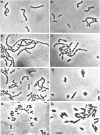
Abstract
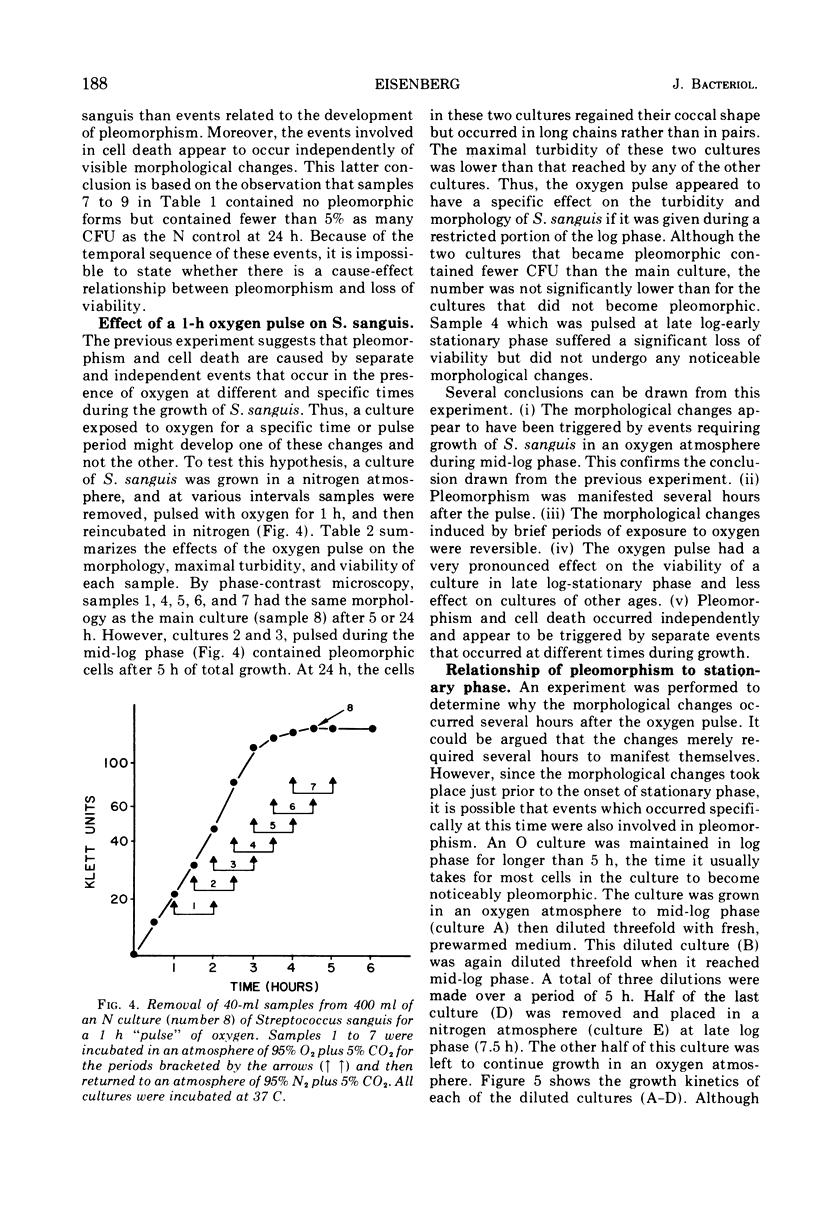
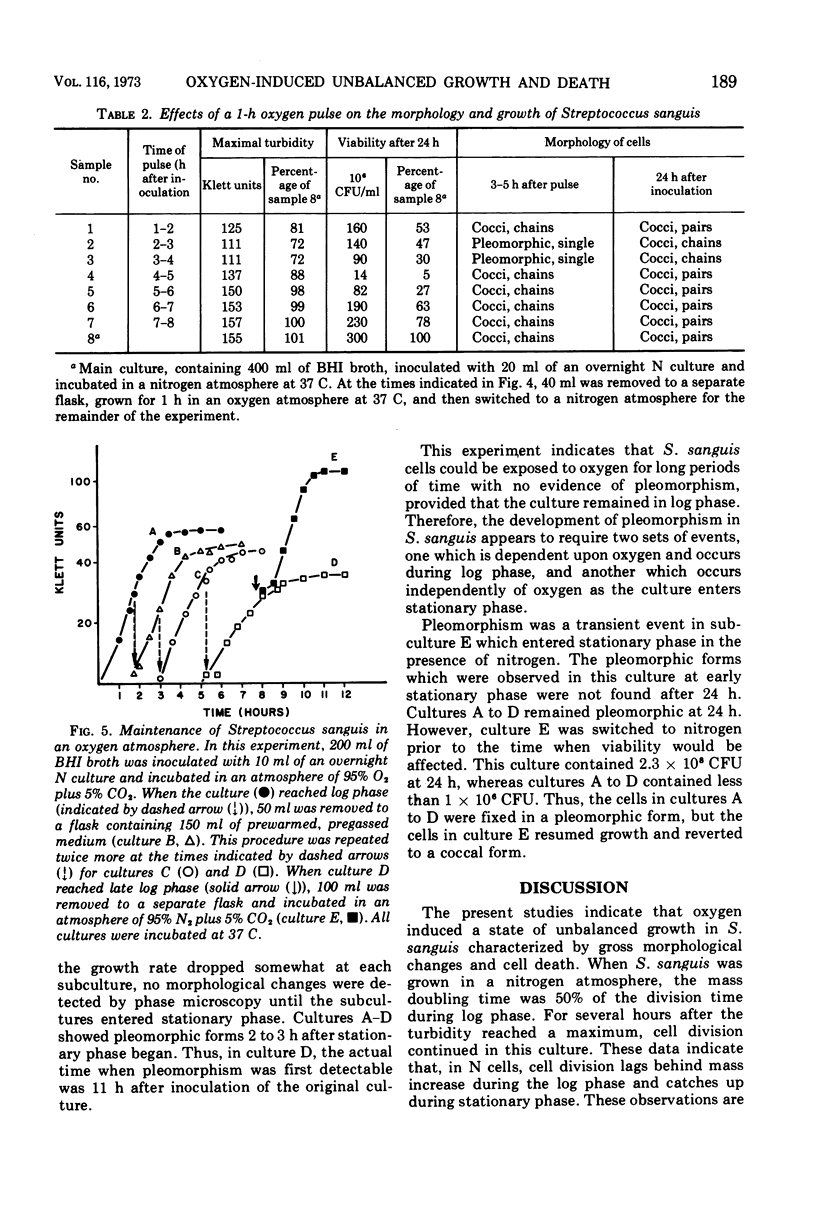
Streptococcus sanguis has normal streptococcal morphology when grown in a nitrogen atmosphere but undergoes gross morphological alterations when grown in the presence of oxygen (O cells). Studies were made of the relationship between the development of pleomorphism and the loss of viability which accompanied it in O cultures of S. sanguis. The development of pleomorphism appears to involve two events. The first step, or triggering event, occurs in log-phase cultures in the presence of oxygen. The second step, or manifestation of pleomorphism, occurs at stationary phase in the presence or absence of oxygen. The loss of viability appears to be related both to the length of time the culture is exposed to oxygen as well as to a specific event which occurs at the transition from log to stationary phase. Oxygen also induced a state of unbalanced growth in S. sanguis in which the proportions of deoxyribonucleic acid, ribonucleic acid, and protein synthesized were altered. The relationship of these alterations to the development of pleomorphism is discussed.
