Abstract
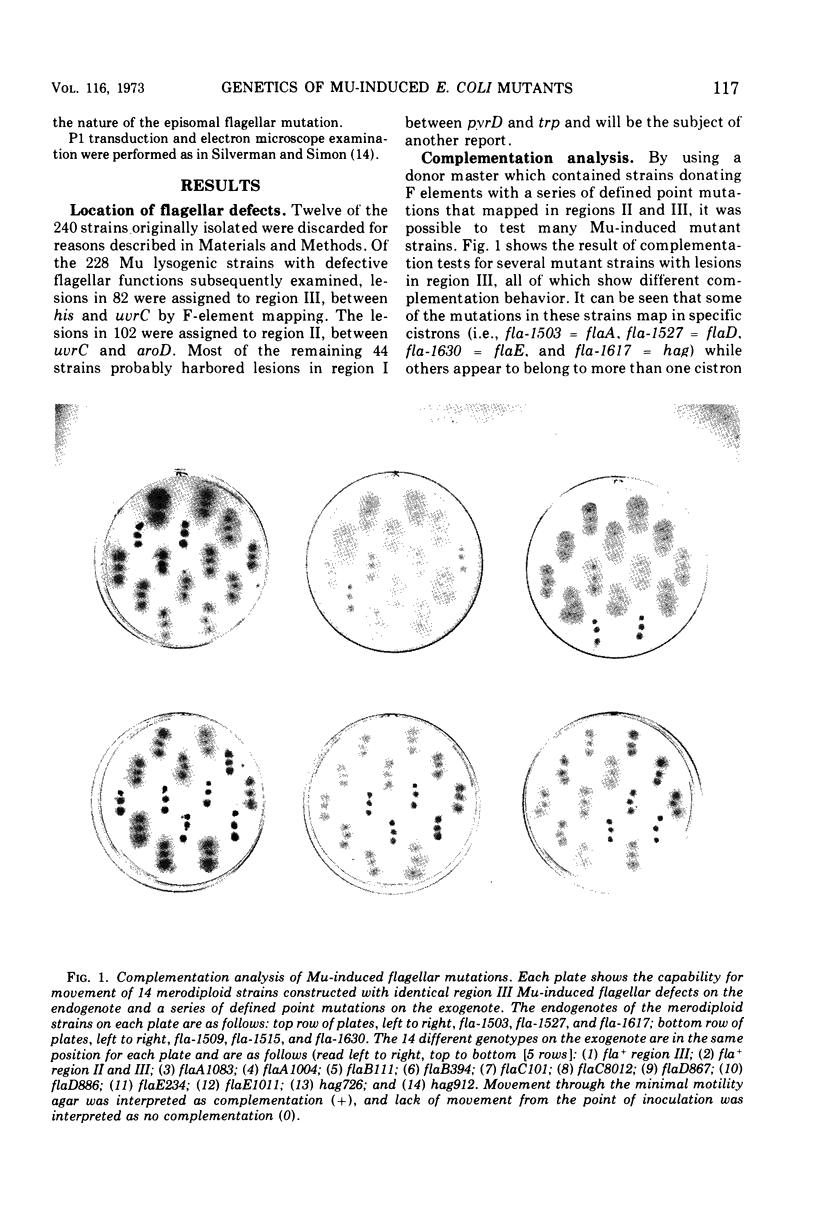
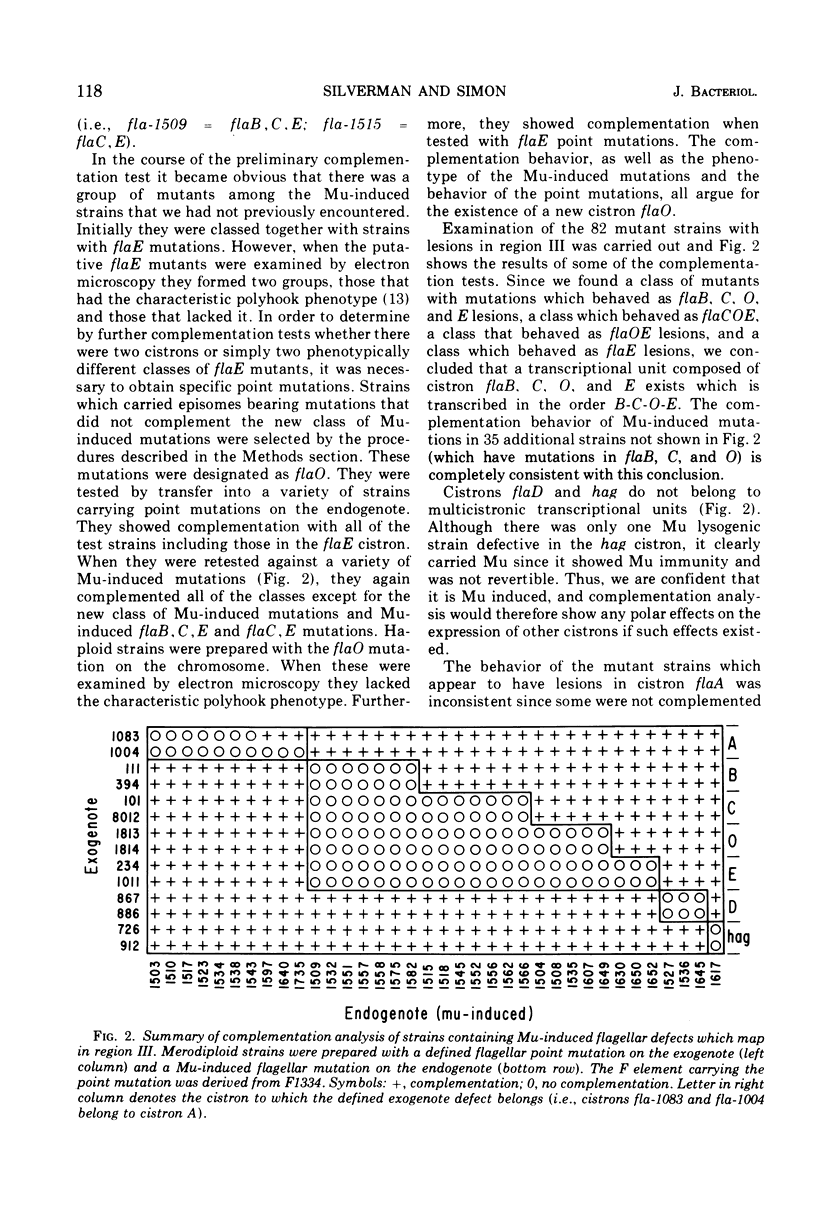
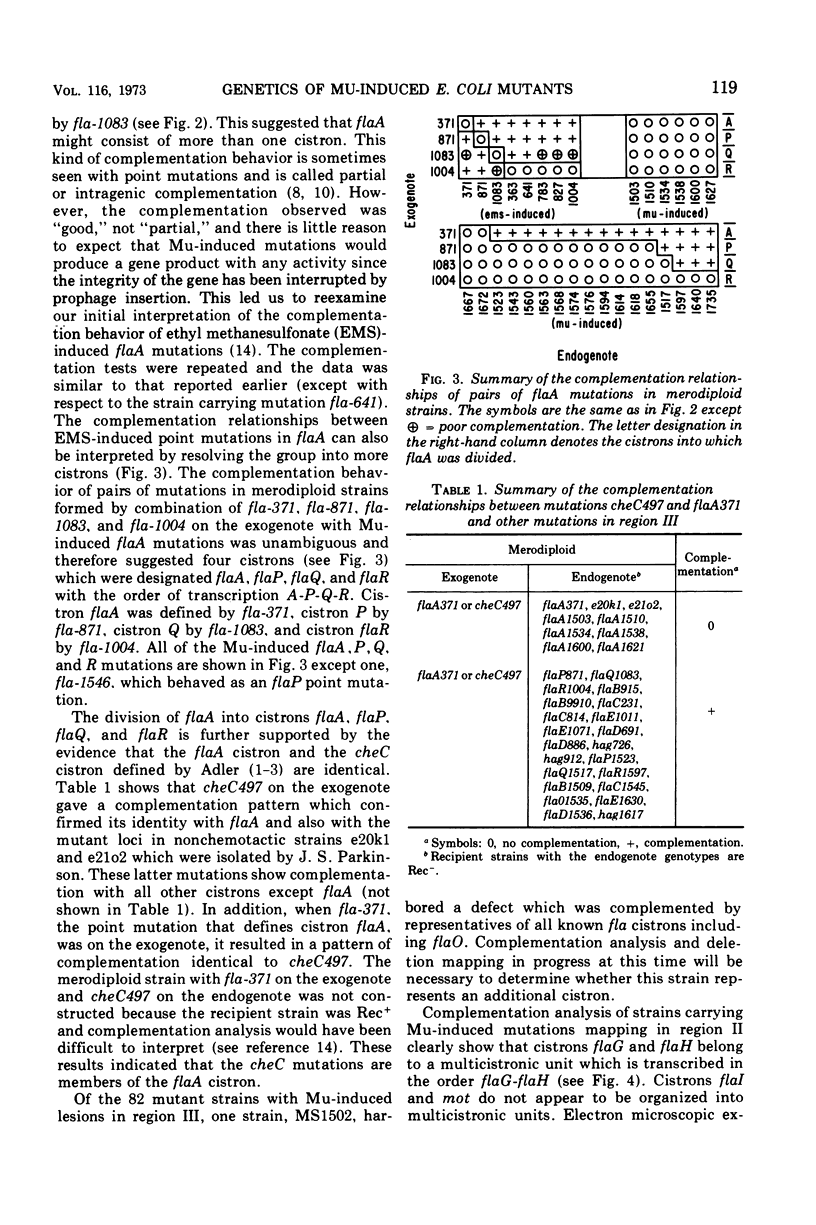
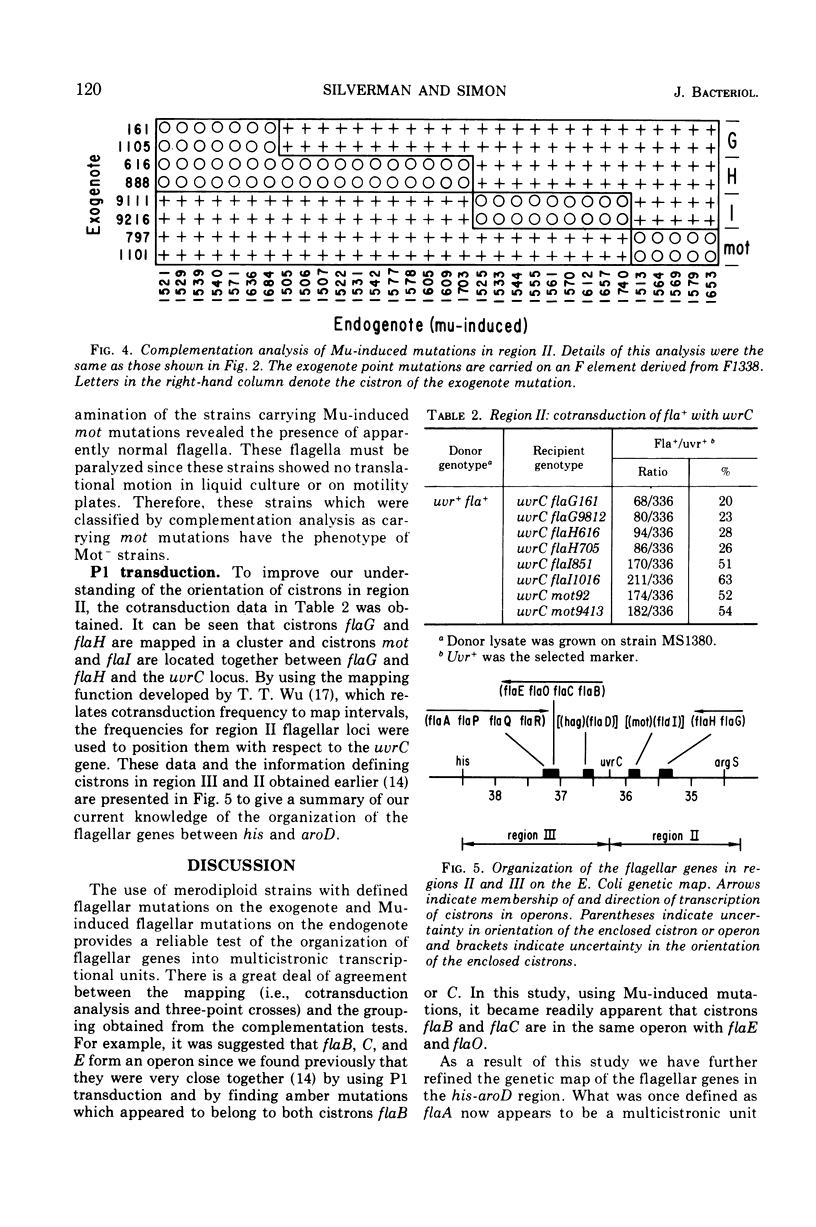
In previous work, at least 10 discrete cistrons involved in the synthesis of flagella in Escherichia coli were described. Six cistrons were located between his and uvrC on the genetic map. These were referred to as hag, flaA, flaB, flaC, flaD, and flaE. Four cistrons referred to as mot, flaG, flaH, and flaI were located between uvrC and aroD. In order to determine whether these genes are organized into transcriptional units, a series of Mu phage-induced flagellar mutants was studied. The mutant strains behaved as if they were carrying strong polar mutations. Of 228 independent Mu-induced mutants, 114 with mutations in the his-aroD region of the genetic map were tested by preparing partial diploid strains with episomes carrying a variety of previously defined mutations. The pattern of complementation that emerged indicated that cistrons flaB, flaC, and flaE form a transcriptional unit. Cistron flaO, defined in the course of this study, is also a member of this transcriptional unit. The order of transcription is