Abstract
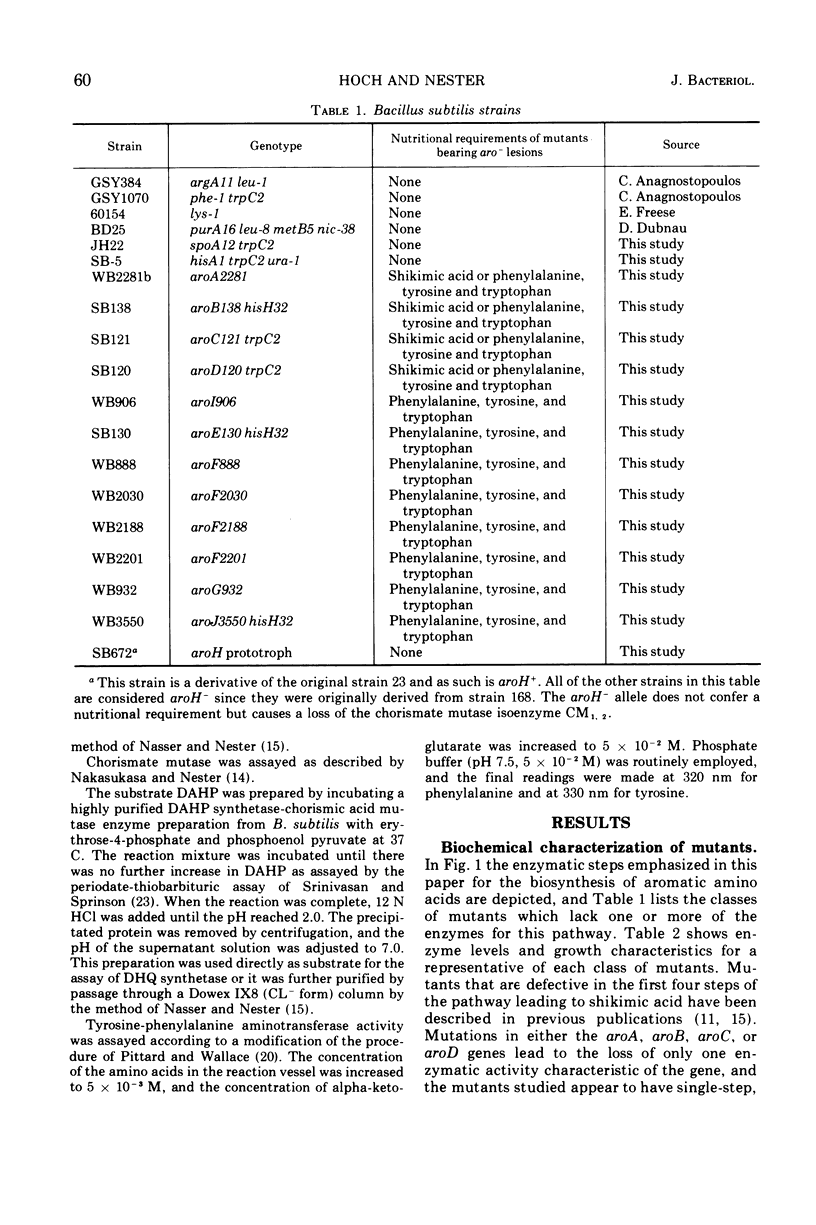
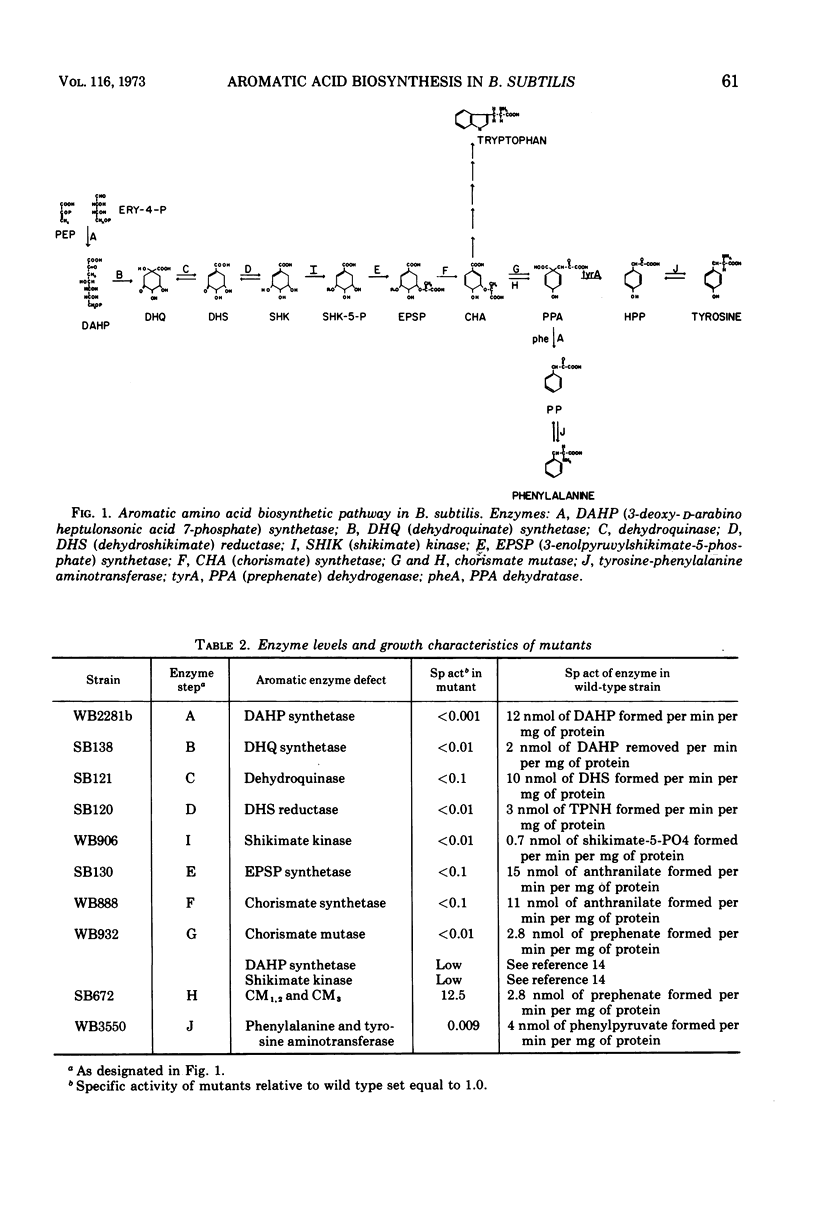
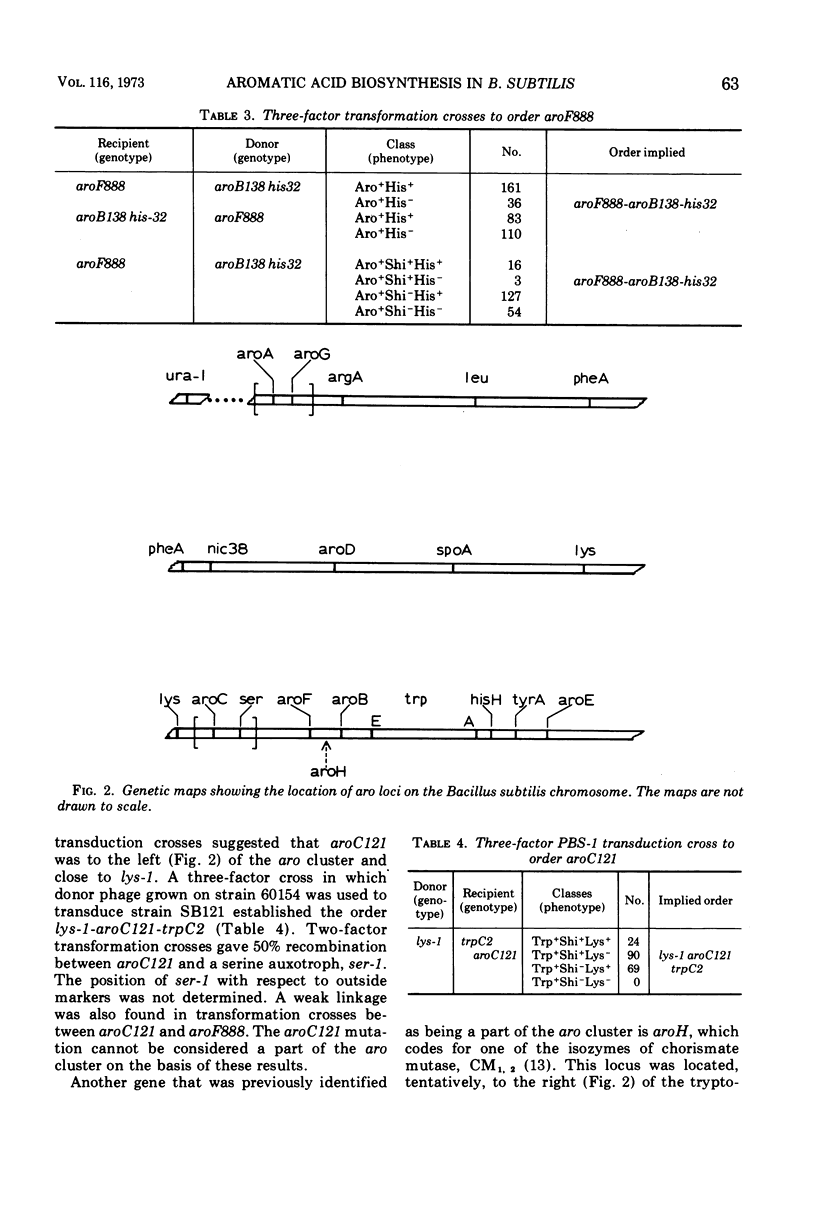
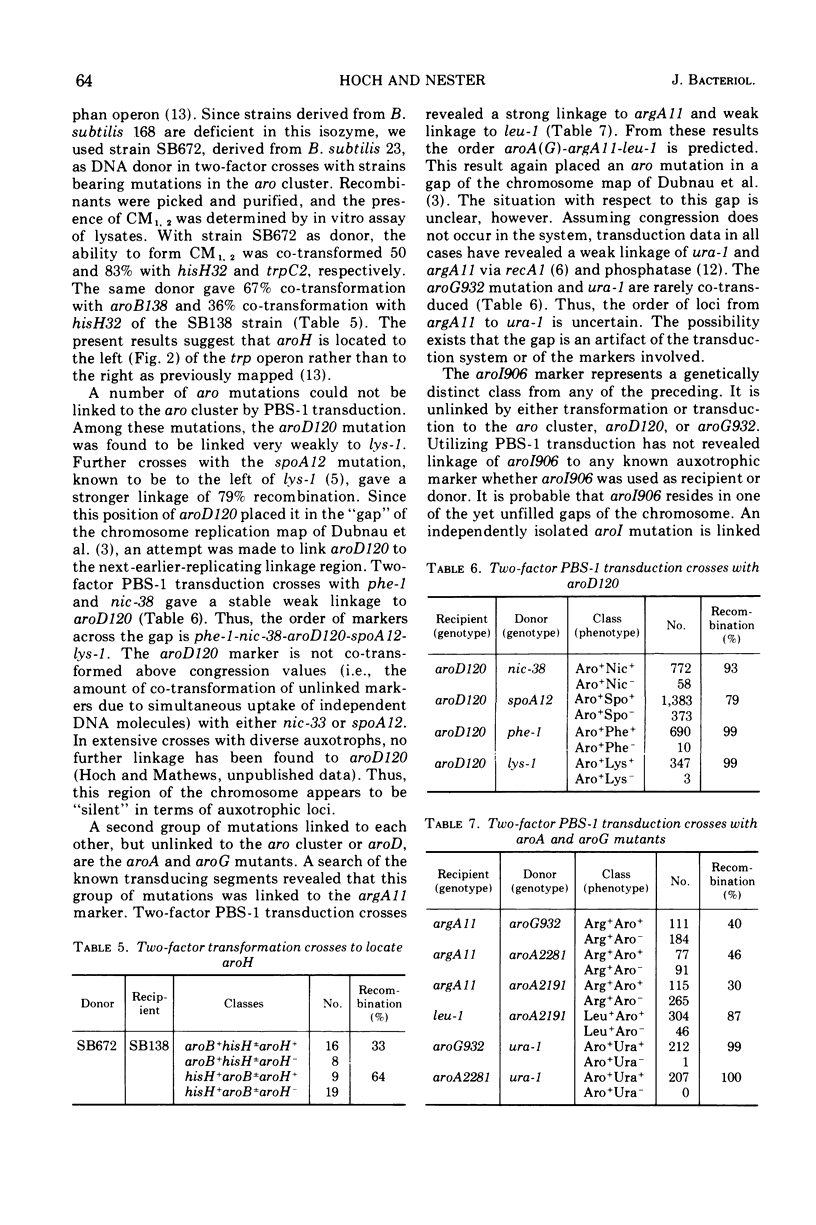
Mutants have been isolated which correspond to every step concerned with the biosynthesis of the aromatic amino acids in Bacillus subtilis. Each mutant has been characterized, and the lesion it bore was analyzed by deoxyribonucleic acid transformation and PBS-1 mediated transduction. The biochemical analysis revealed that each of the mutations appears to have affected a single enzyme, except for two groups of pleiotropic mutations. All aroF mutants (chorismic acid synthetase) lack dehydroquinic acid synthetase (aroB) activity. The gene that specifies aroB is closely linked to the gene coding for the aroF enzyme. Both genes are a part of the aro cluster. Mutants lacking chorismate mutase activity also lack d-arabino-heptulosonic acid-7-phosphate synthetase and shikimate kinase activity, presumably as a result of these three activities forming a multi-enzyme complex. Another mutant, previously undescribed, had been isolated. The affected gene codes for the tyrosine and phenylalanine aminotransferase activity. All of the mutations have been located on the B. subtilis