Abstract
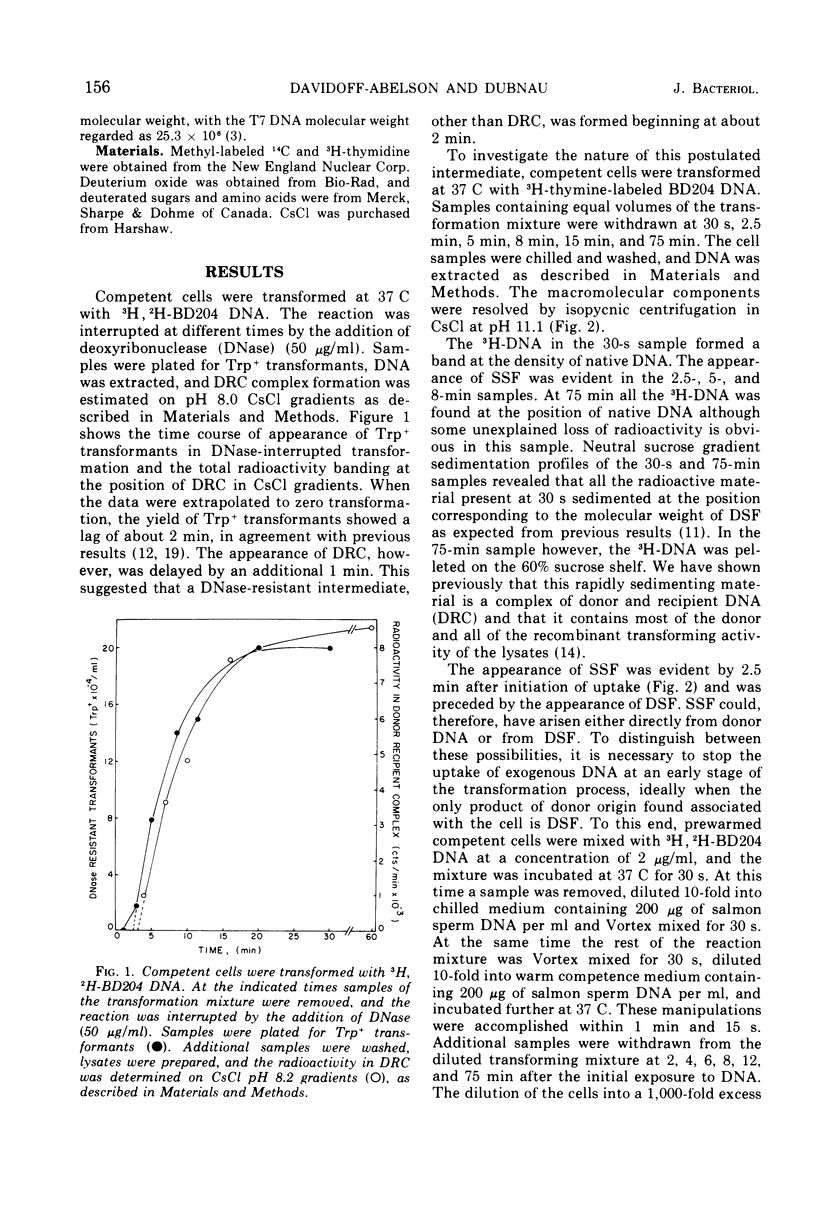
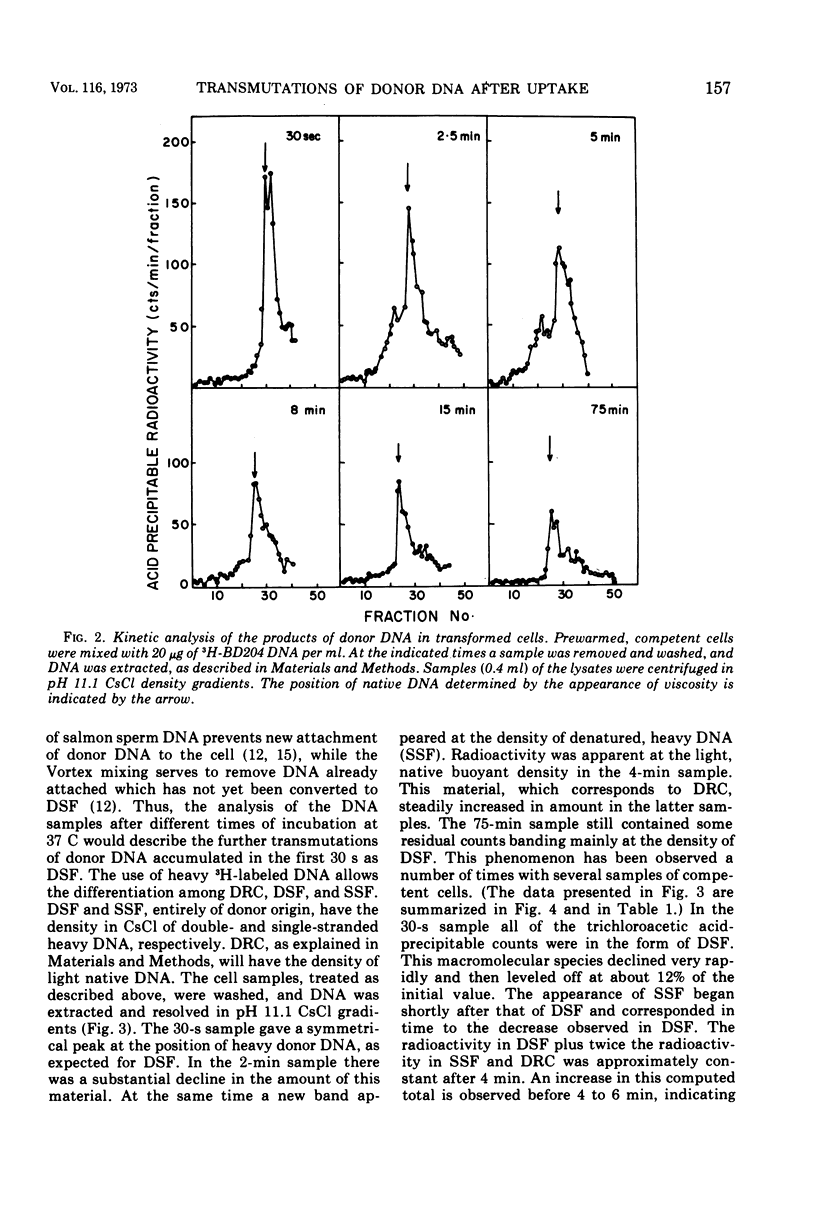
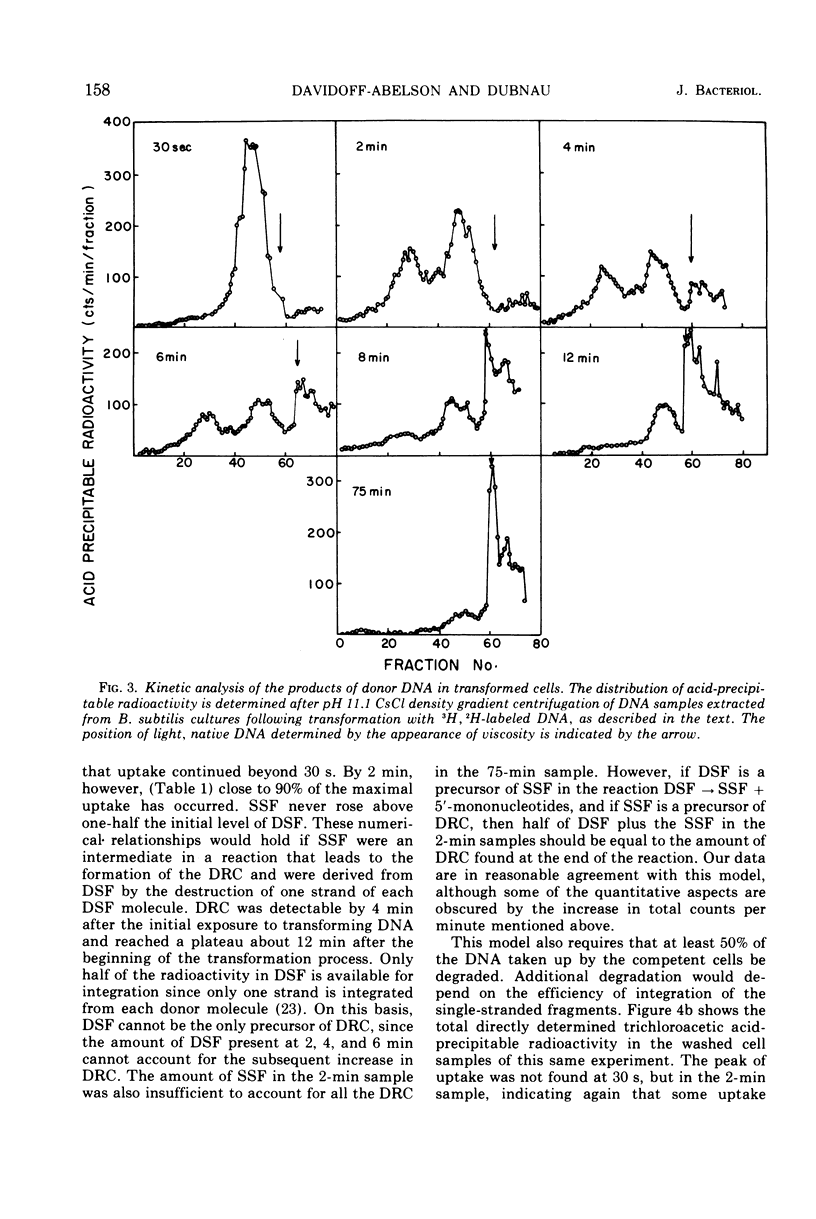
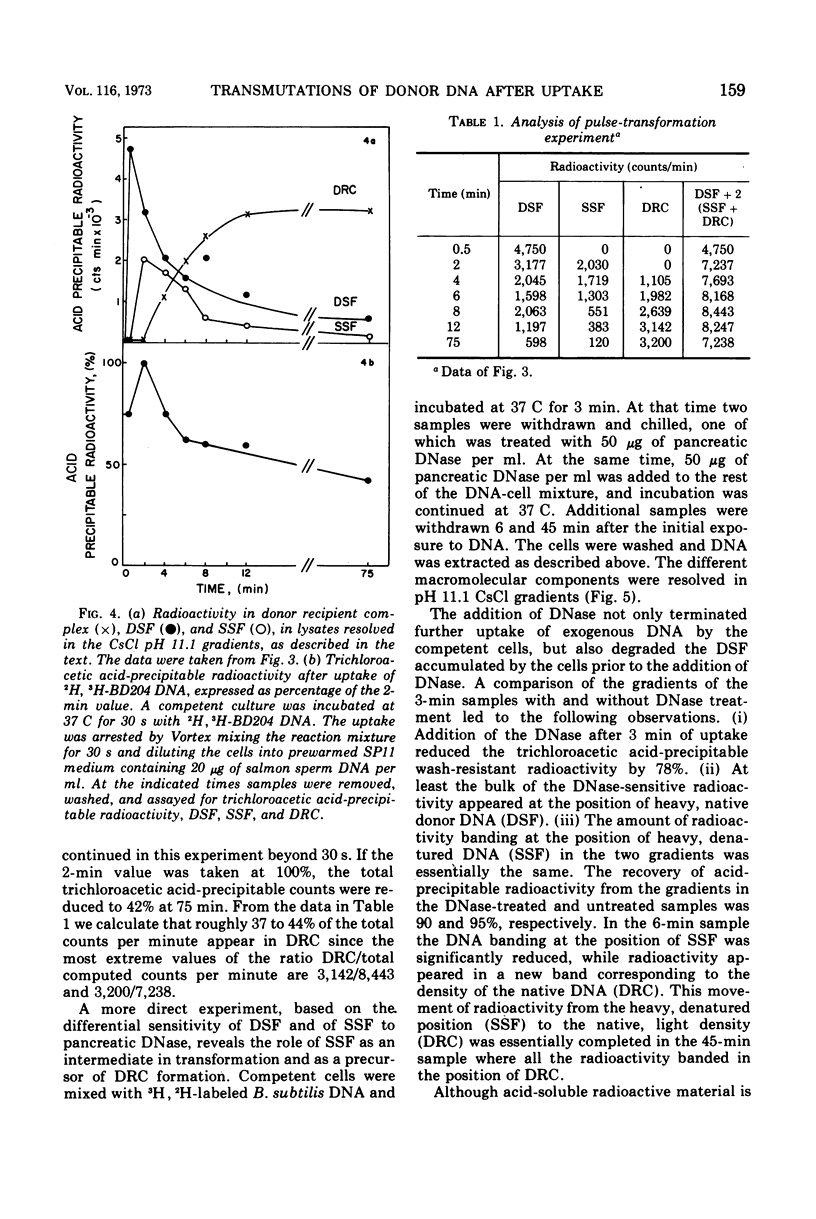
This paper describes the major transmutations of donor deoxyribonucleic acid (DNA) after uptake by competent Bacillus subtilis cells. Kinetic experiments confirm that after exposure to competent cells, donor DNA is converted to double-stranded fragments (DSF) which can be isolated as early as 30 s from the beginning of the reaction. At this time, DSF represent the only identifiable product of donor origin. After 1 to 2 min, DSF are converted to deoxyribonuclease-resistant forms, identified as single-stranded DNA fragments (SSF). SSF are intermediates in the transformation process leading to the formation of donor-recipient complex. This component makes its appearance between 2 to 4 min from the beginning of the transformation process. All the donor-recipient complexes found at the end of the reaction can be accounted for quantitatively by the DSF and the SSF found in the initial stages of transformation. A quantitative discussion of the transformation process is included.