B-C-O-E. flaA was found to be complex, and it included four cistrons, flaA, flaP, flaQ, and flaR, with the transcriptional order A-P-Q-R. Cistrons flaG and flaH are cotranscribed with the transcriptional order G-H. The remaining genes, flaD, flaI, hag, and mot do not belong to multicistronic transcriptional units. Complementation analysis suggested that the cheC locus is the same as cistron flaA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. B., Adler J. Complementation of nonchemotactic mutants of Escherichia coli. Genetics. 1969 Jan;61(1):61–66. doi: 10.1093/genetics/61.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J., Dahl M. M. Nonchemotactic mutants of Escherichia coli. J Bacteriol. 1967 Jan;93(1):390–398. doi: 10.1128/jb.93.1.390-398.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J. Location of genes for motility and chemotaxis on the Escherichia coli genetic map. J Bacteriol. 1969 Jan;97(1):156–161. doi: 10.1128/jb.97.1.156-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boram W., Abelson J. Bacteriophage Mu integration: on the mechanism of Mu-induced mutations. J Mol Biol. 1971 Nov 28;62(1):171–178. doi: 10.1016/0022-2836(71)90137-9. [DOI] [PubMed] [Google Scholar]
- Daniell E., Roberts R., Abelson J. Mutations in the lactose operon caused by bacteriophage Mu. J Mol Biol. 1972 Aug 14;69(1):1–8. doi: 10.1016/0022-2836(72)90019-8. [DOI] [PubMed] [Google Scholar]
- Franklin N. C. Mutation in gal U gene of E. coli blocks phage P1 infection. Virology. 1969 May;38(1):189–191. doi: 10.1016/0042-6822(69)90144-5. [DOI] [PubMed] [Google Scholar]
- Iino T., Enomoto M. Genetical studies of non-flagellate mutants of Salmonella. J Gen Microbiol. 1966 Jun;43(3):315–327. doi: 10.1099/00221287-43-3-315. [DOI] [PubMed] [Google Scholar]
- Jordan E., Saedler H., Starlinger P. O0 and strong-polar mutations in the gal operon are insertions. Mol Gen Genet. 1968;102(4):353–363. doi: 10.1007/BF00433726. [DOI] [PubMed] [Google Scholar]
- Joys T. M., Stocker B. A. Complementation of non-flagellate Salmonella mutants. J Gen Microbiol. 1965 Oct;41(1):47–55. doi: 10.1099/00221287-41-1-47. [DOI] [PubMed] [Google Scholar]
- MEYNELL E. W. A phage, phi chi, which attacks motile bacteria. J Gen Microbiol. 1961 Jun;25:253–290. doi: 10.1099/00221287-25-2-253. [DOI] [PubMed] [Google Scholar]
- Nomura M., Engbaek F. Expression of ribosomal protein genes as analyzed by bacteriophage Mu-induced mutations. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1526–1530. doi: 10.1073/pnas.69.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M. R., Simon M. I. Flagellar assembly mutants in Escherichia coli. J Bacteriol. 1972 Nov;112(2):986–993. doi: 10.1128/jb.112.2.986-993.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Genetic analysis of flagellar mutants in Escherichia coli. J Bacteriol. 1973 Jan;113(1):105–113. doi: 10.1128/jb.113.1.105-113.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR A. L. BACTERIOPHAGE-INDUCED MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1043–1051. doi: 10.1073/pnas.50.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint A. Insertion of phage Mu. 1 within prophage lambda. A new approach for studying the control of the late functions in bacteriophage lambda. Mol Gen Genet. 1969;106(1):89–92. doi: 10.1007/BF00332824. [DOI] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Iino T., Horiguchi T., Ota K. Genetic analysis of fla and mot cistrons closely linked to H1 in Salmonella abortusequi and its derivatives. J Gen Microbiol. 1972 Apr;70(1):59–75. doi: 10.1099/00221287-70-1-59. [DOI] [PubMed] [Google Scholar]



