Abstract
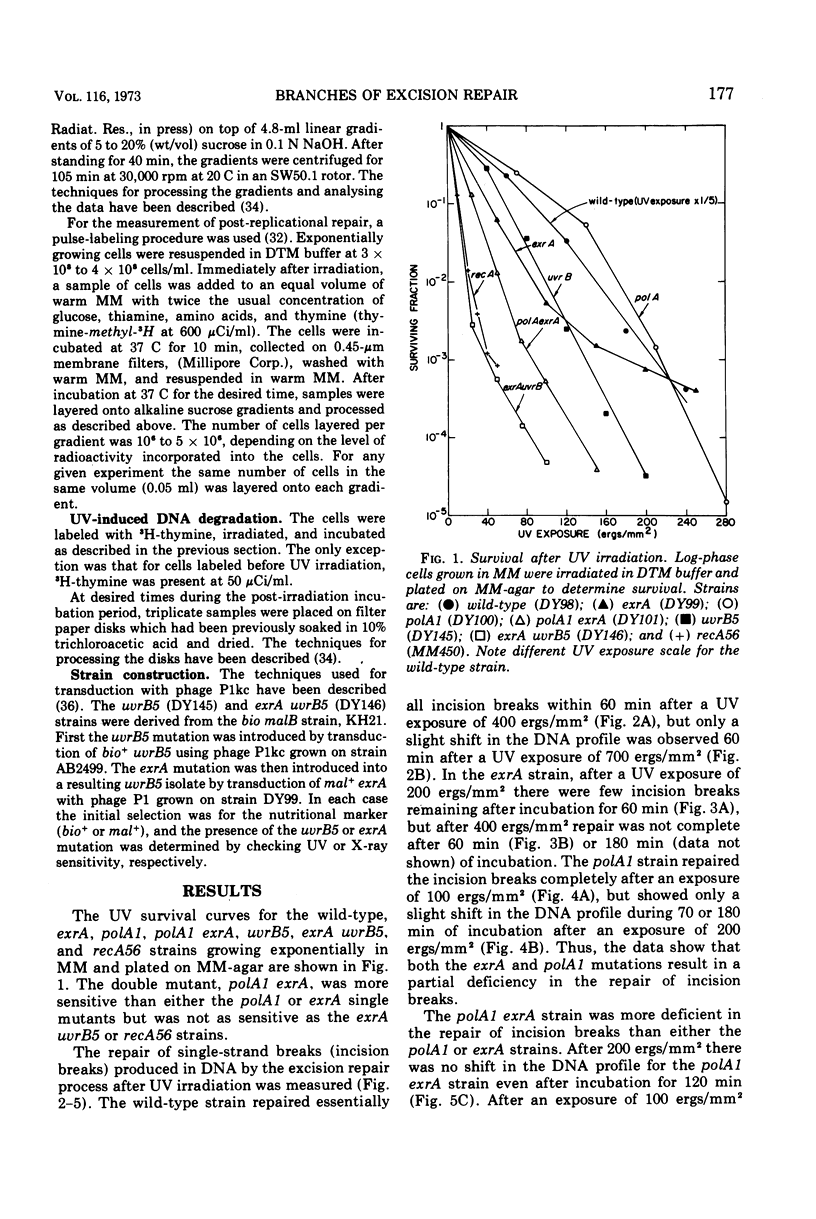
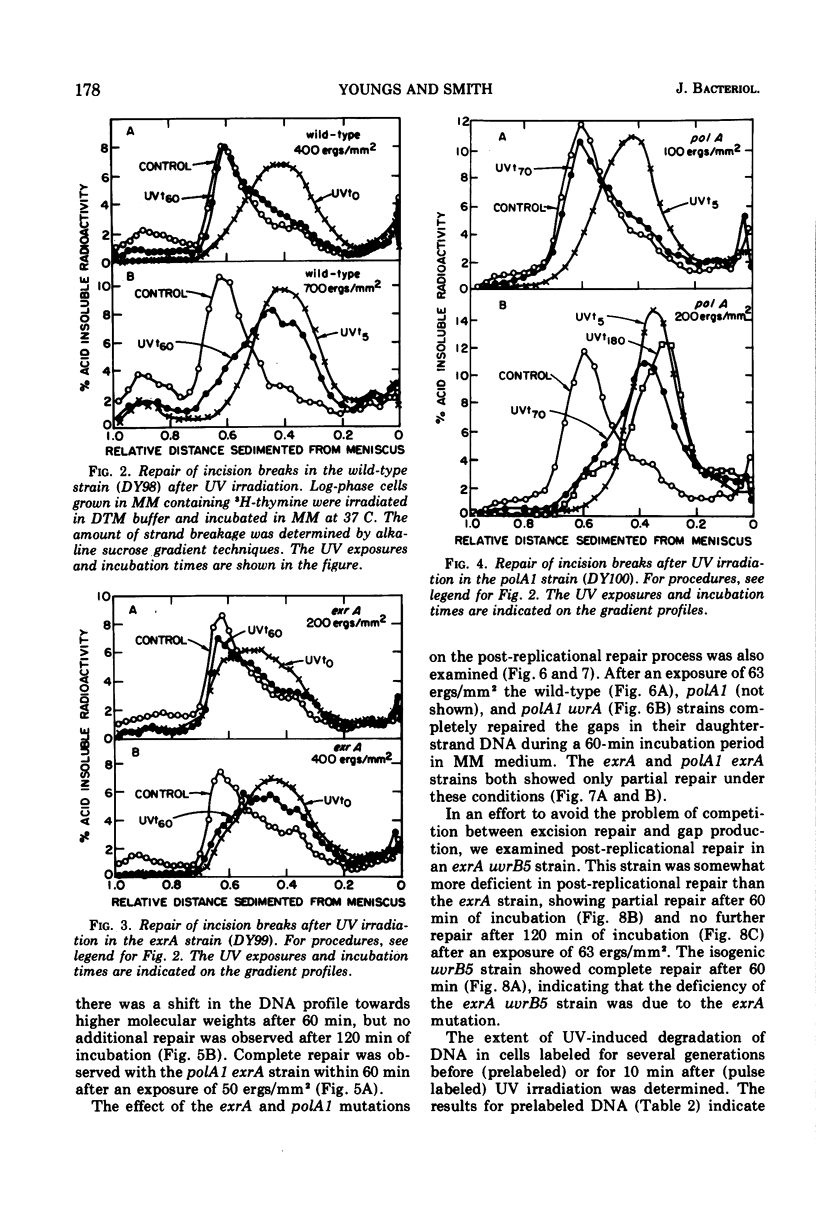
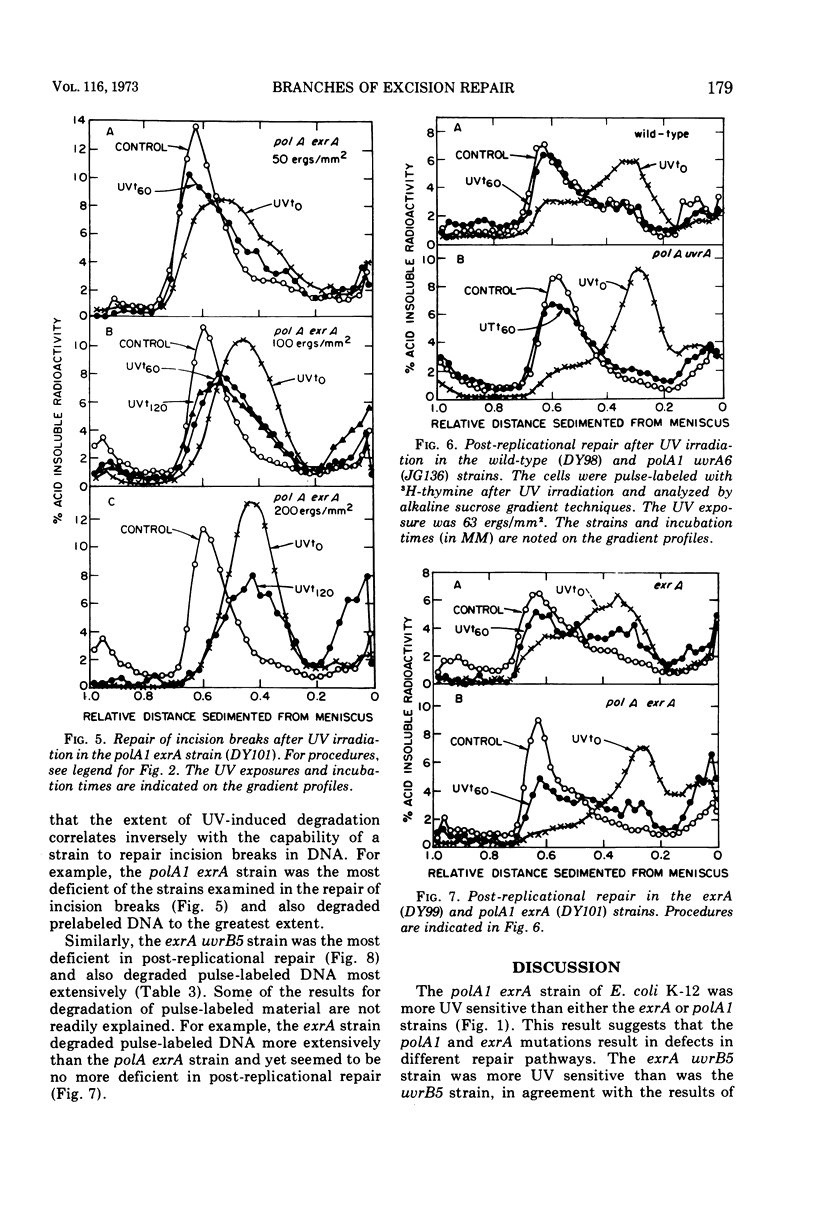
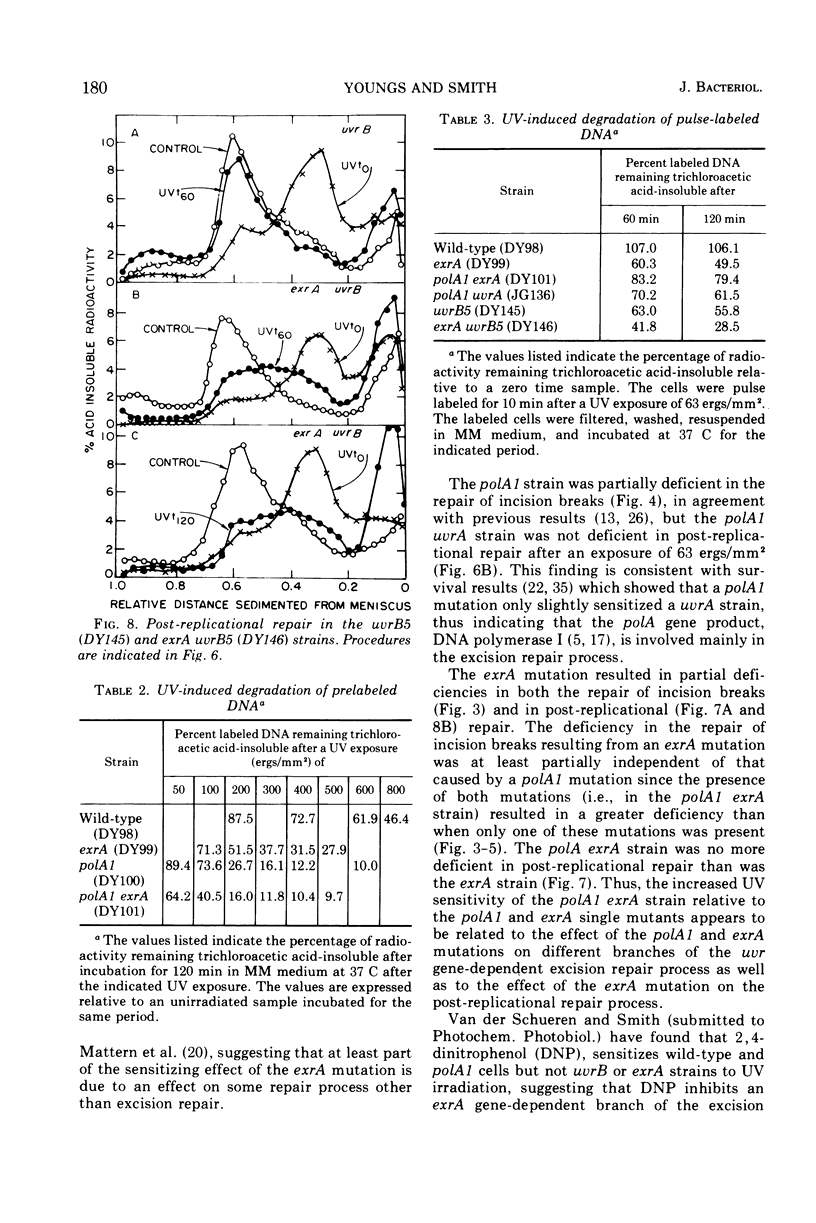
A polA1 exrA strain of Escherichia coli K-12 was found to be more sensitive to ultraviolet radiation than the closely related polA1 or exrA strains, but not as sensitive as either the exrA uvrB or recA strains. The exrA and polA1 mutations both resulted in a deficiency in the repair of single-strand breaks arising in the deoxyribonucleic acid as a result of the excision repair process (incision breaks). These deficiencies were at least partially independent since the double mutant, polA1 exrA, was more deficient than a strain containing either the polA1 or exrA mutation alone. These results suggest that the polA1 and exrA mutations result in defects in two different branches of the uvr gene-dependent excision repair process. The repair of incision breaks was still observed in the polA1 exrA strain after low exposures of ultraviolet radiation, suggesting the existence of a third branch of the excision repair process which is dependent on neither the polA nor exrA genes. The polA1 and polA1 uvrA strains were not deficient in post-replicational repair. The exrA strain was partially deficient in post-replicational repair, but the polA1 exrA strain was no more deficient than the exrA strain in this repair process. Thus, the increased ultraviolet irradiation sensitivity of the polA1 exrA strain relative to the polA1 and exrA strains appears to be