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayad S. R., Barker G. R. The integration of donor and recipient deoxyribonucleic acid during transformation of Bacillus subtilis. Biochem J. 1969 Jun;113(1):167–174. doi: 10.1042/bj1130167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BODMER W. F., GANESAN A. T. BIOCHEMICAL AND GENETIC STUDIES OF INTEGRATION AND RECOMBINATION IN BACILLUS SUBTILIS TRANSFORMATION. Genetics. 1964 Oct;50:717–738. doi: 10.1093/genetics/50.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BODMER W., SCHILDKRAUT C. PREPARATION AND CHARACTERIZATION OF 15N2H3H-LABELED DNA FROM BACILLUS SUBTILIS AND ESCHERICHIA COLI PHAGE T2. Anal Biochem. 1964 Jun;8:229–243. doi: 10.1016/0003-2697(64)90051-x. [DOI] [PubMed] [Google Scholar]
- BRESLER S. E., KRENEVA R. A., KUSHEV V. V., MOSEVITSKII M. I. MOLECULAR MECHANISM OF GENETIC RECOMBINATION IN BACTERIAL TRANSFORMATION. Z Vererbungsl. 1964 Nov 11;95:288–297. doi: 10.1007/BF00897013. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft F. C., Freifelder D. Molecular weights of coliphages and coliphage DNA. I. Measurement of the molecular weight of bacteriophage T7 by high-speed equilibrium centrifugation. J Mol Biol. 1970 Dec 28;54(3):537–546. doi: 10.1016/0022-2836(70)90124-5. [DOI] [PubMed] [Google Scholar]