genome except those in the genes specifying shikimate kinase activity and tyrosine-phenylalanine aminotransferase activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case M. E., Giles N. H. Partial enzyme aggregates formed by pleiotropic mutants in the arom gene cluster of Neurospora crassa. Proc Natl Acad Sci U S A. 1971 Jan;68(1):58–62. doi: 10.1073/pnas.68.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Goldthwaite C., Smith I., Marmur J. Genetic mapping in Bacillus subtilis. J Mol Biol. 1967 Jul 14;27(1):163–185. doi: 10.1016/0022-2836(67)90358-0. [DOI] [PubMed] [Google Scholar]
- Gibson F., Pittard J. Pathways of biosynthesis of aromatic amino acids and vitamins and their control in microorganisms. Bacteriol Rev. 1968 Dec;32(4 Pt 2):465–492. [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Anagnostopoulos C. Chromosomal location and properties of radiation sensitivity mutations in Bacillus subtilis. J Bacteriol. 1970 Aug;103(2):295–301. doi: 10.1128/jb.103.2.295-301.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A. Genetic analysis of pleiotropic negative sporulation mutants in Bacillus subtilis. J Bacteriol. 1971 Mar;105(3):896–901. doi: 10.1128/jb.105.3.896-901.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch S. O., Anagnostopoulos C., Crawford I. P. Enzymes of the tryptophan operon of Bacillus subtilis. Biochem Biophys Res Commun. 1969 Jun 27;35(6):838–844. doi: 10.1016/0006-291x(69)90700-1. [DOI] [PubMed] [Google Scholar]
- Hoch S. O., Roth C. W., Crawford I. P., Nester E. W. Control of tryptophan biosynthesis by the methyltryptophan resistance gene in Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):38–45. doi: 10.1128/jb.105.1.38-45.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionesco H., Michel J., Cami B., Schaeffer P. Symposium on bacterial spores: II. Genetics of sporulation in Bacillus subtilis Marburg. J Appl Bacteriol. 1970 Mar;33(1):13–24. doi: 10.1111/j.1365-2672.1970.tb05230.x. [DOI] [PubMed] [Google Scholar]
- JENSEN R. A., NESTER E. W. THE REGULATORY SIGNIFICANCE OF INTERMEDIARY METABOLITES: CONTROL OF AROMATIC ACID BIOSYNTHESIS BY FEEDBACK INHIBITION IN BACILLUS SUBTILIS. J Mol Biol. 1965 Jun;12:468–481. doi: 10.1016/s0022-2836(65)80270-4. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Nester E. W. Regulatory enzymes of aromatic amino acid biosynthesis in Bacillus subtilis. I. Purification and properties of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase. J Biol Chem. 1966 Jul 25;241(14):3365–3372. [PubMed] [Google Scholar]
