Abstract
The rat mitochondrial outer membrane-localized benzodiazepine receptor (MBR) was expressed in wild-type and TspO− (tryptophan-rich sensory protein) strains of the facultative photoheterotroph, Rhodobacter sphaeroides 2.4.1, and was shown to retain its structure within the bacterial outer membrane as assayed by its binding properties with a variety of MBR ligands. Functionally, it was able to substitute for TspO by negatively regulating the expression of photosynthesis genes in response to oxygen. This effect was reversed pharmacologically with the MBR ligand PK11195. These results suggest a close evolutionary and functional relationship between the bacterial TspO and the MBR. This relationship provides further support for the origin of the mammalian mitochondrion from a “photosynthetic” precursor. Finally, these findings provide novel insights into the physiological role that has been obscure for the MBR in situ.
Rhodobacter sphaeroides is a metabolically versatile photosynthetic proteobacterium capable of growth under a wide variety of environmental conditions. A decrease in oxygen availability leads to the induction of the membranous photosynthetic apparatus. Expression of the genes encoding components of the photosynthetic complexes (structural polypeptides, bacteriochlorophyll, and carotenoids) is tightly regulated in response to oxygen tension and/or light intensity. We have shown the presence of a tryptophan-rich sensory protein (TspO), previously designated CrtK/crtK (1), localized to the outer membrane of R. sphaeroides, which functions as a negative regulator of expression of some photosynthesis genes in this bacterium (2). TspO has an unusually high content of l-tryptophan and other aromatic amino acids and is characterized by a high degree of homology to the 18-kDa outer membrane component (pk18) of the mammalian mitochondrial benzodiazepine receptors (MBRs). Alignment of the amino acid sequences of TspO and pk18 revealed ≈30% identity and a further ≈32% conservative replacements between the bacterial and mammalian proteins (Fig. 1). pk18, which is the major drug-binding component of the MBR, is probably associated with the voltage-dependent anion channel in the outer mitochondrial membrane (4, 8).
Figure 1.
Alignment of R. sphaeroides 2.4.1 TspO (RsTspO) (2), Rhodobacter capsulatus CrtK (RcCrtK) (1), pk18 from rat (Rpk18) (3), mouse (Mpk18) (4), human (Hpk18) (5), bovine (Bpk18) (6), and hypothetical protein (ScTspO) from Synechocystis sp. strain PCC6803 (7). The alignments were generated by the seqvu computer program and by sight. Residues that are identical in five or more sequences are framed; residues conservatively changed relative to the RsTspO are shaded; dashes indicate gaps to maximize alignments. Numbers indicate the amino acid positions of the aligned sequences.
MBR, also known as the peripheral-type benzodiazepine receptor, is present in many types of mammalian tissues, and is characterized by its ability to bind with nanomolar affinity to a variety of benzodiazepine-like drugs as well as dicarboxylic tetrapyrrole intermediates of the heme biosynthetic pathway. Although the MBR was discovered more than a decade ago, its biological role is not yet understood. Depending upon the tissue, it was shown to be involved in steroidogenesis, heme biosynthesis, cell growth and differentiation, mitochondrial respiratory control, and immune and stress response (9). However, the striking similarity between the mammalian MBR and bacterial TspO suggests that both proteins may have a similar functional activity for their respective cells (10). The α-3 subdivision of the purple bacteria is considered to be a likely source of the endosymbiont that ultimately gave rise to the mitochondrion (11). Therefore, it is possible that the mammalian MBR remains both evolutionarily and functionally related to the TspO of R. sphaeroides.
Here we show that both the functional and structural relationship between the MBR and the bacterial outer membrane protein, TspO, remain intact providing exclusive insights into the physiological role for the MBR in the mitochondrial outer membrane.
MATERIALS AND METHODS
Bacterial Strains and Growth Conditions.
R. sphaeroides 2.4.1 as well as its TspO− derivative, TSPO1 (tspO::Kmr) were used throughout this study. Cultures of R. sphaeroides were grown in Sistrom’s minimal medium A (12) containing 0.4% succinate as the carbon source as described previously (13). Cell growth was monitored spectrophotometrically at 660 nm. To minimize antibiotic photooxidation (14), liquid cultures of R. sphaeroides grown photoheterotrophically in the presence of tetracycline were placed behind a CS 7–69 filter (620–1100 nm; Corning).
