Abstract
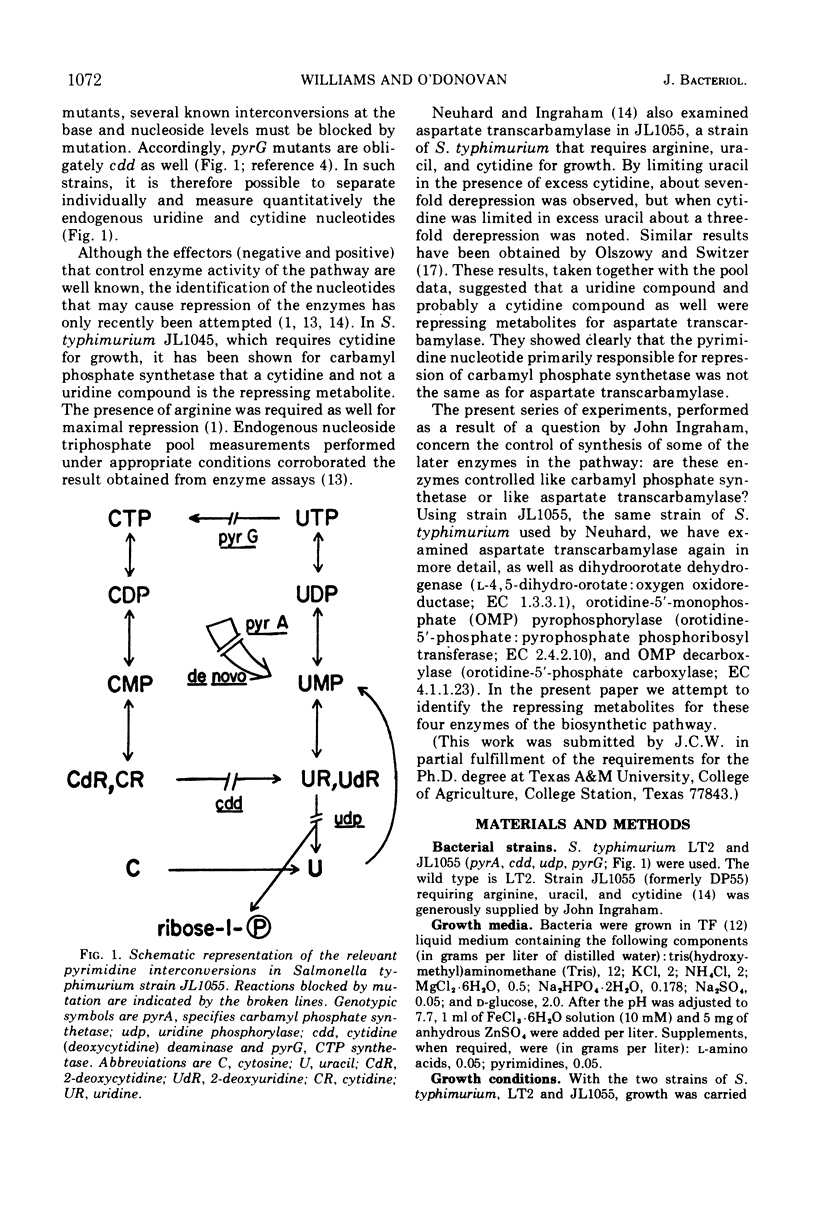
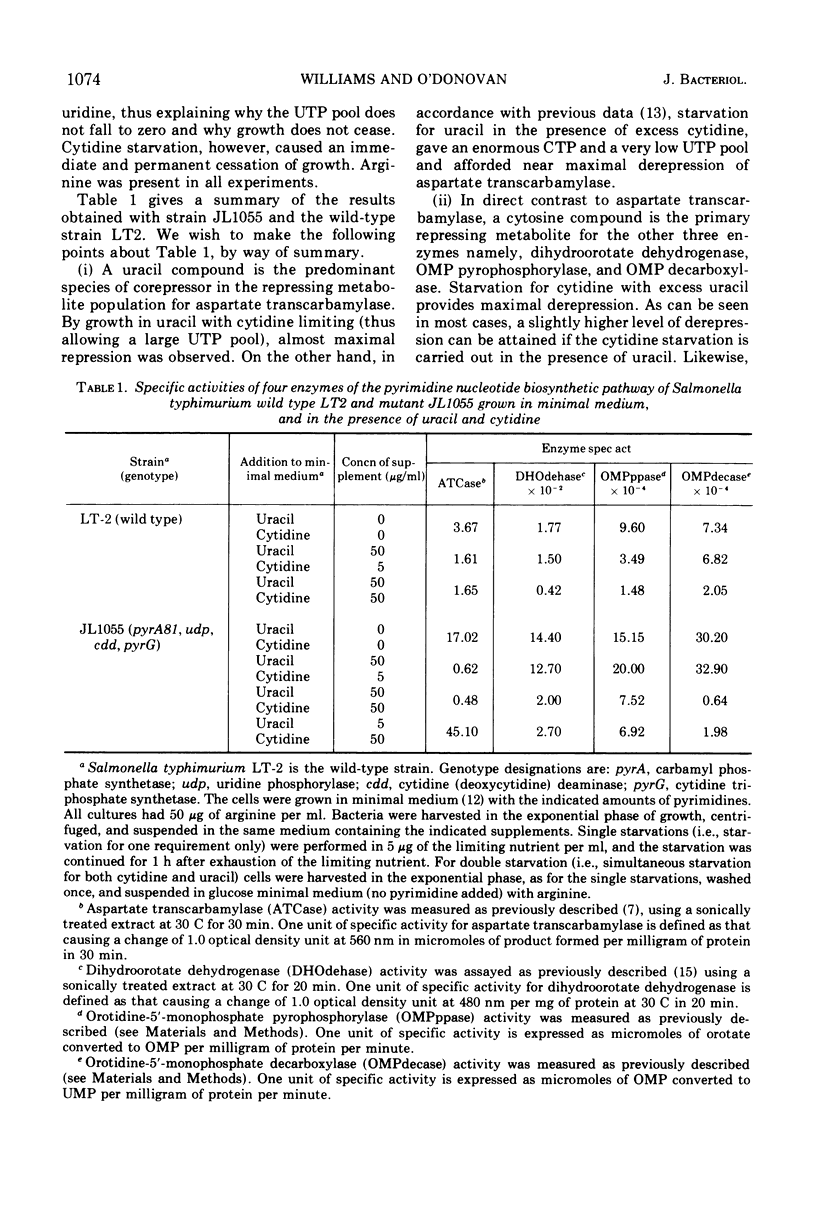
It has been reported by other workers that a uridine and probably also a cytidine nucleotide are required for maximal repression of aspartate transcarbamylase encoded by the gene pyrB in Salmonella typhimurium. We have identified the repressing metabolites for three more biosynthetic enzymes, namely, dihydroorotate dehydrogenase (encoded by pyrD), orotidine-5′-monophosphate pyrophosphorylase (encoded by pyrE), and orotidine-5′-monophosphate decarboxylase (encoded by pyrF), as well as examining the repression profiles of aspartate transcarbamylase in more detail. Using a specially constructed strain of S. typhimurium (JL1055) which lacks the enzymes for the interconversion of cytidine and uridine compounds, thus allowing the independent manipulation of endogenous cytidine and uridine nucleotides, we found that a cytidine compound is the primary effector of repression in all cases except for aspartate transcarbamylase where little repression is observed in excess cytidine. For aspartate transcarbamylase, we found that the primary repressing metabolite is a uridine compound.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abd-el-Al A., Ingraham J. L. Control of carbamyl phosphate synthesis in Salmonella typhimurium. J Biol Chem. 1969 Aug 10;244(15):4033–4038. [PubMed] [Google Scholar]
- Anderson P. M., Marvin S. V. Effect of allosteric effectors and adenosine triphosphate on the aggregation and rate of inhibition by N-ethylmaleimide of carbamyl phosphate synthetase of Escherichia coli. Biochemistry. 1970 Jan 6;9(1):171–178. doi: 10.1021/bi00803a022. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Meister A. Control of Escherichia coli carbamyl phosphate synthetase by purine and pyrimidine nucleotides. Biochemistry. 1966 Oct;5(10):3164–3169. doi: 10.1021/bi00874a013. [DOI] [PubMed] [Google Scholar]