related to the effect of the polA1 and exrA mutations on different branches of the uvr gene-dependent excision repair process as well as to the effect of the exrA mutation on the post-replicational repair process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achey P., Billen D. Saturation of dark repair synthesis: accumulation of strand breaks. Biophys J. 1969 May;9(5):647–653. doi: 10.1016/S0006-3495(69)86409-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. M., Paterson M. C., Setlow R. B. Excision-repair properties of an Escherichia coli mutant deficient in DNA polymerase. Nature. 1970 May 23;226(5247):708–710. doi: 10.1038/226708a0. [DOI] [PubMed] [Google Scholar]
- Cooper P. K., Hanawalt P. C. Heterogeneity of patch size in repair replicated DNA in Escherichia coli. J Mol Biol. 1972 Jun 14;67(1):1–10. doi: 10.1016/0022-2836(72)90381-6. [DOI] [PubMed] [Google Scholar]
- Cooper P. K., Hanawalt P. C. Role of DNA polymerase I and the rec system in excision-repair in Escherichia coli. Proc Natl Acad Sci U S A. 1972 May;69(5):1156–1160. doi: 10.1073/pnas.69.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Donch J., Greenberg J., Green M. H. Repression of induction by U.V. of lambda phage by exrA mutations in Escherichia coli. Genet Res. 1970 Feb;15(1):87–97. doi: 10.1017/s0016672300001397. [DOI] [PubMed] [Google Scholar]