Strains of Escherichia coli were grown as described previously (15). When appropriate, antibiotics were added to the following final concentrations: ampicillin (Ap), 100 μg/ml; kanamycin (Km), 25 μg/ml; spectinomycin (Sm), 50 μg/ml; streptomycin (Sp), 50 μg/ml; and tetracycline (Tc), 10 μg/ml for E. coli; 1 μg/ml for R. sphaeroides. Conjugal matings between E. coli and R. sphaeroides were performed as described by Moore and Kaplan (16).
Molecular Techniques.
Standard procedures were used for plasmid isolation, restriction endonuclease digestion, PCR, isolation of DNA fragments from gels, ligation, and other molecular biological techniques (15, 17). Sequencing was done with an ABI 373A automatic DNA sequencer (Applied Biosystems) at the DNA Core Facility of the Department of Microbiology and Molecular Genetics (University of Texas, Houston).
Construction of the Plasmid.
pAY13 was constructed by introducing the rat pk18 gene (3) with a PCR-generated upstream sequence into the broad host range vector pRK415 (2). pBluescript SK(+) harboring the pk18 gene was used as a template, and the primers 5′-AGGGATCCGGAGGCACTATGTCTCAATCCTGGG-3′ and 3′-GCTATGGCAGCTGGAGC-5′ were used. The 5′ primer contains the GGAGG sequence 4 bp upstream of the ATG start codon, which is known to be an effective Shine-Dalgarno site in R. sphaeroides. The 0.8-kb PCR product was digested with EcoRV and BamHI, and introduced into the BamHI–Ecl136II cut pRK415 derivative, pUI2701, lacking the 0.7-kb BamHI–Ecl136II fragment, placing the pk18 gene under the control of the rrnB promoter. E. coli DH5α cells were transformed with the plasmid DNA, and pAY13 was introduced into R. sphaeroides cells by conjugal matings essentially as described earlier (16).
β-Galactosidase Activity in Cell Extracts.
R. sphaeroides cultures were grown to a cell density of approximately 1.8 × 108 cells per ml, and chloramphenicol was added to a final concentration of 80 μg/ml to terminate protein synthesis. Assay of β-galactosidase activity in cell extracts was performed as described previously (2, 16).
Preparation of Membranes of R. sphaeroides and Binding Assays.
Cells were grown semiaerobically, by sparging with a gas mixture of 95% N2, 3% O2, and 2% CO2, collected by centrifugation, washed twice with PBS (pH 7.5) containing protease inhibitors, and resuspended in the same buffer to an OD660 of 1.0. Cells were fixed by adding glutaraldehyde to a final concentration of 0.2%, incubated on a shaker at 200 rpm for 40 min at 30°C, and washed twice with cold 0.1 M glycine in PBS (pH 7.5) to remove excess cross-linking reagent. Cells were disrupted by French press and fractionated by centrifugation (15 min, 20,000 × g; 1.5 h, 150,000 × g), and the resulting membrane pellet was resuspended in 10% sucrose in PBS and kept at −70°C prior to binding assays.
Binding of [3H]PK11195 [1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide] and photolabeling with [3H]PK14105 [2-fluoro-5-nitrophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide] using glutaraldehyde-fixed membranes (100 μg of protein per assay) were performed as described elsewhere (18).
RESULTS
Expression of Rat pk18 in R. sphaeroides.
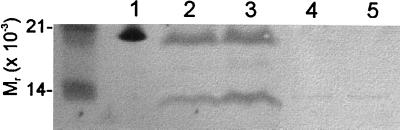
To explore the possibility that TspO is evolutionarily and functionally related to the mammalian MBR, we expressed the cDNA encoding rat pk18 (3) on a plasmid, pAY13, in R. sphaeroides 2.4.1 (wild type) and in the TspO− mutant strain TSPO1(2). Thirty-five percent of the codons of the rat pk18 DNA are rarely used in the G/C rich R. sphaeroides. To increase the efficiency of pk18 expression in R. sphaeroides, the gene was cloned under the control of a strong rrnB promoter, and a Shine-Dalgarno (19) sequence (GGAGG) 4 bp upstream of the ATG start codon was introduced. Expression of the rat protein was monitored by a Western blot assay using a polyclonal antiserum raised against an internal peptide sequence of rat pk18 (ref. 18; Fig. 2). pk18 was found to be relatively labile when expressed in the bacterial host: the 18-kDa band was observed together with a series of lower molecular weight bands that apparently correspond to partial degradation products of pk18. Following subcellular fractionation of 2.4.1 (pAY13) and TSPO1 (pAY13) (20, 21), pk18 was shown to be localized to the outer membrane of R. sphaeroides, thus mimicking the cellular localization of the bacterial TspO.