Full text
PDF

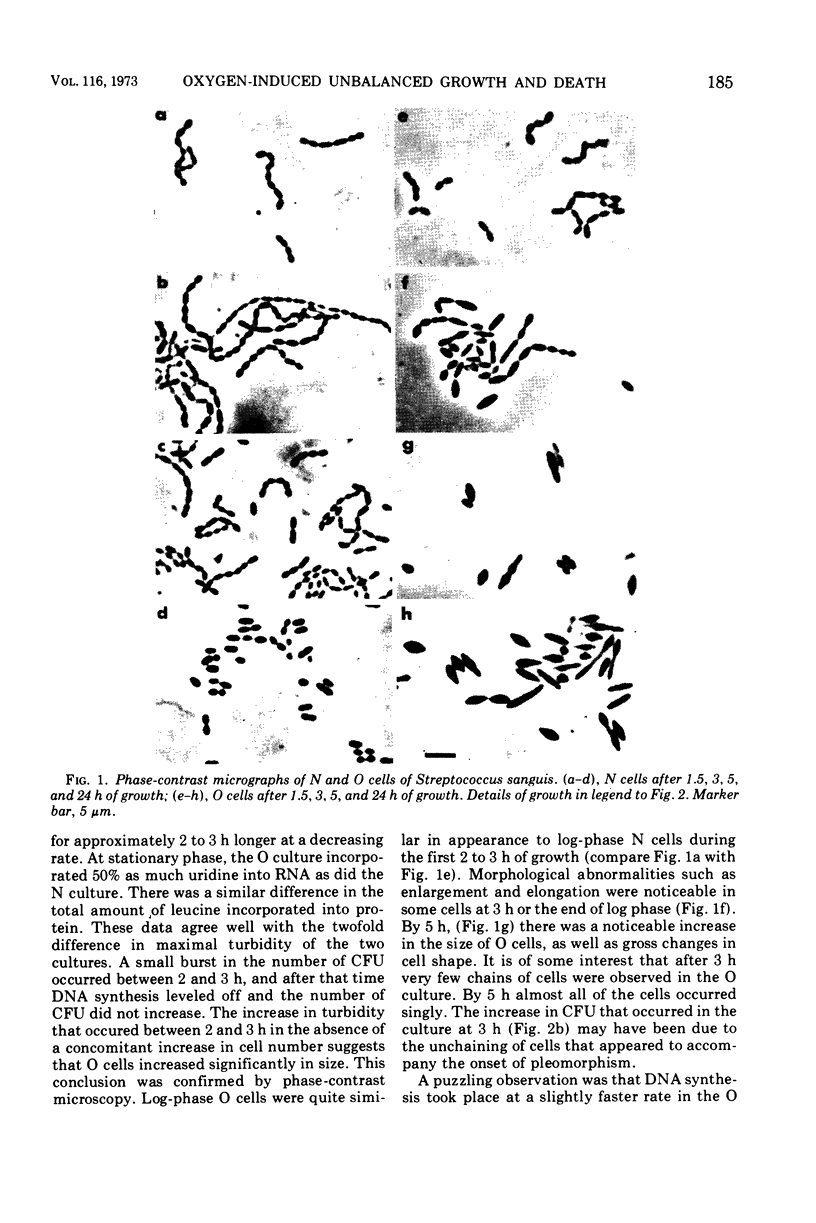
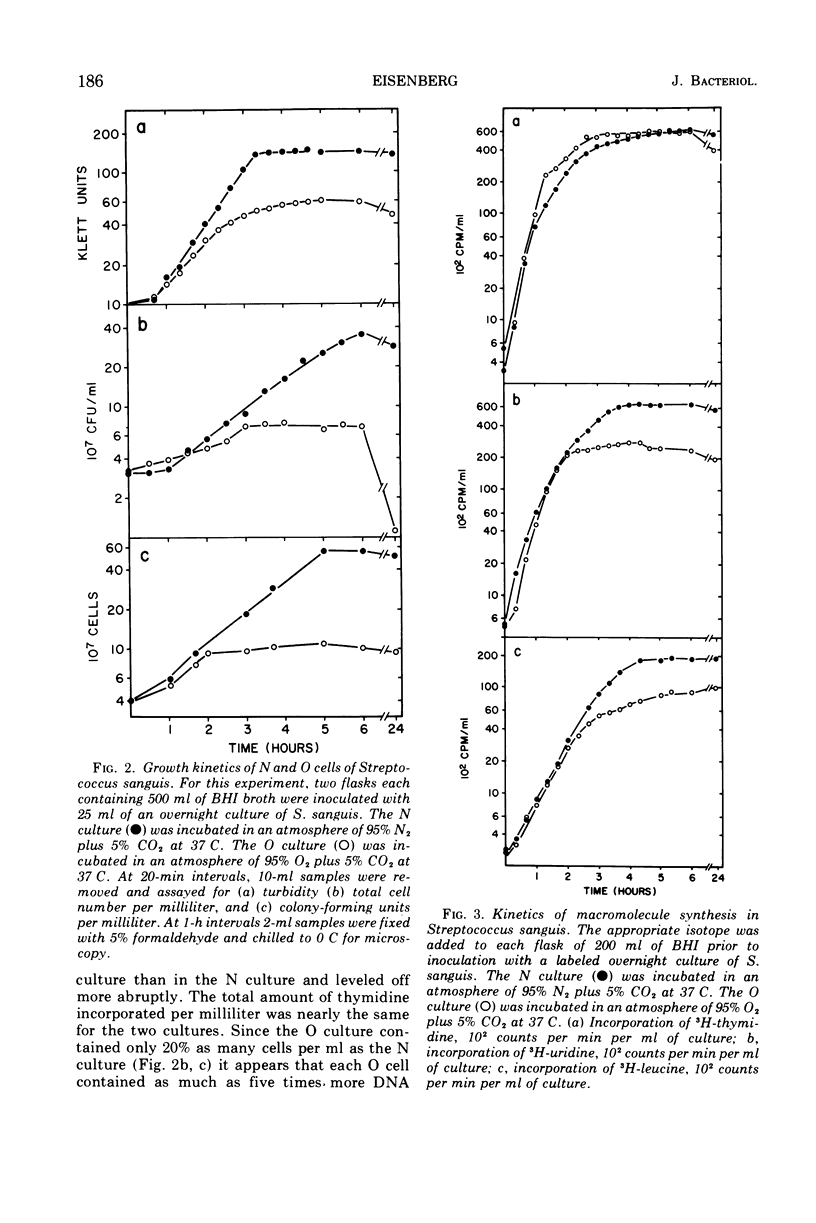
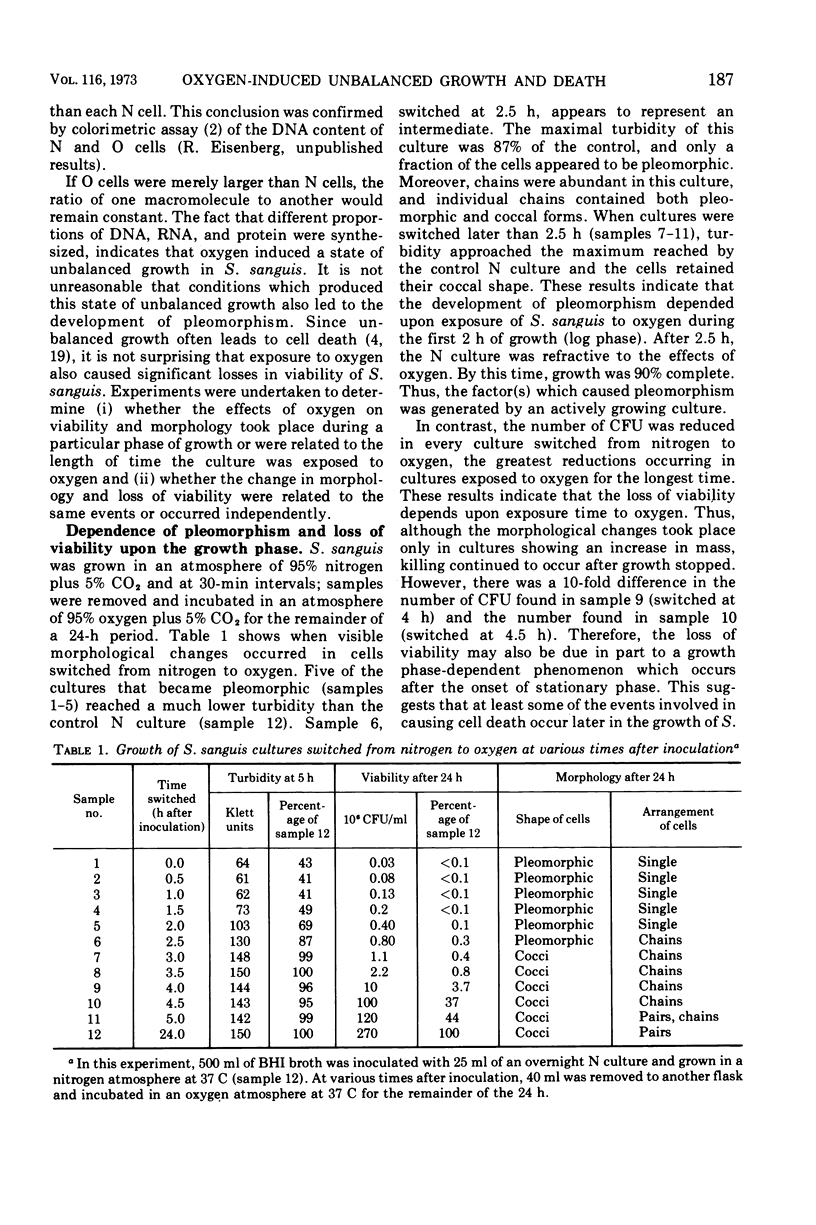


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLLUM F. J. Thermal conversion of nonpriming deoxyribonucleic acid to primer. J Biol Chem. 1959 Oct;234:2733–2734. [PubMed] [Google Scholar]
- Carlsson J. A numerical taxonomic study of human oral streptococci. Odontol Revy. 1968;19(2):137–160. [PubMed] [Google Scholar]
- Cohen S. S., Barner H. D. STUDIES ON UNBALANCED GROWTH IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1954 Oct;40(10):885–893. doi: 10.1073/pnas.40.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EKSTEDT R. D., STOLLERMAN G. H. Factors affecting the chain length of group A streptococci. I. Demonstration of a metabolically active chain-splitting system. J Exp Med. 1960 Oct 1;112:671–686. doi: 10.1084/jem.112.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P. Autolysin(s) of Bacillus subtilis as dechaining enzyme. J Bacteriol. 1970 Aug;103(2):494–499. doi: 10.1128/jb.103.2.494-499.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Inouye M. Reversal by sodium chloride of envelope protein changes related to DNA replication and cell division of Escherichia coli. J Mol Biol. 1972 Feb 14;63(3):597–600. doi: 10.1016/0022-2836(72)90451-2. [DOI] [PubMed] [Google Scholar]