- BECKWITH J. R., PARDEE A. B., AUSTRIAN R., JACOB F. Coordination of the synthesis of the enzymes in the pyrimidine pathway of E. coli. J Mol Biol. 1962 Dec;5:618–634. doi: 10.1016/s0022-2836(62)80090-4. [DOI] [PubMed] [Google Scholar]
- Beck C. F., Ingraham J. L. Location on the chromosome of Salmonella typhimurium of genes governing pyrimidine metabolism. Mol Gen Genet. 1971;111(4):303–316. doi: 10.1007/BF00569782. [DOI] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis J. A., Ames B. N. Histidine regulation in Salmonella typhimurium. XI. The percentage of transfer RNA His charged in vivo and its relation to the repression of the histidine operon. J Mol Biol. 1972 Apr 28;66(1):131–142. doi: 10.1016/s0022-2836(72)80011-1. [DOI] [PubMed] [Google Scholar]
- Long C. W., Levitzki A., Koshland D. E., Jr The subunit structure and subunit interactions of cytidine triphosphate synthetase. J Biol Chem. 1970 Jan 10;245(1):80–87. [PubMed] [Google Scholar]
- Long C. W., Pardee A. B. Cytidine triphosphate synthetase of Escherichia coli B. I. Purification and kinetics. J Biol Chem. 1967 Oct 25;242(20):4715–4721. [PubMed] [Google Scholar]
- MUNCH-PETERSEN A., NEUHARD J. STUDIES ON THE ACID-SOLUBLE NUCLEOTIDE POOL IN THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI DURING THYMINE STARVATION. I. ACCUMULATION OF DEOXYADENOSINE TRIPHOSPHATE IN ESCHERICHIA COLI 15 T-A-U-. Biochim Biophys Acta. 1964 Apr 27;80:542–551. doi: 10.1016/0926-6550(64)90298-1. [DOI] [PubMed] [Google Scholar]
