Abstract
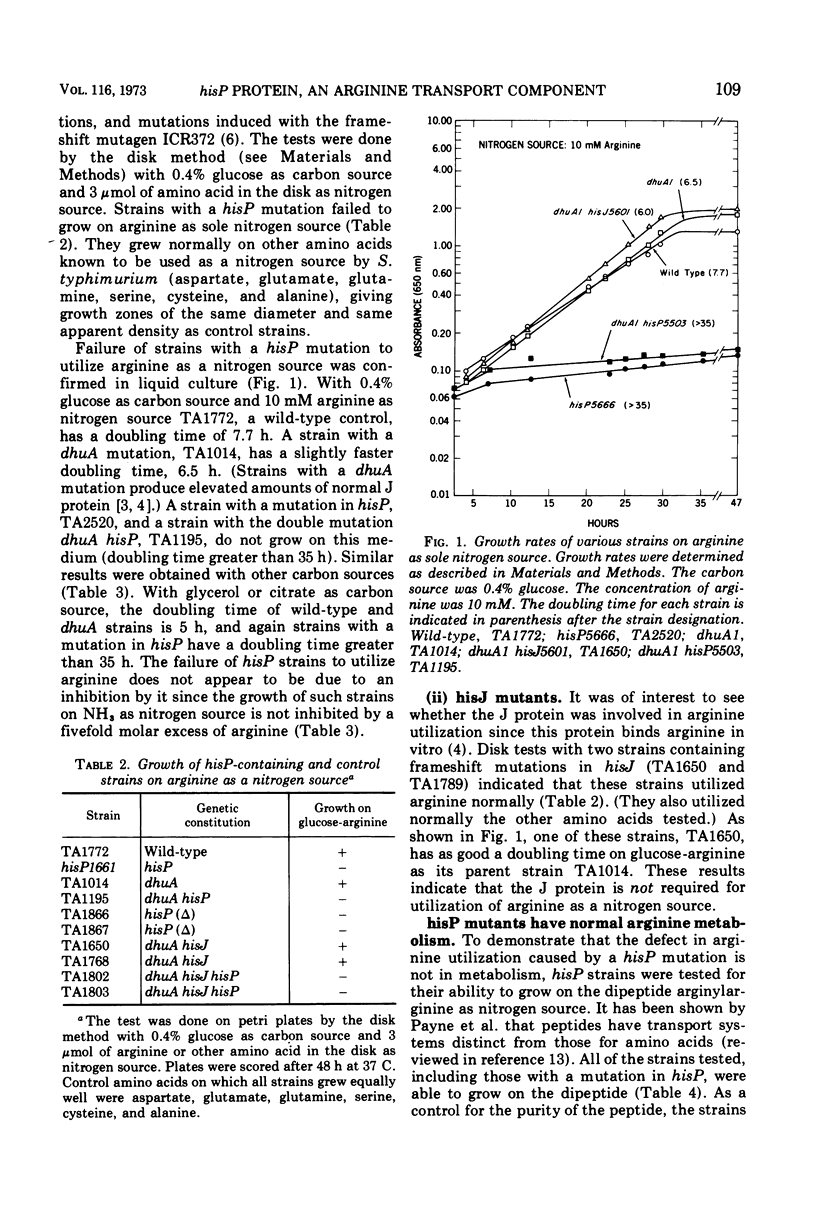
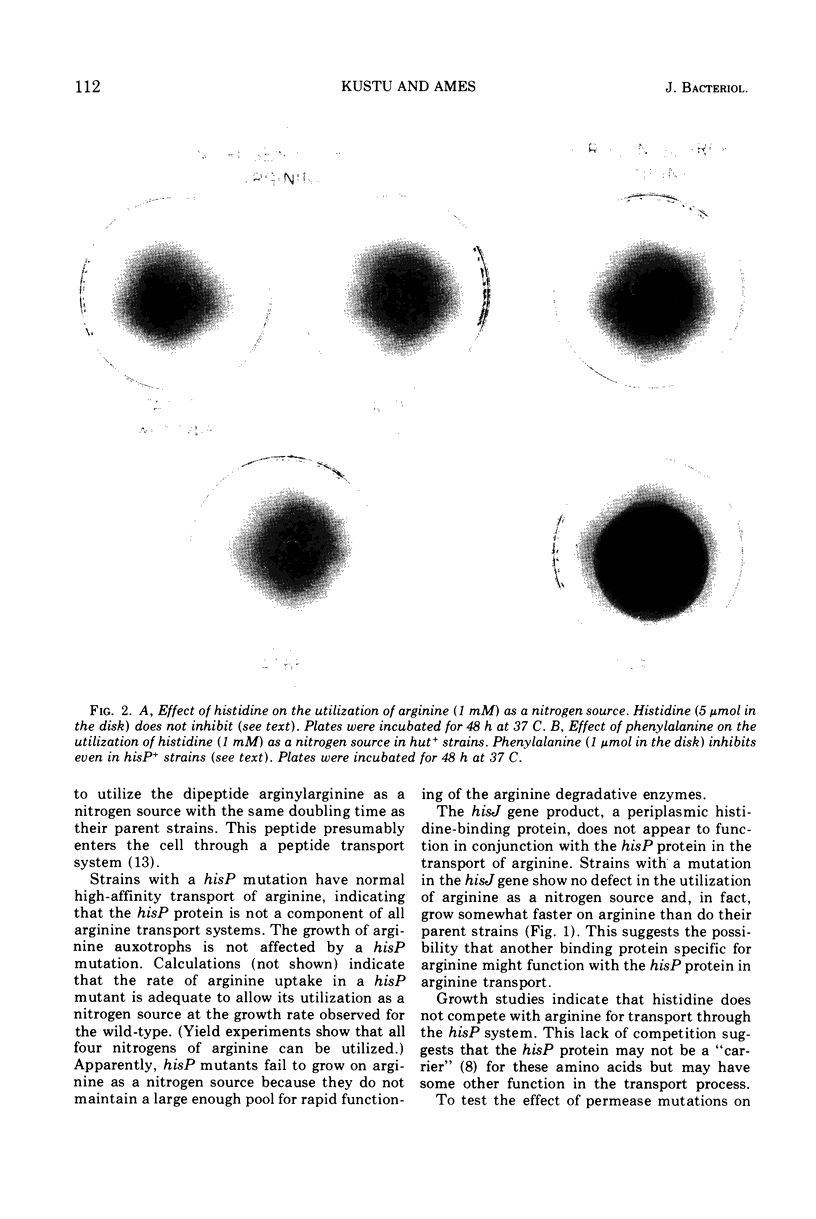
The hisP protein is an essential component of the high-affinity histidine transport system in Salmonella typhimurium. Our present studies demonstrate that this protein is also an essential component of an arginine transport system. Strains with a mutation in the hisP gene are unable to transport arginine for use as a sole nitrogen source. However, such strains have normal high-affinity transport of arginine, indicating that the hisP protein is not required for all arginine transport systems. Histidine does not appear to compete with arginine for transport through the hisP system, suggesting that the hisP protein may not be a “carrier” for these amino acids. The hisJ protein, a periplasmic histidine-binding protein, is known to function in conjunction with the hisP protein in the transport of histidine. The hisJ protein does not function with the hisP protein in arginine transport.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Whitfield H. J., Jr Frameshift mutagenesis in Salmonella. Cold Spring Harb Symp Quant Biol. 1966;31:221–225. doi: 10.1101/sqb.1966.031.01.030. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Lever J. E. The histidine-binding protein J is a component of histidine transport. Identification of its structural gene, hisJ. J Biol Chem. 1972 Jul 10;247(13):4309–4316. [PubMed] [Google Scholar]
- Ames G. F., Lever J. Components of histidine transport: histidine-binding proteins and hisP protein. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1096–1103. doi: 10.1073/pnas.66.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Roth J. R. Histidine and aromatic permeases of Salmonella typhimurim. J Bacteriol. 1968 Nov;96(5):1742–1749. doi: 10.1128/jb.96.5.1742-1749.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill W. J., Magasanik B. Genetic and metabolic control of histidase and urocanase in Salmonella typhimurium, strain 15-59. J Biol Chem. 1969 Oct 10;244(19):5392–5402. [PubMed] [Google Scholar]