Figure 2.
Western blot analysis of rat pk18 expressed in R. sphaeroides. The proteins (8.5 μg of protein per lane) were separated by SDS/Tricine electrophoresis, electroblotted onto nitrocellulose membrane, and probed with antibodies raised against rat pk18. Lane 1, urea-treated rat adrenal mitochondria; lanes 2–5, outer membrane preparations of TSPO1 (pAY13), 2.4.1(pAY13), TSPO1 (pRK415), and 2.4.1 (pRK415), respectively.
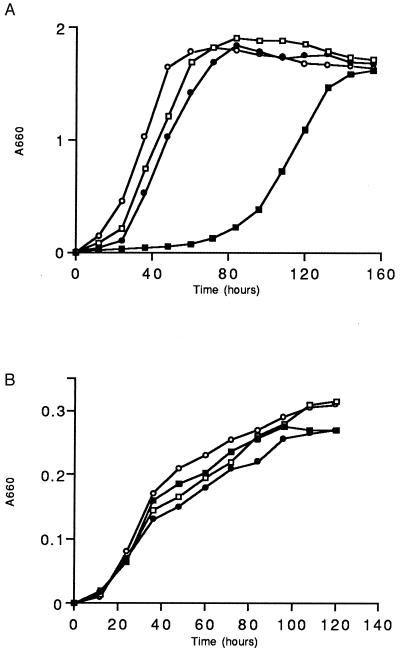
Expression of pk18 did not affect the growth of R. sphaeroides 2.4.1 or TSPO1 under aerobic or semiaerobic conditions. However, when the cells were adapted to photosynthetic growth conditions (Fig. 3A), the presence of the pAY13 plasmid in TSPO1 resulted in a significantly longer lag phase as well as in a 2.5- to 3-fold increase in the culture doubling time when compared with the wild type. R. sphaeroides 2.4.1 harboring pAY13 did not reveal any alterations in growth under any conditions tested. When bacteria were grown anaerobically in the dark in the presence of the alternative electron acceptor dimethyl sulfoxide there was no difference between the wild type or TSPO1 expressing pk18 (Fig. 3B). Therefore, pk18, when expressed in R. sphaeroides growing photoheterotrophically, exerted a differential phenotypic effect depending on the presence (wild type) or absence (TSPO1) of its bacterial counterpart (TspO) in the membrane. This data may indicate that pk18 and TspO occupy similar environments in the bacterial outer membrane, and negative effects of pk18 on growth under photosynthetic conditions are recessive to a wild-type copy of TspO.
Figure 3.
Relative growth rates of R. sphaeroides strains under photosynthetic, 10 W/m2 (A), and anaerobic dark/dimethyl sulfoxide (B) conditions. (○), R. sphaeroides 2.4.1; (•), 2.4.1 (pAY13); (□), TSPO1; (▪), TSPO1 (pAY13). Growth conditions were as described (1).
We were able to show that this effect was not due to any changes in the structure of the plasmid or host genome during the course of these studies. We tested the possibility that plasmid pAY13 acquired a mutation when introduced into TSPO1, thus leading to the altered growth characteristics during the photosynthetic growth mode. We sequenced the pk18 gene as well as the upstream region from this plasmid, which was isolated from photosynthetically grown cells; however, no sequence alterations, when compared with the parental pAY13 plasmid, were detected. When the TSPO1 (pAY13), following adaptation to photosynthetic growth, was cured of the plasmid harboring pk18, and subsequently transformed with the parental pAY13 DNA, it exhibited the same growth characteristics under all cultivation conditions tested as did the parental TSPO1 (pAY13) strain. This suggests that alterations in the growth of TSPO1 (pAY13) are the result of the presence of pk18 in the cells (i.e., a phenotypic effect), and not because of a genetic alteration, which could occur in either plasmid or genomic DNA. Control experiments with the vector pRK415 were conducted; no alterations in growth were observed due to the vector DNA in cells grown either photosynthetically or aerobically.
