Abstract
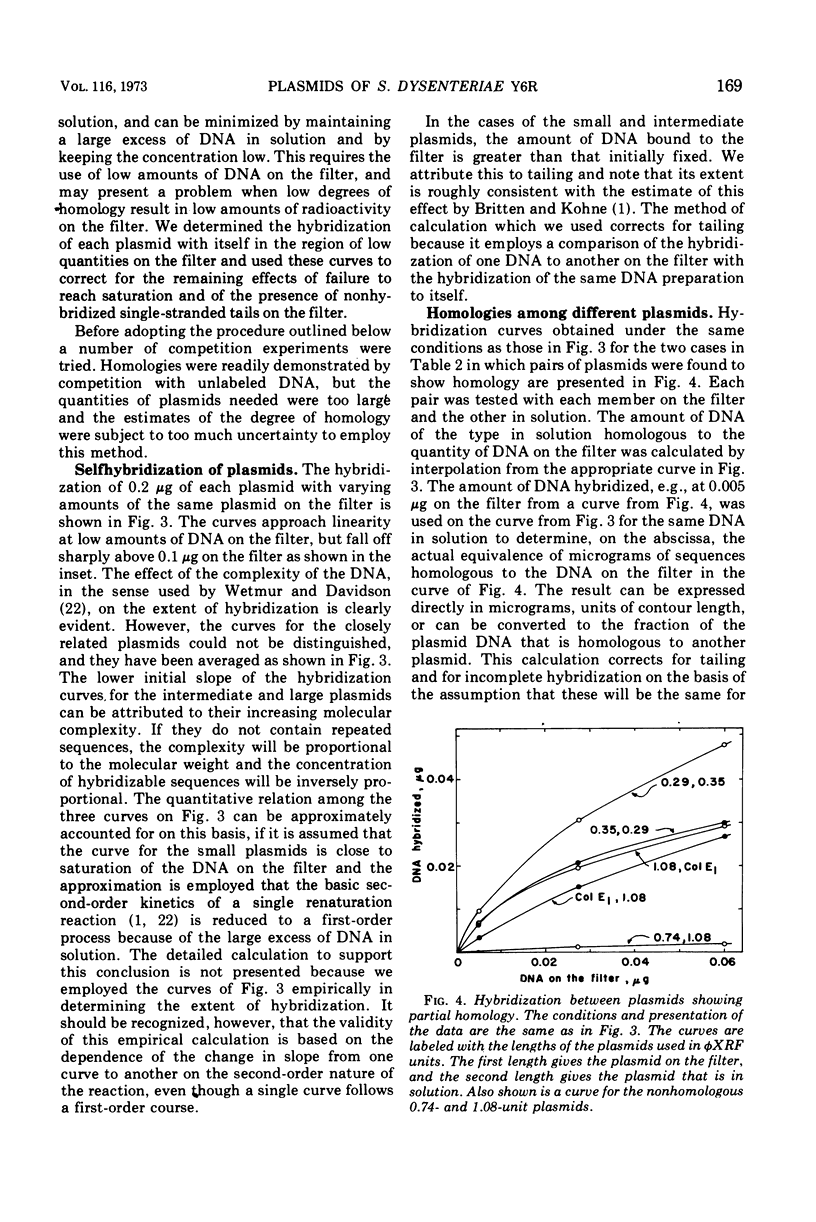
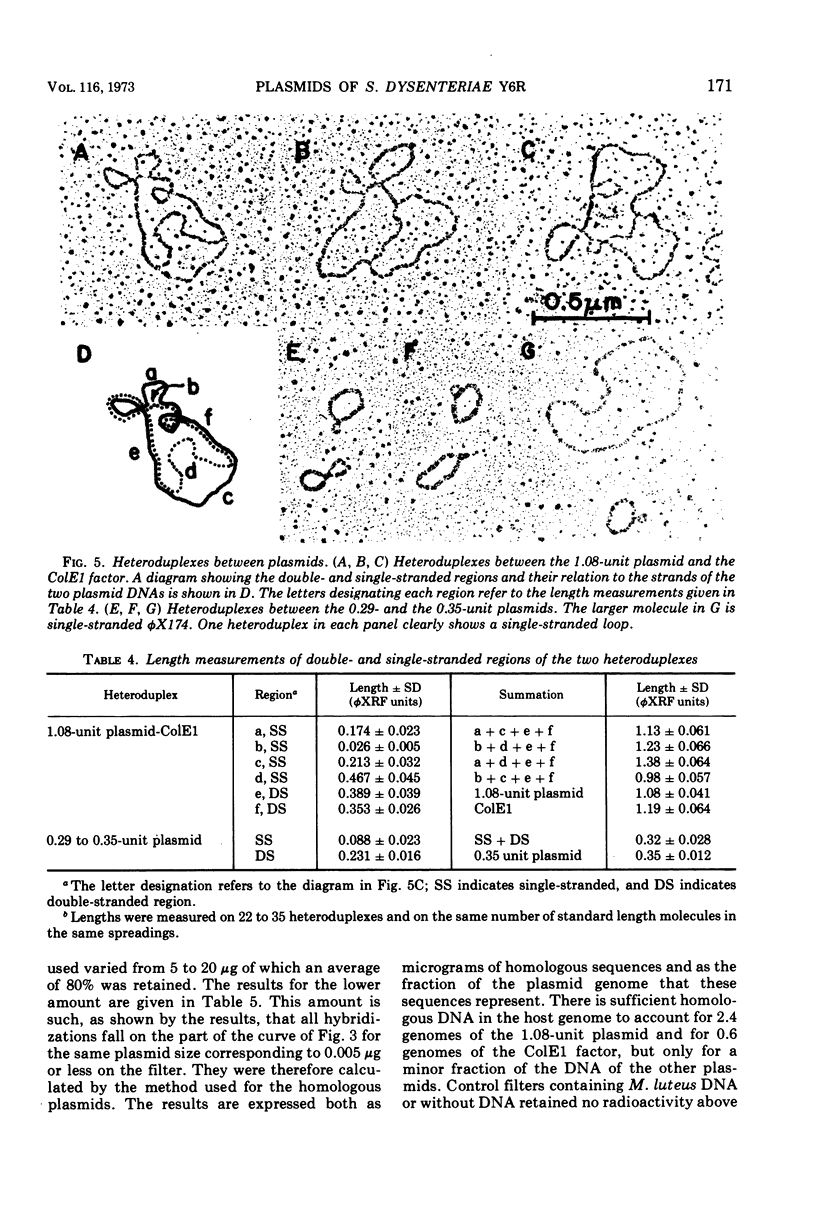
The six plasmids of Shigella dysenteriae Y6R were separated by sucrose gradients into five fractions containing deoxyribonucleic acid (DNA), having contour lengths (expressed in units equal to the fraction of the length of the replicative form of φX174), respectively, of 0.29, 0.35, 0.74, 1.08, and a mixture of 5.7 and 7.2. DNA-DNA hybridization on nitrocellulose filters between each of the plasmids and between plasmid-free S. dysenteriae Y6R host DNA and plasmids was investigated. There was a high degree of homology between the 0.29- and 0.35-unit plasmids. No significant homology was found between any of the other pairs of plasmids. Homologous DNA to the extent of 2.4 copies of the 1.08-unit plasmid was found in the host genome. Homology between the other plasmids and the host genome is very slight, but appears to be significant. About 0.7 of the 1.08-unit plasmid is homologous to the ColE1 façtor of Escherichia coli JC411 (ColE1). This plasmid may be defective ColE1 factor with the immunity function intact, but with a defect in the gene leading to the production of active colicin. Electron microscope examination of heteroduplexes formed between the two smallest plasmids and between the 1.08-unit plasmid and the ColE1 factor yielded independent determinations of the extent of homology in agreement with the values determined by hybridization. In the latter case, two nonhomologous regions of substitution of DNA were detected.