- Gordon A. S., Lombardi F. J., Kaback H. R. Solubilization and partial purification of amino acid-specific components of the D-lactate dehydrogenase-coupled amino acid-transport systems (E. coli-cell membranes-sephadex-detergent-solubilized-vesicles). Proc Natl Acad Sci U S A. 1972 Feb;69(2):358–362. doi: 10.1073/pnas.69.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnick D., Calvo J. M., Klopotowski T., Ames B. N. Compounds which serve as the sole source of carbon or nitrogen for Salmonella typhimurium LT-2. J Bacteriol. 1969 Oct;100(1):215–219. doi: 10.1128/jb.100.1.215-219.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewska-Grynkiewicz K., Walczak W., Klopotowski T. Mutants of Salmonella typhimurium able to utilize D-histidine as a source of L-histidine. J Bacteriol. 1971 Jan;105(1):28–37. doi: 10.1128/jb.105.1.28-37.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever J. E. Purification and properties of a component of histidine transport in Salmonella typhimurium. The histidine-binding protein J. J Biol Chem. 1972 Jul 10;247(13):4317–4326. [PubMed] [Google Scholar]
- Meiss H. K., Brill W. J., Magasanik B. Genetic control of histidine degradation in Salmonella typhimurium, strain LT-2. J Biol Chem. 1969 Oct 10;244(19):5382–5391. [PubMed] [Google Scholar]
- Rosen B. P. Basic amino acid transport in Escherichia coli. J Biol Chem. 1971 Jun 10;246(11):3653–3662. [PubMed] [Google Scholar]
- Shifrin S., Ames B. N., FerroLuzzi-Ames G. Effect of the alpha-hydrazino analogue of histidine on histidine transport and arginine biosynthesis. J Biol Chem. 1966 Jul 25;241(14):3424–3429. [PubMed] [Google Scholar]
- Sletzinger M., Firestone R. A., Reinhold D. F., Rooney C. S., Nicholson W. H. The alpha-hydrazino analog of histidine. J Med Chem. 1968 Mar;11(2):261–263. doi: 10.1021/jm00308a015. [DOI] [PubMed] [Google Scholar]