Function of Rat pk18 in R. sphaeroides.
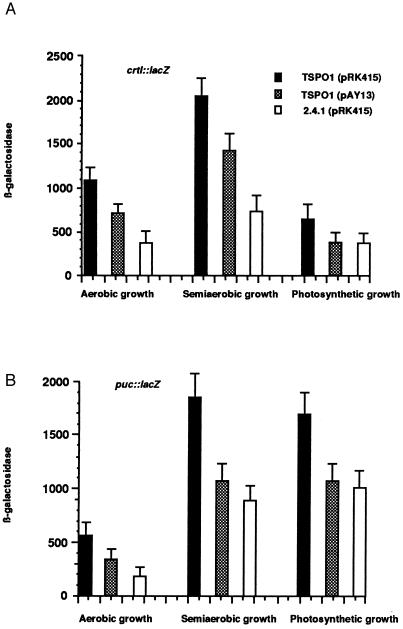
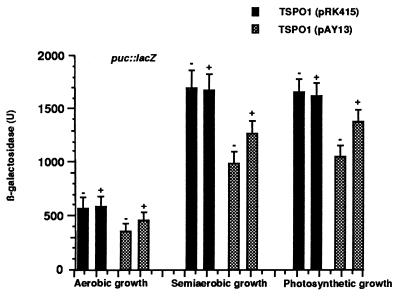
We further assessed the physiological activity of pk18 in R. sphaeroides by comparing its presence to that of TspO, which we have shown (2) behaves as a negative regulator of specific photosynthesis gene expression. TspO is active under a variety of cultivation conditions including aerobic, semiaerobic, or photosynthetic (2). Because of its location in the outer membrane, TspO is believed to be a sensor of oxygen and perhaps light, transmitting a signal to a cytoplasmic regulator. In turn, the accumulation of carotenoid and bacteriochlorophyll pigments is significantly increased in the TspO− background in semiaerobic grown cells. When incubated under limited aeration, TSPO1 (pAY13) cells accumulated approximately 35–40% (370 ± 33 μg/100 ml cell suspension) less carotenoid pigments than the corresponding TSPO1 strain containing only vector DNA (560 ± 48 μg/100 ml cell suspension), suggesting a negative effect of pk18 on the expression of the carotenoid biosynthetic genes. This effect is similar to what we observed for the presence and absence of TspO (2). To gain additional insight into the question of how pk18 affects the production of carotenoid pigments, we studied the expression of the crtI::lacZ transcriptional fusion (2) in the TspO− background in the presence or absence of pk18. When pAY13 was present in mutant TSPO1, it caused an approximate 35–40% decrease in crtI expression, under all growth conditions tested (Fig. 4A). pk18 also exerted a similar effect on the expression of the puc operon, which encodes the structural polypeptides of the B800–850 light harvesting complex (ref. 22; Fig. 4B). Therefore, pk18, when present in R. sphaeroides TspO−, behaved similarly to its bacterial counterpart, negatively affecting the expression of several photosynthesis genes.
Figure 4.
Analysis of the transcriptional effect of pk18 expressed in R. sphaeroides. Cells were grown to an OD660 of 0.2–0.3 and collected by centrifugation. Aerobic and semiaerobic growth was maintained by sparging with a gas mixture of 20% O2/77% N2/3% CO2 or 2% O2/95% N2/3% CO2, respectively. Photosynthetic cells were grown in front of the light at 10 W/m2 (for crtI::lacZ assay) or 100 W/m2 (for puc::lacZ assay) by sparging with a gas mixture of 97% N2/3% CO2, and β-Galactosidase activity was determined as described. Data represent the mean value ± SD of three independent experiments.