- Ganesan A. K., Smith K. C. Dark recovery processes in Escherichia coli irradiated with ultraviolet light. I. Effect of rec mutations on liquid holding recovery. J Bacteriol. 1968 Aug;96(2):365–373. doi: 10.1128/jb.96.2.365-373.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. D., Grunstein J., Witkin E. M. Inviability of recA- derivatives of the DNA polymerase mutant of De Lucia and Cairns. J Mol Biol. 1971 Jun 14;58(2):631–634. doi: 10.1016/0022-2836(71)90377-9. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P. Genes that control DNA repair and genetic recombination in Escherichia coli. Adv Biol Med Phys. 1968;12:299–317. doi: 10.1016/b978-1-4831-9928-3.50016-3. [DOI] [PubMed] [Google Scholar]
- Kanner L., Hanawalt P. Repair deficiency in a bacterial mutant defective in DNA polymerase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):149–155. doi: 10.1016/0006-291x(70)90770-9. [DOI] [PubMed] [Google Scholar]
- Kapp D. S., Smith K. C. Repair of radiation-induced damage in Escherichia coli. II. Effect of rec and uvr mutations on radiosensitivity, and repair of x-ray-induced single-strand breaks in deoxyribonucleic acid. J Bacteriol. 1970 Jul;103(1):49–54. doi: 10.1128/jb.103.1.49-54.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T. Excision repair characteristics of recB - res - and uvrC - strains of Escherichia coli. J Bacteriol. 1972 Dec;112(3):1237–1246. doi: 10.1128/jb.112.3.1237-1246.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley W. S., Whitfield H. J. Purification of an altered DNA polymerase from an E. coli strain with a pol mutation. Nature. 1971 Mar 5;230(5288):33–36. doi: 10.1038/230033a0. [DOI] [PubMed] [Google Scholar]