- Le Hegarat J. C., Anagnostopoulos C. Localisation chromosomique d'un gène gouvernant la synthèse d'une phosphatase alcaline chez Bacillus subtilis. C R Acad Sci Hebd Seances Acad Sci D. 1969 Nov 17;269(20):2048–2050. [PubMed] [Google Scholar]
- Lorence J. H., Nester E. W. Multiple molecular forms of chorismate mutase in Bacillus subtillis. Biochemistry. 1967 May;6(5):1541–1553. doi: 10.1021/bi00857a041. [DOI] [PubMed] [Google Scholar]
- NESTER E. W., LEDERBERG J. Linkage of genetic units of Bacillus subtilis in DNA transformation. Proc Natl Acad Sci U S A. 1961 Jan 15;47:52–55. doi: 10.1073/pnas.47.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsukasa W. M., Nester E. W. Regulation of aromatic amino acid biosynthesis in Bacillus subtilis 168. I. Evidence for and characterization of a trifunctional enzyme complex. J Biol Chem. 1972 Sep 25;247(18):5972–5979. [PubMed] [Google Scholar]
- Nasser D., Nester E. W. Aromatic amino acid biosynthesis: gene-enzyme relationships in Bacillus subtilis. J Bacteriol. 1967 Nov;94(5):1706–1714. doi: 10.1128/jb.94.5.1706-1714.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E W, Schafer M, Lederberg J. Gene Linkage in DNA Transfer: A Cluster of Genes Concerned with Aromatic Biosynthesis in Bacillus Subtilis. Genetics. 