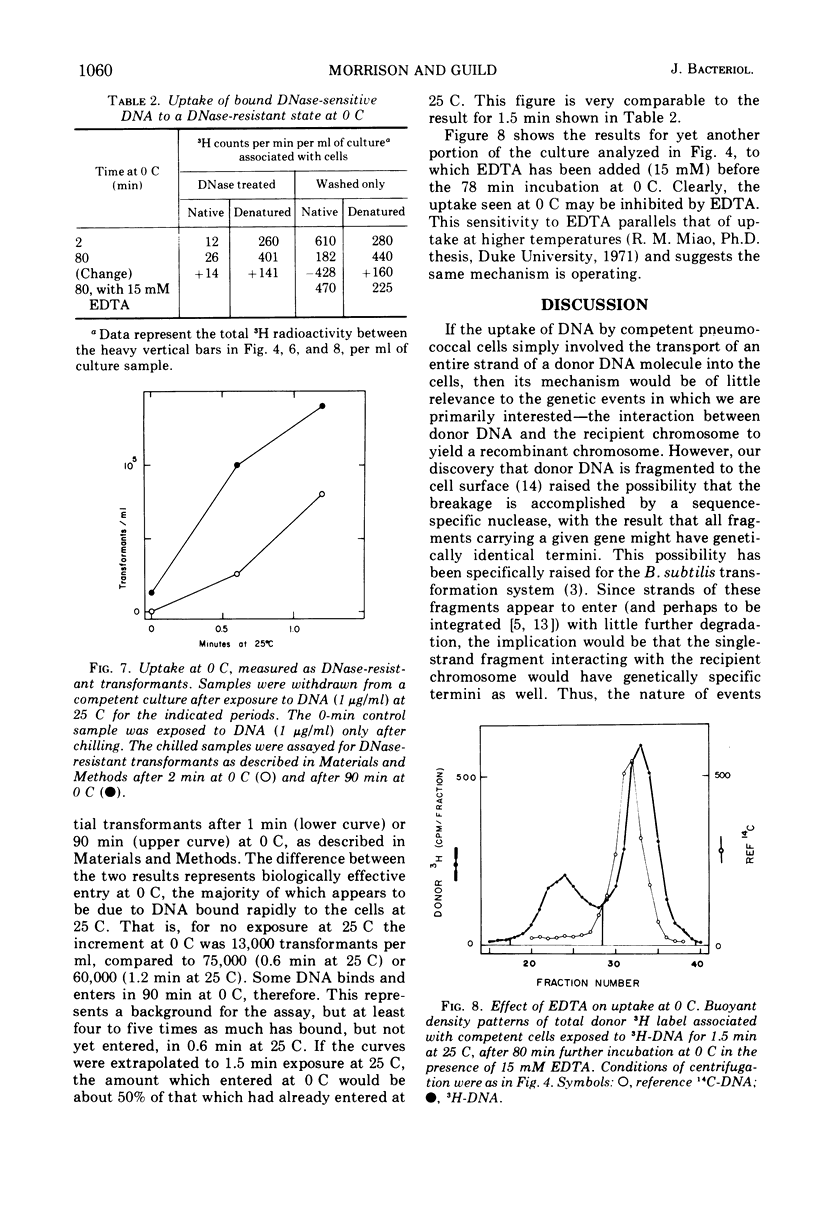
We exposed competent cells of Diplococcus pneumoniae to high-molecular-weight donor deoxyribonucleate (DNA) and examined the state of the DNA bound to them in forms sensitive to deoxyribonuclease I. The portion elutable with 5 M guanidine hydrochloride was shown to be native, of much lower molecular weight (4 × 106 to 5 × 106) than the donor, and as active in further transformation as sheared DNA of the same size. The portion resistant to release by guanidine hydrochloride was also shown to be native and active in transformation. These results, along with previous ones, imply that the breaks produced outside the cell are not at genetically specific sites. Furthermore, it was found that entry past the cell barrier to deoxyribonuclease could occur at 0 C by a process sensitive to ethylenediaminetetraacetate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cato A., Jr, Guild W. R. Transformation and DNA size. I. Activity of fragments of defined size and a fit to a random double cross-over model. J Mol Biol. 1968 Oct 14;37(1):157–178. doi: 10.1016/0022-2836(68)90080-6. [DOI] [PubMed] [Google Scholar]
- Collins C. J., Guild W. R. Events occurring near the time of synapsis during transformation in Diplococcus pneumoniae. J Bacteriol. 1972 Jan;109(1):266–275. doi: 10.1128/jb.109.1.266-275.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming DNA following uptake by competent Bacillus subtilis. Formation and properties of products isolated from transformed cells which are derived entirely from donor DNA. J Mol Biol. 1972 Feb 28;64(1):9–29. doi: 10.1016/0022-2836(72)90318-x. [DOI] [PubMed] [Google Scholar]
- GUILD W. R., DEFILIPPES F. M. Ionizing radiation and ultrasonic evidence for a minimum unit of transforming principle DNA. Biochim Biophys Acta. 1957 Nov;26(2):241–251. doi: 10.1016/0006-3002(57)90002-1. [DOI] [PubMed] [Google Scholar]
- Gurney T., Jr, Fox M. S. Physical and genetic hybrids formed in bacterial transformation. J Mol Biol. 1968 Feb 28;32(1):83–100. doi: 10.1016/0022-2836(68)90147-2. [DOI] [PubMed] [Google Scholar]
- Haseltine F. P., Fox M. S. Bacterial inactivation of transforming deoxyribonucleate. J Bacteriol. 1971 Sep;107(3):889–899. doi: 10.1128/jb.107.3.889-899.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgpeth J., Goodman H. M., Boyer H. W. DNA nucleotide sequence restricted by the RI endonuclease. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3448–3452. doi: 10.1073/pnas.69.11.