- Bodmer W. F. Integration of deoxyribonuclease-treated DNA in bacillus subtilis transformation. J Gen Physiol. 1966 Jul;49(6):233–258. doi: 10.1085/jgp.49.6.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton M. D., Hall B. D. Transforming activity in single-stranded DNA from Bacillus subtilis. J Mol Biol. 1968 Jun 28;34(3):439–451. doi: 10.1016/0022-2836(68)90171-x. [DOI] [PubMed] [Google Scholar]
- Davidoff-Abelson R., Dubnau D. Conditions affecting the isolation from transformed cells of Bacillus subtilis of high-molecular-weight single-stranded deoxyribonucleic acid of donor origin. J Bacteriol. 1973 Oct;116(1):146–153. doi: 10.1128/jb.116.1.146-153.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming DNA following uptake by competent Bacillus subtilis. Formation and properties of products isolated from transformed cells which are derived entirely from donor DNA. J Mol Biol. 1972 Feb 28;64(1):9–29. doi: 10.1016/0022-2836(72)90318-x. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming DNA following uptake by competent Bacillus subtilis. IV. The endwise attachment and uptake of transforming DNA. J Mol Biol. 1972 Feb 28;64(1):31–46. doi: 10.1016/0022-2836(72)90319-1. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming deoxyribonucleic acid after uptake by competent Bacillus subtilis: size and distribution of the integrated donor segments. J Bacteriol. 