Full text
PDF

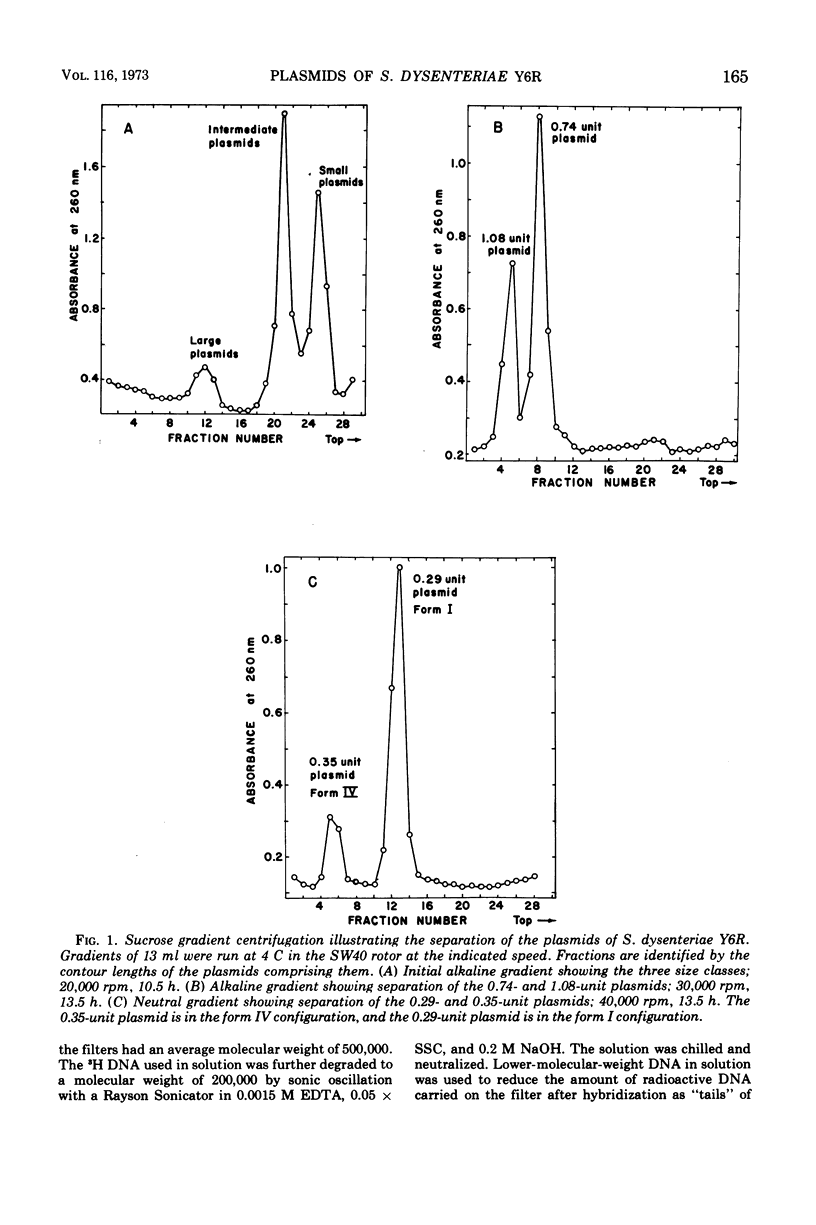
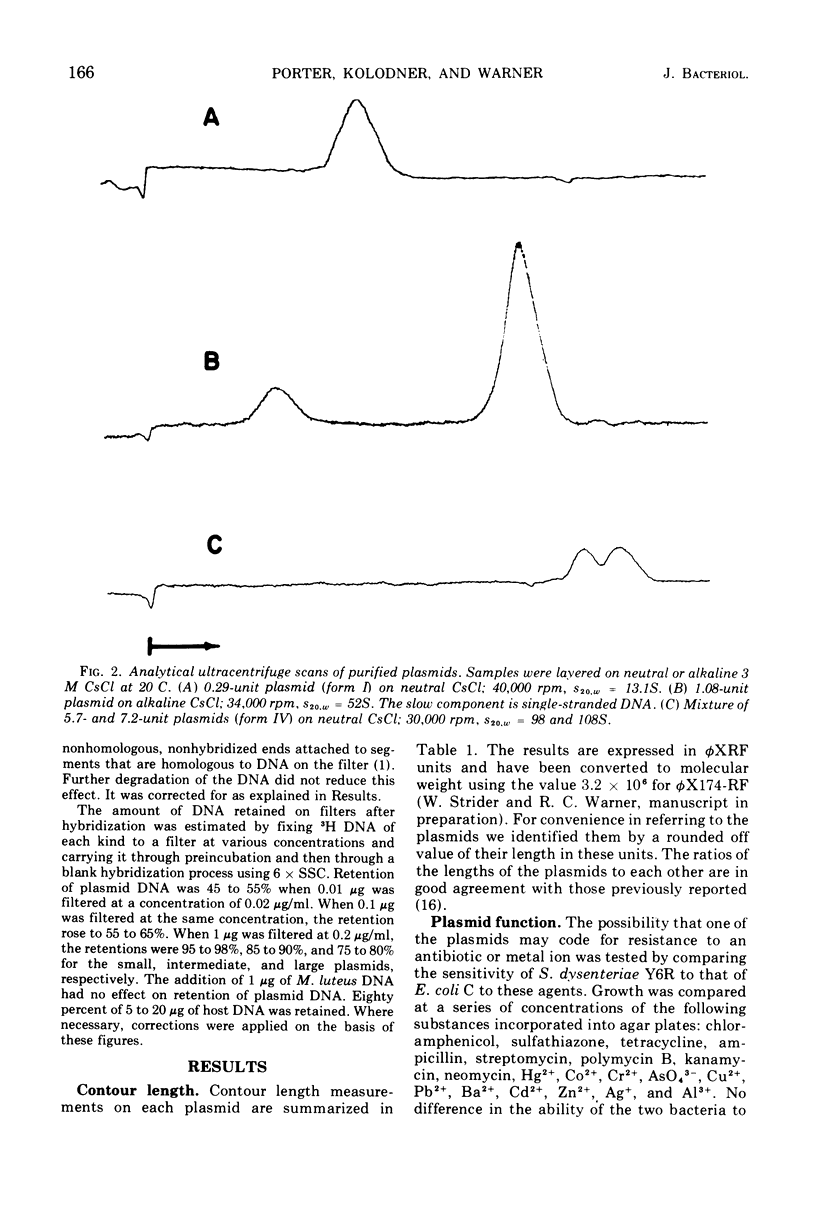

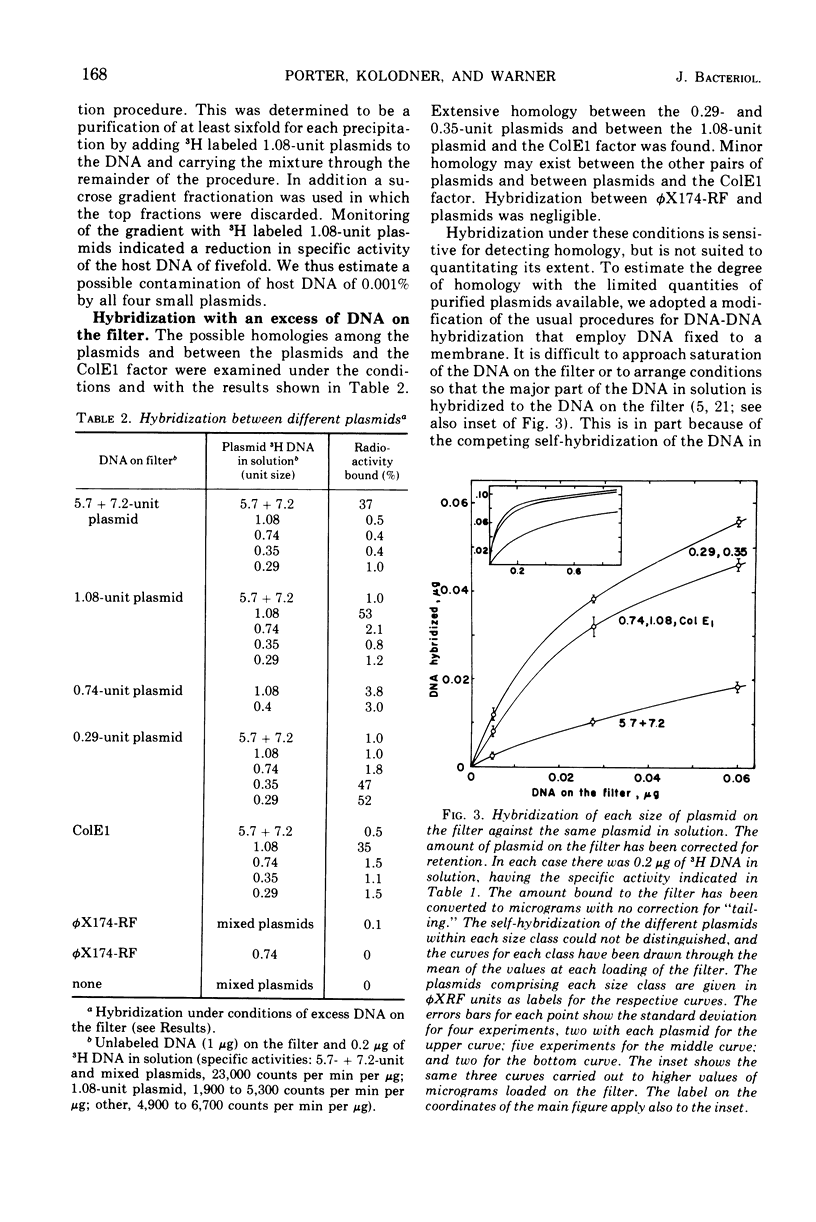




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Domingo E., Gordon C. N., Warner R. C. Azotobacter phages: properties of phages A12, A21, A31, A41 and their constituent DNAs. Virology. 1972 Aug;49(2):439–452. doi: 10.1016/0042-6822(72)90496-5. [DOI] [PubMed] [Google Scholar]
- FREDERICQ P. Colicins. Annu Rev Microbiol. 1957;11:7–22. doi: 10.1146/annurev.mi.11.100157.000255. [DOI] [PubMed] [Google Scholar]
- Goebel W., Schrempf H. Isolation of minicircular deoxyribonucleic acids from wild strains of Escherichia coli and their relationship to other bacterial plasmids. J Bacteriol. 1972 Sep;111(3):696–704. doi: 10.1128/jb.111.3.696-704.