- Mattern I. E., Zwenk H., Rörsch A. The genetic constitution of the radiation-sensitive mutant Escherichia coli Bs-1. Mutat Res. 1966 Oct;3(5):374–380. doi: 10.1016/0027-5107(66)90047-9. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Monk M., Kinross J. Conditional lethality of recA and recB derivatives of a strain of Escherichia coli K-12 with a temperature-sensitive deoxyribonucleic acid polymerase I. J Bacteriol. 1972 Mar;109(3):971–978. doi: 10.1128/jb.109.3.971-978.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk M., Peacey M., Gross J. D. Repair of damage induced by ultraviolet light in DNA polymerase-defective Escherichia coli cells. J Mol Biol. 1971 Jun 14;58(2):623–630. doi: 10.1016/0022-2836(71)90376-7. [DOI] [PubMed] [Google Scholar]
- Mount D. W., Kosel C. Properties of strains of Escherichia coli K12 carrying mutant lex-1 and uvrA6 alleles. Mol Gen Genet. 1973;120(4):291–299. doi: 10.1007/BF00268143. [DOI] [PubMed] [Google Scholar]
- Mount D. W., Low K. B., Edmiston S. J. Dominant mutations (lex) in Escherichia coli K-12 which affect radiation sensitivity and frequency of ultraviolet lght-induced mutations. J Bacteriol. 1972 Nov;112(2):886–893. doi: 10.1128/jb.112.2.886-893.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson M. C., Boyle J. M., Setlow R. B. Ultraviolet- and X-ray-induced responses of a deoxyribonucleic acid polymerase-deficient mutant of Escherichia coli. J Bacteriol. 1971 Jul;107(1):61–67. doi: 10.1128/jb.107.1.61-67.