1963 Apr;48(4):529–551. doi: 10.1093/genetics/48.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Lorence J. H., Nasser D. S. An enzyme aggregate involved in the biosynthesis of aromatic amino acids in Bacillus subtilis. Its possible function in feedback regulation. Biochemistry. 1967 May;6(5):1553–1563. doi: 10.1021/bi00857a042. [DOI] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Gene controlling the uptake of shikimic acid by Escherichia coli. J Bacteriol. 1966 Oct;92(4):1070–1075. doi: 10.1128/jb.92.4.1070-1075.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth C. W., Nester E. W. Co-ordinate control of tryptophan, histidine and tyrosine enzyme synthesis in Bacillus subtilis. J Mol Biol. 1971 Dec 28;62(3):577–589. doi: 10.1016/0022-2836(71)90157-4. [DOI] [PubMed] [Google Scholar]
- SRINIVASAN P. R., ROTHSCHILD J., SPRINSON D. B. THE ENZYMIC CONVERSION OF 3-DEOXY-D-ARABINO-HEPTULOSONIC ACID 7-PHOSPHATE TO 5-DEHYDROQUINATE. J Biol Chem. 1963 Oct;238:3176–3182. [PubMed] [Google Scholar]
- SRINIVASAN P. R., SPRINSON D. B. 2-Keto-3-deoxy-D-arabo-heptonic acid 7-phosphate synthetase. J Biol Chem. 1959 Apr;234(4):716–722. [PubMed] [Google Scholar]