- Mosteller R. D., Yanofsky C. Evidence that tryptophanyl transfer ribonucleic acid is not the corepressor of the tryptophan operon of Escherichia coli. J Bacteriol. 1971 Jan;105(1):268–275. doi: 10.1128/jb.105.1.268-275.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhard J., Ingraham J. Mutants of Salmonella typhimurium requiring cytidine for growth. J Bacteriol. 1968 Jun;95(6):2431–2433. doi: 10.1128/jb.95.6.2431-2433.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhard J. Pyrimidine nucleotide metabolism and pathways of thymidine triphosphate biosynthesis in Salmonella typhimurium. J Bacteriol. 1968 Nov;96(5):1519–1527. doi: 10.1128/jb.96.5.1519-1527.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Gerhart J. C. Isolation and partial characterization of regulatory mutants of the pyrimidine pathway in Salmonella typhimurium. J Bacteriol. 1972 Mar;109(3):1085–1096. doi: 10.1128/jb.109.3.1085-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszowy J., Switzer R. L. Specific repression of phosphoribosylpyrophosphate synthetase by uridine compounds in Salmonella typhimurium. J Bacteriol. 1972 Apr;110(1):450–451. doi: 10.1128/jb.110.1.450-451.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer C. E., Smith G. R., Cortese R., Ames B. N. [Mutant tRNA His ineffective in repression and lacking two pseudouridine modifications]. Nat New Biol. 1972 Jul 19;238(81):72–74. doi: 10.1038/newbio238072a0. [DOI] [PubMed] [Google Scholar]
- TAYLOR W. H., TAYLOR M. L. ENZYMES OF THE PYRIMIDINE PATHWAY IN ESCHERICHIA COLI. II. INTRACELLULAR LOCALIZATION AND PROPERTIES OF DIHYDROOROTIC DEHYDROGENASE. J Bacteriol. 1964 Jul;88:105–110. doi: 10.1128/jb.88.1.105-110.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]