We further assessed the specificity of the pk18 effect upon photosynthesis gene expression, using the puf::lacZ transcriptional fusion, which is known not to be affected by TspO (2). Under both aerobic [235 ± 28 units of β-galactosidase activity for TSPO1 and 247 ± 32 for TSPO1 (pAY13)] and semiaerobic [612 ± 51 units of β-galactosidase activity for TSPO1 and 634 ± 62 for TSPO1 (pAY13)] conditions, the presence of pk18 did not affect puf operon expression. Therefore, the effect of pk18 is quite specific, both positive and negative, and mimics the role of the native bacterial TspO. The restoration of the wild-type phenotype to TSPO1 by pk18 was only partial, unlike the effect of tspO, which, when present in trans, reduced the expression of these same photosynthesis genes to below the wild-type level (i.e., by a factor of 2.5–5; ref. 2). Nonetheless, the effect of pk18 under these conditions is highly reproducible and statistically significant, and as discussed later, its partial effect is probably due to the instability of the protein in the outer membrane and/or its divergent sequence relative to TspO.
Ligand Binding of Rat pk18 in R. sphaeroides.
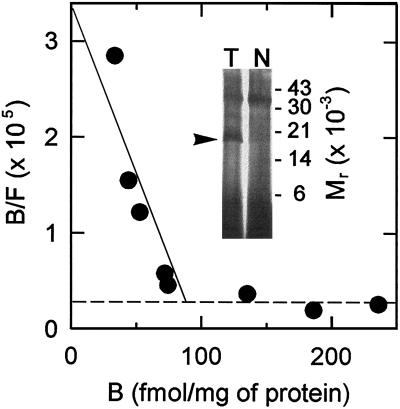
Despite the identification of a rat pk18 protein product with a smaller Mr than the normal protein, we attempted to determine if specific PK11195 (23) binding could be detected in cells expressing this protein. Membrane preparations did not reveal significant high affinity specific binding, presumably the result of the lability of the rat protein in the bacterial outer membrane. In various attempts to stabilize pk18 against proteolytic degradation we discovered that a very mild glutaraldehyde fixation of the cells, prior to membrane isolation, permitted the detection of a significant amount of specific ligand binding at low nanomolar concentrations of [3H]PK11195. TSPO1 and 2.4.1 cells harboring the pAY13 plasmid exhibited this high affinity binding toward PK11195 (Fig. 5). The dissociation constant ranged from 4 to 12 nM in different sample preparations with densities of about 100–200 fmol/mg of receptor in the isolated outer membrane. This density is likely to be a marked underestimate, because recovery of membranes following fixation compromises the purity and recovery of outer membranes coupled with the probability that degradation of the receptors may be only partially prevented. Concomitantly, pk18 was identified by specific photolabeling with [3H]PK14105. The expected Mr of 18,000 for this photolabeled adduct is indicative of a proportion, albeit low, of undegraded pk18 preserved by fixation and apparently responsible for the high affinity binding sites. TSPO1 and 2.4.1 cells that did not contain pAY13 did not display these high affinity binding sites nor any specifically photolabeled protein. An apparent low affinity, high density site was observed in all cells independent of pAY13, which may result from “pooling” of the radioligand in membrane vesicles. This site had an apparent Kd in the micromolar range and a Bmax in excess of 10 pmol/mg of protein. Further evidence that the low affinity site is not related to MBR is made obvious by the fact that only PK11195, and not other MBR ligands described below, competed for “binding” of [3H]PK11195 to this site.
Figure 5.
Scatchard analysis of [3H]PK11195 binding and [3H]PK14105 photolabeling in TSPO1 (pAY13) membranes. A representative Scatchard plot is shown where specific binding of [3H]PK11195 was calculated as total binding (in the absence of competitor) minus nonspecific binding (determined in the presence of 10 μM PK11195). Radioligand concentrations were in the range of 0.8–185 nM; however, the points shown here extend only to 15 nM to highlight the contribution from the high affinity sites (Kd = 4 nM) detected with expression of rat pk18, as represented by the solid line. Each point represents the average of triplicate determinations at each concentration. The dashed line indicates low affinity, high density sites that are not dependent upon rat pk18 expression. Inset shows autoradiography of membrane proteins photolabeled with 100 nM [3H]PK14105 in the absence (T) or presence (N) of 10 μM PK11195. The arrow points to the specifically photolabeled 18-kDa protein.