- LOMINSKI I., CAMERON J., WYLLIE G. Chaining and unchaining Streptococcus faecalis; a hypothesis of the mechanism of bacterial cell separation. Nature. 1958 May 24;181(4621):1477–1477. doi: 10.1038/1811477a0. [DOI] [PubMed] [Google Scholar]
- MITCHISON J. M. The growth of single cells. III. Streptococcus faecalis. Exp Cell Res. 1961 Jan;22:208–225. doi: 10.1016/0014-4827(61)90099-4. [DOI] [PubMed] [Google Scholar]
- Mendelson N. H., Cole R. M. Genetic regulation of cell division initiation in Bacillus subtilis. J Bacteriol. 1972 Nov;112(2):994–1003. doi: 10.1128/jb.112.2.994-1003.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Tamura G. Mutant of Escherichia coli with thermosensitive protein in the process of cellular division. J Bacteriol. 1972 Nov;112(2):959–966. doi: 10.1128/jb.112.2.959-966.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D. Relationship between the latent form and the active form of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Nov;100(2):617–624. doi: 10.1128/jb.100.2.617-624.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Groves D. J., Clark D. J. Regulation of Cell Division in Escherichia coli: Characterization of Temperature-Sensitive Division Mutants. J Bacteriol. 1970 Dec;104(3):1052–1064. doi: 10.1128/jb.104.3.1052-1064.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., McConnell M., Burdett I. D. The isolation and characterization of mutants of Bacillus subtilis and Bacillus licheniformis with disturbed morphology and cell division. J Gen Microbiol. 1970 May;61(2):155–171. doi: 10.1099/00221287-61-2-155. [DOI] [PubMed] [Google Scholar]
- Shaw M. K. Formation of filaments and synthesis of macromolecules at temperatures below the minimum for growth of Escherichia coli. J Bacteriol. 1968 Jan;95(1):221–230. doi: 10.1128/jb.95.1.221-230.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. S., Pardee A. B. Accumulation of a protein required for division during the cell cycle of Escherichia coli. J Bacteriol. 1970 Mar;101(3):901–909. doi: 10.1128/jb.101.3.901-909.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alstyne D., Simon M. I. Division mutants of Bacillus subtilis: isolation and PBS1 transduction of division-specific markers. J Bacteriol. 1971 Dec;108(3):1366–1379. doi: 10.1128/jb.108.3.1366-1379.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]