Abstract
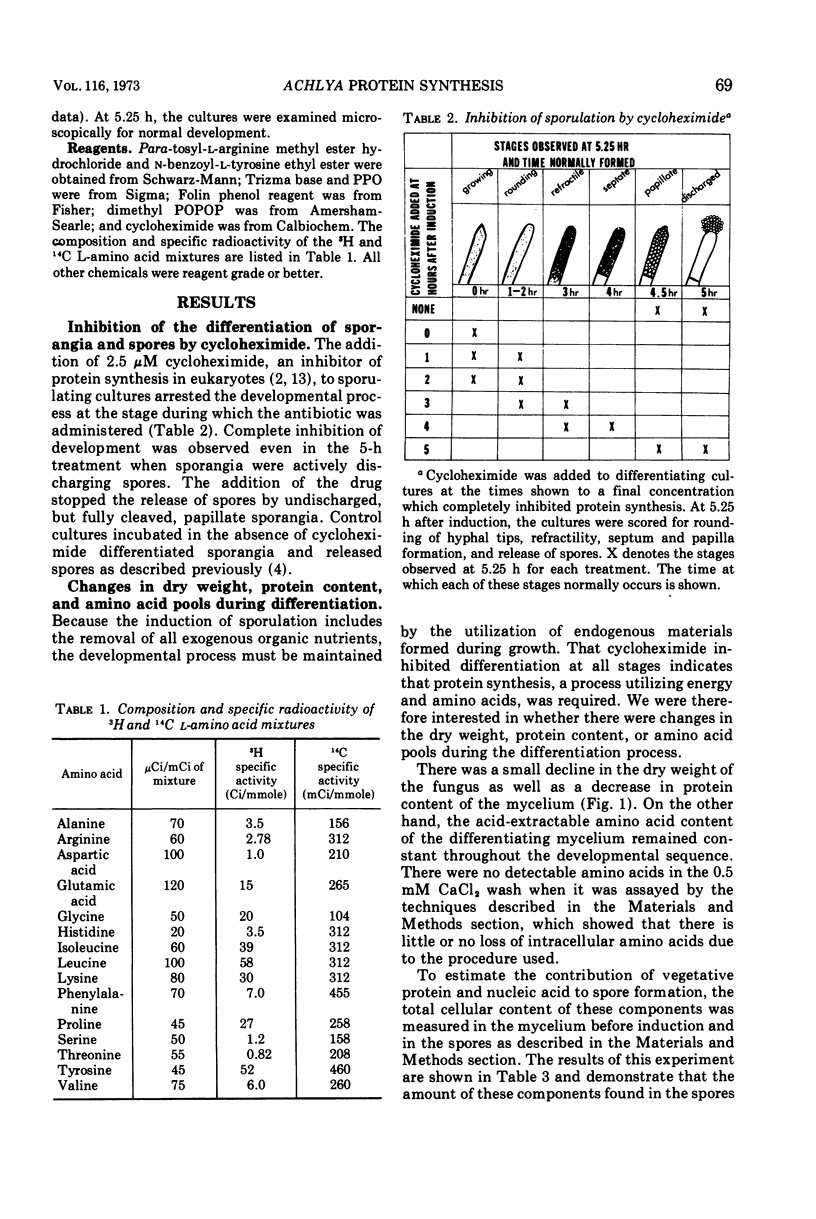
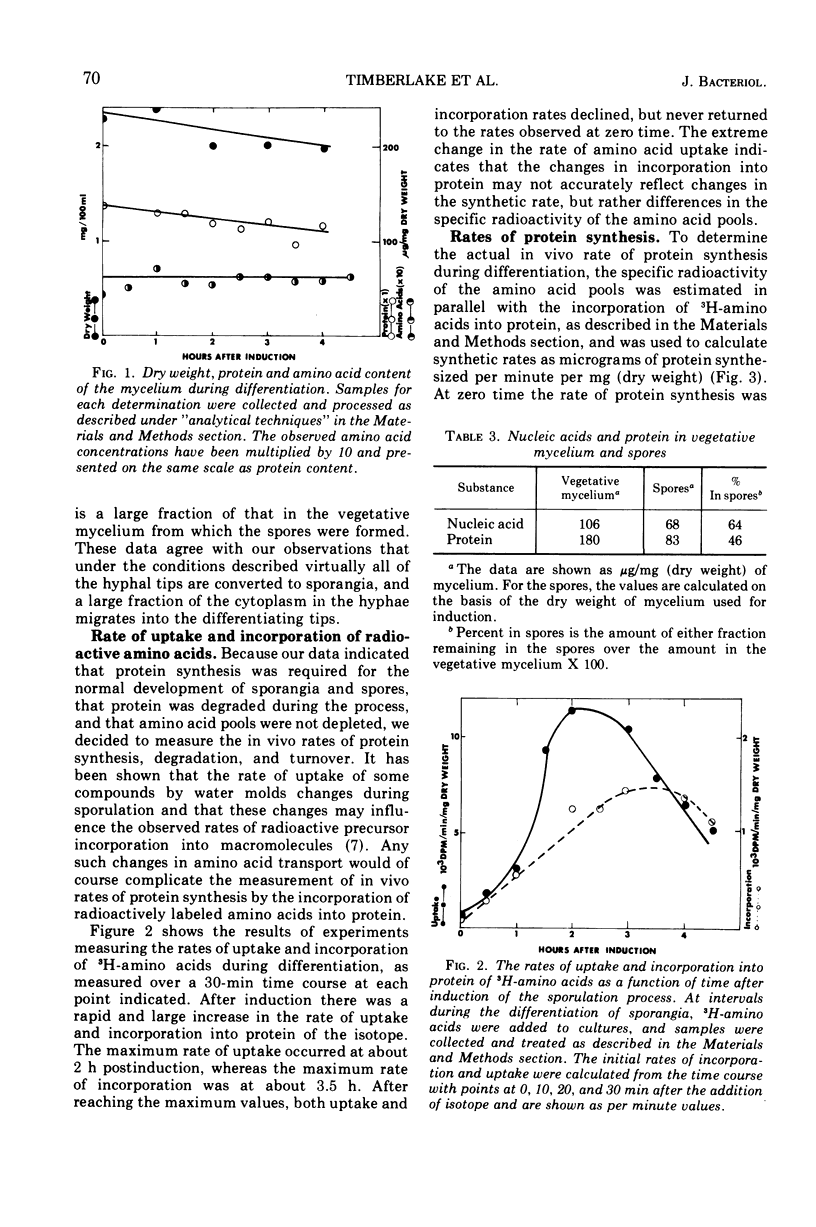
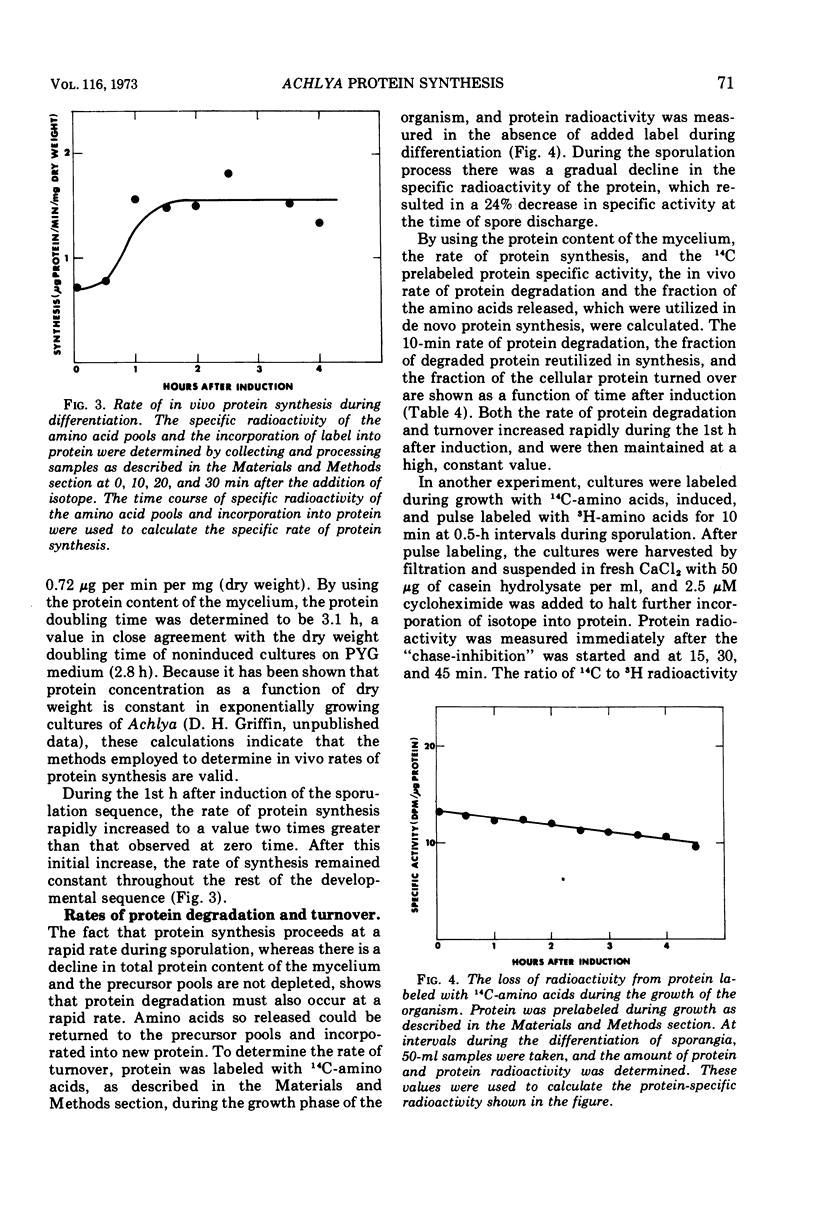
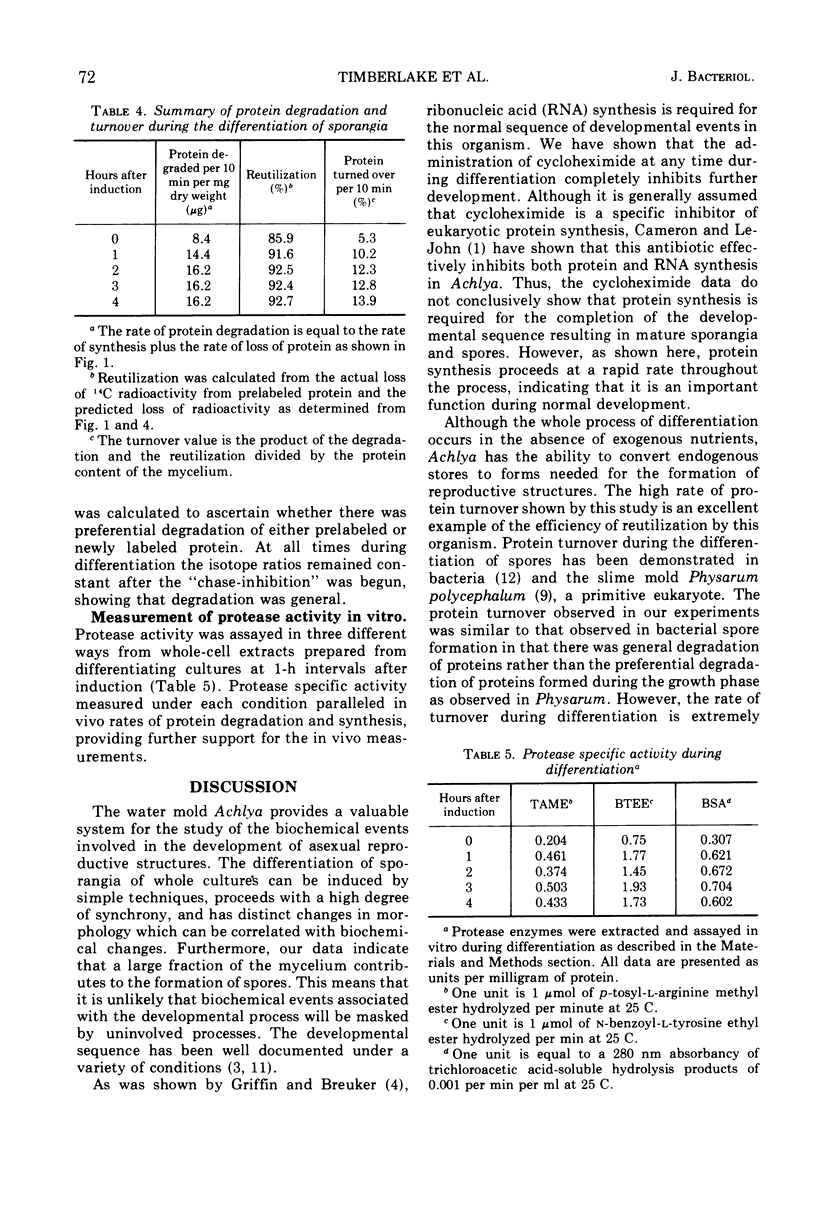
During the synchronous differentiation of sporangia in the absence of added nutrients, the water mold Achlya bisexualis (Coker and Couch) actively synthesized protein. Inhibition of protein synthesis at any time during the sporulation process completely inhibited further differentiation. Large changes in the rate of radioactive amino acid uptake resulted in changes in the specific activity of the cellular amino acid pool. The rate of protein synthesis was calculated from the amino acid