To provide further support that the high affinity site mimics the rat MBR, specific binding of [3H]PK11195 was measured in the presence of different MBR ligands. A radioligand concentration of 5 nM was used in this competition binding assay, in which it was determined that more than 70% of the specifically bound ligand is at the high affinity site. Under these conditions, in addition to PK11195, alpidem and (−) PK14067 showed potent capabilities (IC50s from 10 to 70 nM) to compete against [3H]PK11195 binding (Table 1). In correspondence with the specificity of the rat MBR, zolpidem, the imidazopyridine congener of alpidem, displayed a much higher IC50. Likewise, the quinoline propanamide (+) PK14068 was about an order of magnitude less potent than its stereoisomer (−) PK14067. The fact that these compounds, which are structurally distinct from the isoquinoline carboxamide PK11195, displayed potencies that paralleled the pattern found with native rat MBR, provides strong evidence that expression of pk18 gives rise to the high affinity PK11195 binding site. The benzodiazepines Ro5–4864 and diazepam showed IC50s near 1 μM. It is not unusual to observe that these values are considerably higher than those found for native rat MBR, because benzodiazepine binding is quite sensitive to many forms of physicochemical disturbance (for a review see ref. 9), as might be encountered with the incorporation of pk18 into a foreign environment such as the bacterial membrane. Lastly, conforming with MBR specificity, the central benzodiazepine ligand flumazenil was not effective at higher micromolar concentrations in competing against PK11195 binding.
Table 1.
Competition of specific [3H]PK11195 binding by MBR ligands. Specific binding was measured at 5 nM [3H]PK11195, as described for Fig. 5, in the presence of different concentrations of the competing ligands indicated. Assays at five different ligand concentrations were performed in triplicate. The IC50s were estimated by nonlinear regression analysis as described earlier (18, 24)
| Class | Ligand | IC50 (μM) |
|---|---|---|
| Isoquinoline carboxamide | PK11195 | 0.042 |
| Quinoline propanamide | (−)PK14067 | 0.079 |
| (+)PK14068 | 0.54 | |
| Imidazopyridine | Alpidem | 0.010 |
| Zolpidem | 3.6 | |
| Benzodiazepine | Ro5-4864 | 1.0 |
| Diazepam | 0.63 | |
| Flumazenil | >10 |
PK11195-Induced Physiological Changes in R. sphaeroides (pAY13).
The finding that pk18, when introduced into the bacterial membrane, effectively binds to a number of ligands suggests that these drugs may exhibit some physiological effect in R. sphaeroides following a challenge. To explore this possibility, TSPO1 and TSPO1 (pAY13) cells were grown in the presence of 10 μM PK11195, and the expression of the puc::lacZ transcriptional fusion was monitored (Fig. 6). PK11195 was found to significantly increase expression of the reporter gene by 30–40% in TSPO1 (pAY13) grown under aerobic, semiaerobic, or photosynthetic conditions. No alteration of puc::lacZ expression in the TSPO1 (pRK415) control background could be detected. A similar effect of PK11195 on TSPO1 (pAY13) cells was observed when expression of the crtI::lacZ transcriptional fusion was monitored (data not shown). We have also demonstrated that PK11195 did not exert its effect upon the puc::lacZ transcriptional fusion expression when the wild-type copy of TspO was present. Under both aerobic growth conditions [190 ± 22 units of β-galactosidase activity for 2.4.1 (pRK415); 185 ± 16 for 2.4.1 (pRK415) + 10 μM PK11195; 188 ± 19 for 2.4.1 (pAY13); 191 ± 22 for 2.4.1 (pAY13) + 10 μM PK11195] and semiaerobic growth conditions [950 ± 87 units of β-galactosidase activity for 2.4.1 (pRK415); 978 ± 77 for 2.4.1 (pRK415) + 10 μM PK11195; 932 ± 81 for 2.4.1 (pAY13); 938 ± 77 for 2.4.1 (pAY13) + 10 μM PK11195], the expression of the reporter gene was not affected by the presence of isoquinoline carboxamide in the incubation medium. Therefore, these results suggest that the PK11195 binding site may be allosterically coupled with a site normally occupied by an endogenous ligand for pk18/TspO, which may affect the physiological activity of the former.
Figure 6.
Effect of PK11195 on puc::lacZ expression. TSPO1 (filled columns) and TSPO1 (pAY13) (hatched columns) were grown in the presence (+) or absence (−) of 10 μM PK11195. Growth conditions and β-Galactosidase assay are the same as in Fig. 4. Data are the mean value ± SD of at least three independent assays.