1972 Aug;111(2):488–494. doi: 10.1128/jb.111.2.488-494.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971 Mar 14;56(2):209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Gabor M., Hotchkiss R. D. Manifestation of linear organization in molecules of pneumococcal transforming DNA. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1441–1448. doi: 10.1073/pnas.56.5.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson F., Hales H. B. Mechanism of the sensitization of bacterial transforming DNA to ultraviolet light by the incorporation of 5-bromouracil. J Mol Biol. 1970 May 28;50(1):59–69. doi: 10.1016/0022-2836(70)90103-8. [DOI] [PubMed] [Google Scholar]
- Kelly M. S. The causes of instability of linkage in transformation of Bacillus subtilis. Mol Gen Genet. 1967;99(4):350–361. doi: 10.1007/BF00330910. [DOI] [PubMed] [Google Scholar]
- LEVINE J. S., STRAUSS N. LAG PERIOD CHARACTERIZING THE ENTRY OF TRANSFORMING DEOXYRIBONUCLEIC ACID INTO BACILLUS SUBTILIS. J Bacteriol. 1965 Feb;89:281–287. doi: 10.1128/jb.89.2.281-287.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Early intermediate state of transforming deoxyribonucleic acid during uptake by Bacillus subtilis. J Bacteriol. 1971 Oct;108(1):38–44. doi: 10.1128/jb.108.1.38-44.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Transformation and deoxyribonucleic acid size: extent of degradation on entry varies with size of donor. J Bacteriol. 1972 Dec;112(3):1157–1168. doi: 10.1128/jb.112.3.1157-1168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechowska M., Fox M. S. Fate of transforming deoxyribonucleate in Bacillus subtilis. J Bacteriol. 1971 Nov;108(2):680–689. doi: 10.1128/jb.108.2.680-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss N. Transformation of Bacillus subtilis using hybrid DNA molecules constructed by annealing resolved complementary strands. Genetics. 1970 Dec;66(4):583–593. doi: 10.1093/genetics/66.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevethia M. J., Mandel M. Nature of the ethylenediaminetetraacetic acid requirement for transformation of Bacillus subtilis with single-stranded deoxyribonucleic acid. J Bacteriol. 1970 Mar;101(3):844–850. doi: 10.1128/jb.101.3.844-850.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENEMA G., PRITCHARD R. H., VENEMA-SCHROEDER T. PROPERTIES OF NEWLY INTRODUCED TRANSFORMING DEOXYRIBONUCLEIC ACID IN BACILLUS SUBTILIS. J Bacteriol. 1965 Aug;90:343–346. doi: 10.1128/jb.90.2.343-346.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]