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habich A., Weissmann C., Libonati M., Warner R. C. Isolation of a fraction of Bacillus megaterium DNA enriched in "minus" sequences. J Mol Biol. 1966 Nov 14;21(2):255–264. doi: 10.1016/0022-2836(66)90096-9. [DOI] [PubMed] [Google Scholar]
- Hamon Y., Péron Y. Nouvelle classification des colicines appartenant au groupe E. Zentralbl Bakteriol Orig. 1966 Jul;200(3):375–379. [PubMed] [Google Scholar]
- LINN S., LEHMAN I. R. AN ENDONUCLEASE FROM NEUROSPORA CRASSA SPECIFIC FOR POLYNUCLEOTIDES LACKING AN ORDERED STRUCTURE. I. PURIFICATION AND PROPERTIES OF THE ENZYME. J Biol Chem. 1965 Mar;240:1287–1293. [PubMed] [Google Scholar]
- Lee C. S., Davidson N. Physicochemical studies on the minicircular DNA in Escherichia coli 15. Biochim Biophys Acta. 1970 Apr 15;204(2):285–295. doi: 10.1016/0005-2787(70)90146-2. [DOI] [PubMed] [Google Scholar]
- Puga A., Shleser R., Kohne D. E. Homology between the genomes of bacteriophage S13 and Escherichia coli. Virology. 1971 Feb;43(2):507–510. doi: 10.1016/0042-6822(71)90323-0. [DOI] [PubMed] [Google Scholar]
- Rabin E. Z., Preiss B., Fraser M. J. A nuclease from Neurospora crassa conidia specific for single-stranded nucleic acids. Prep Biochem. 1971;1(4):283–307. doi: 10.1080/00327487108081946. [DOI] [PubMed] [Google Scholar]
- Rush M. G., Gordon C. N., Warner R. C. Circular deoxyribonucleic acid from Shigella dysenteriae Y6R. J Bacteriol. 1969 Nov;100(2):803–808. doi: 10.1128/jb.100.2.803-808.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush M. G., Warner R. C. Alkali denaturation of covalently closed circular duplex deoxyribonucleic acid. J Biol Chem. 1970 May 25;245(10):2704–2708. [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmaar S. O., Cohen J. A. A quantitative assay for DNA-DNA hybrids using membrane filters. Biochem Biophys Res Commun. 1966 Aug 23;24(4):554–558. doi: 10.1016/0006-291x(66)90356-1. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]