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. M., Marmur J. The 5'-ends of the DNA of defective bacteriophages of Bacillus subtilis. J Mol Biol. 1970 Feb 14;47(3):591–593. doi: 10.1016/0022-2836(70)90326-8. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Smith H. O. A restriction enzyme from Hemophilus influenzae. II. J Mol Biol. 1970 Jul 28;51(2):393–409. doi: 10.1016/0022-2836(70)90150-6. [DOI] [PubMed] [Google Scholar]
- Linn S., Arber W. Host specificity of DNA produced by Escherichia coli, X. In vitro restriction of phage fd replicative form. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1300–1306. doi: 10.1073/pnas.59.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R., Guild W. R. Competent Diplococcus pneumoniae accept both single- and double-stranded deoxyribonucleic acid. J Bacteriol. 1970 Feb;101(2):361–364. doi: 10.1128/jb.101.2.361-364.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Breakage prior to entry of donor DNA in Pneumococcus transformation. Biochim Biophys Acta. 1973 Apr 11;299(4):545–556. doi: 10.1016/0005-2787(73)90226-8. [DOI] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Transformation and deoxyribonucleic acid size: extent of degradation on entry varies with size of donor. J Bacteriol. 1972 Dec;112(3):1157–1168. doi: 10.1128/jb.112.3.1157-1168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Mudd J. A., Marmur J. Conversion of Bacillus subtilis DNA to phage DNA following mitomycin C induction. J Mol Biol. 1968 Jun 28;34(3):429–437. doi: 10.1016/0022-2836(68)90170-8. [DOI] [PubMed] [Google Scholar]
- RICHARDSON C. C., INMAN R. B., KORNBERG A. ENZYMIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. 18. THE REPAIR OF PARTIALLY SINGLE-STRANDED DNA TEMPLATES BY DNA POLYMERASE. J Mol Biol. 1964 Jul;9:46–69. doi: 10.1016/s0022-2836(64)80090-5. [DOI] [PubMed] [Google Scholar]
- Shoemaker N. B., Guild W. R. Kinetics of integration of transforming DNA in pneumococcus. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3331–3335. doi: 10.1073/pnas.69.11.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINOGRAD J., MORRIS J., DAVIDSON N., DOVE W. F., Jr The bouyant behavior of viral and bacterial DNA in alkaline CsCl. Proc Natl Acad Sci U S A. 1963 Jan 15;49:12–17. doi: 10.1073/pnas.49.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R., Meselson M. A specific complex between a restriction endonuclease and its DNA substrate. Proc Natl Acad Sci U S A. 1970 Feb;65(2):357–362. doi: 10.1073/pnas.65.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]