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick S. G., Bridges B. A. Survival, mutation and capacity to repair single-strand DNA breaks after gamma irradiation in different Exr - strains of Escherichia coli. Mol Gen Genet. 1972;119(2):93–102. doi: 10.1007/BF00269129. [DOI] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Deoxyribonucleic acid polymerase: two distinct enzymes in one polypeptide. II. A proteolytic fragment containing the 5' leads to 3' exonuclease function. Restoration of intact enzyme functions from the two proteolytic fragments. J Biol Chem. 1972 Jan 10;247(1):232–240. [PubMed] [Google Scholar]
- Setlow R. B., Setlow J. K. Effects of radiation on polynucleotides. Annu Rev Biophys Bioeng. 1972;1:293–346. doi: 10.1146/annurev.bb.01.060172.001453. [DOI] [PubMed] [Google Scholar]
- Smith K. C., Meun D. H. Repair of radiation-induced damage in Escherichia coli. I. Effect of rec mutations on post-replication repair of damage due to ultraviolet radiation. J Mol Biol. 1970 Aug;51(3):459–472. doi: 10.1016/0022-2836(70)90001-x. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. Production and repair of radiochemical damage in Escherichia coli deoxyribonucleic acid; its modification by culture conditions and relation to survival. J Bacteriol. 1971 Jan;105(1):127–135. doi: 10.1128/jb.105.1.127-135.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. X-ray sensitivity and repair capacity of a polA1 exrA strain of Escherichia coli K-12. J Bacteriol. 1973 Apr;114(1):121–127. doi: 10.1128/jb.114.1.121-127.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