DISCUSSION
Taken together, the data presented here suggest a close functional and structural relationship between the bacterial TspO and the mammalian pk18 protein. The data suggest the potential to generate a mitochondrion-like environment within this bacterial cell type to study the properties and functional activities of both the mammalian and bacterial receptors. Thus, it is of critical importance to investigate both genetic and physiologic conditions that can lead to the stabilization of the rat protein in its transfer from the cytoplasm to the outer membrane. This raises another interesting observation: these proteins, lacking a definable signal sequence, are imported to the bacterial outer membrane. For the rat protein it might appear paradoxical, because it is encoded by a nuclear gene. By contrast, when the rat protein is expressed in E. coli, this protein was not found in the outer membrane, but was evident in cell membrane fractions.
A database search revealed the presence of a tspO homolog in the genome of the unicellular cyanobacterium Synechocystis sp. (7). Interestingly, the degree of homology between the R. sphaeroides TspO and the hypothetical protein from Synechocystis is lower (23–25% identical amino acids with a further 20–25% conservative replacements) than that between TspO and mammalian pk18 (Fig. 1), which correlates with the early evolutionary divergence between the α-subdivision of photosynthetic proteobacteria and the cyanobacteria (11). The Synechocystis protein sequence, like that of TspO, is enriched with tryptophan residues, five of which are conserved among all prokaryotic and mammalian TspO homologs. This may indicate the importance of these amino acid residues in maintaining the functional and/or structural properties of both the bacterial and mammalian receptors. It is noteworthy that TspO does not bind PK11195 or PK14105, suggesting that the drug-binding properties are probably restricted to homologs of the animal kingdom.
These results lend further support for the possible evolutionary relationship between the α-3 subdivision of the purple photosynthetic bacteria and the mammalian mitochondrion. In light of the ability of pk18 to respond to an environmental signal when in the outer membrane of R. sphaeroides, it is reasonable to suggest that in situ pk18, like TspO, may be involved as a possible mitochondrial-localized, oxygen-dependent signal generator (25). Depending on the tissue of residence, the MBR would transmit a regulatory signal to different downstream responsive genes, leading to a tissue-specific response. The current literature archives a variety of diverse activities to the MBR, and such a hypothesis would be in keeping with these observations.
The ability of pk18 to interact with definite specificity with a variety of MBR ligands when isolated outer membranes are used suggests that pk18 assumes a conformation in the R. sphaeroides outer membrane similar to that of the native receptor in the mitochondrial outer membrane. That the isoquinoline carboxamide PK11195 can act as an antagonist to the environmental signal to which pk18 is responsive lends additional support to the concept that the function of the rat protein in situ parallels its physiologic effect when present in the outer membrane of R. sphaeroides. This could imply a direct interaction of the MBR with molecular oxygen and the ability of the MBR to interact with additional elements of a signal transduction pathway to effect nuclear gene expression or other molecular events in a tissue-specific manner.
On the other hand, TspO is active under both aerobic and photosynthetic conditions. Therefore, the question arises: was the photosynthetic function of TspO acquired later in the process of evolution, after the divergence of the bacterial progenitor and mitochondrial symbiont, or was this function modified by the mitochondrial receptor? One might expect to find the possible answer to this question in studies of the nonphotosynthetic bacterium Paracoccus denitrificans, which is closely related to R. sphaeroides and which, according to some preliminary observations, may contain a homolog of the TspO protein (A.Y., M. Gomelsky, and S.K., unpublished data).
Although the relative physiologic effect of the rat protein in R. sphaeroides on reporter gene expression is severalfold less than TspO, the data are highly reproducible. Knowing that the rat protein is unstable in R. sphaeroides is in keeping with the quantitative observations on reporter gene expression. Furthermore, the spectrum of reporter genes that are affected by pk18, or unaffected in the case of the puf operon, provides convincing evidence that the rat protein is effectively mimicking TspO.
Acknowledgments
This work was supported by U.S. Public Health Service Grant GM15590 to S.K. and by Grant MH44284 to K.E.K.