pool specific activity and the incorporation of isotope into protein. During the 1st h after induction of the sporulation process, the rate of protein synthesis increased to two times the initial value. The amino acid precursors for this synthesis were supplied by the degradation of preexisting protein. Proteolytic enzyme activity assayed in vitro increased in proportion to the in vivo rates of protein synthesis and degradation. Differentiation was accompanied by a slight decline in dry weight of the mycelium as well as by a decrease in the protein content, whereas the relative size of the amino acid pools remained constant.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cameron L. E., LéJohn H. B. On the involvement of calcium in amino acid transport and growth of the fungus Achlya. J Biol Chem. 1972 Aug 10;247(15):4729–4739. [PubMed] [Google Scholar]
- Griffin D. H., Breuker C. Ribonucleic acid synthesis during the differentiation of sporangia in the water mold Achlya. J Bacteriol. 1969 May;98(2):689–696. doi: 10.1128/jb.98.2.689-696.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D. H. Effect of Electrolytes on Differentiation in Achlya sp. Plant Physiol. 1966 Oct;41(8):1254–1256. doi: 10.1104/pp.41.8.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murphy M. N., Lovett J. S. RNA and protein synthesis during zoospore differentiation in synchronized cultures of Blastocladiella. Dev Biol. 1966 Aug;14(1):68–95. doi: 10.1016/0012-1606(66)90006-6. [DOI] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Kornberg A. Biochemical studies of bacterial sporulation and germaination. VII. Protein turnover during sporulation of Bacillus subtilis. J Biol Chem. 1968 Sep 10;243(17):4600–4605. [PubMed] [Google Scholar]
- Viau J. P., Davis F. F. Effect of cycloheximide on the synthesis and modification of ribosomal RNA in Neurospora crassa. Biochim Biophys Acta. 1970 May 21;209(1):190–195. doi: 10.1016/0005-2787(70)90675-1. [DOI] [PubMed] [Google Scholar]