ABBREVIATIONS
- MBR
mitochondrial benzodiazepine receptor
- pk18
18-kDa protein component of MBR
- TspO
tryptophan-rich sensory protein
- PK11195
1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide
- PK14105
1-(2-fluoro-5-nitrophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide
- PK14067 and PK14068
(−)- and (+)-N,N-diethyl-2-methyl-3-[4-(2-phenyl) quinolinyl] propanamide, respectively
References
- 1.Armstrong G A, Alberti M, Leach F, Hearst J E. Mol Gen Genet. 1989;216:254–268. doi: 10.1007/BF00334364. [DOI] [PubMed] [Google Scholar]
- 2.Yeliseev A A, Kaplan S. J Biol Chem. 1995;270:21167–21175. doi: 10.1074/jbc.270.36.21167. [DOI] [PubMed] [Google Scholar]
- 3.Sprengel R, Werner P, Seeburg P H, Mukhin A G, Santi M R, Grayson D R, Guidotti A, Krueger K E. J Biol Chem. 1989;264:20415–20421. [PubMed] [Google Scholar]
- 4.Garnier M, Dimchev A, Boujrad N, Price M J, Musto N A, Papadopoulos V. Mol Pharmacol. 1994;45:201–211. [PubMed] [Google Scholar]
- 5.Riond J, Mattei M G, Kaghad M, Dumont X, Guillemot J C, Le Fur G, Caput D, Ferrara P. Eur J Biochem. 1991;195:307–312. doi: 10.1111/j.1432-1033.1991.tb15707.x. [DOI] [PubMed] [Google Scholar]
- 6.Parola A L, Stump D G, Pepperl D J, Krueger K E, Regan J W, Laird H E, II. J Biol Chem. 1991;266:14082–14087. [PubMed] [Google Scholar]
- 7.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, et al. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 8.McEnery M W, Snowman A M, Trifiletti R R, Snyder S H. Proc Natl Acad Sci USA. 1992;89:3170–3174. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krueger K E. Biochim Biophys Acta. 1995;1241:453–470. doi: 10.1016/0304-4157(95)00016-x. [DOI] [PubMed] [Google Scholar]
- 10.Baker M E, Fanestil D D. Cell. 1991;65:721–722. doi: 10.1016/0092-8674(91)90379-d. [DOI] [PubMed] [Google Scholar]
- 11.Woese C R. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen-Bazire G, Sistrom W R, Stanier R Y. J Cell Comp Physiol. 1956;49:25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- 13.Davis J, Donohue T J, Kaplan S. J Bacteriol. 1988;170:320–329. doi: 10.1128/jb.170.1.320-329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin J P, Jr, Colina K, Logsdon N. J Bacteriol. 1987;169:2516–2522. doi: 10.1128/jb.169.6.2516-2522.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 16.Moore M D, Kaplan S. J Bacteriol. 1989;171:4385–4394. doi: 10.1128/jb.171.8.4385-4394.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J D, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1987. [Google Scholar]
- 18.Blahos J, Whalin M E, Krueger K E. J Biol Chem. 1995;270:20285–20291. doi: 10.1074/jbc.270.35.20285. [DOI] [PubMed] [Google Scholar]
- 19.Dryden S D, Kaplan S. Nucleic Acids Res. 1990;18:7267–7277. doi: 10.1093/nar/18.24.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss R L. J Bacteriol. 1976;128:668–670. doi: 10.1128/jb.128.2.668-670.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tai S P, Kaplan S. J Bacteriol. 1985;164:181–186. doi: 10.1128/jb.164.1.181-186.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J K, Kaplan S. J Bacteriol. 1992;174:1158–1171. doi: 10.1128/jb.174.4.1158-1171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Fur G, Vaucher N, Perrier M L, Famier A, Benavides J, Renault C, Dubroeucq M C, Gueremy C, Uzan A. Life Sci. 1983;33:449–457. doi: 10.1016/0024-3205(83)90794-4. [DOI] [PubMed] [Google Scholar]
- 24.Papadopoulos V, Nowzari F B, Krueger K E. J Biol Chem. 1991;266:3682–3687. [PubMed] [Google Scholar]
- 25.Hirsch J D, Beyer C F, Malkowitz L, Beer B, Blume A J. Mol Pharmacol. 1989;35:157–163. [PubMed] [Google Scholar]