Abstract
When T cells become infected by the parasite Theileria parva, they acquire a transformed phenotype and no longer require antigen-specific stimulation or exogenous growth factors. This is accompanied by constitutive interleukin 2 (IL-2) and IL-2 receptor expression. Transformation can be reversed entirely by elimination of the parasites using the specific drug BW720c. Extracellular signal-regulated kinase and jun NH2-terminal kinase (JNK) are members of the mitogen-activated protein kinase family, which play a central role in the regulation of cellular differentiation and proliferation and also participate in the regulation of IL-2 and IL-2 receptor gene expression. T. parva was found to induce an unorthodox pattern of mitogen-activated protein kinase expression in infected T cells. JNK-1 and JNK-2 are constitutively active in a parasite-dependent manner, but have altered properties. In contrast, extracellular signal-regulated kinase-2 is not activated even though its activation pathway is functionally intact. Different components of the T cell receptor (TCR)-dependent signal transduction pathways also were examined. The TCRζ or CD3ɛ chains were found not to be phosphorylated and T. parva-transformed T cells were resistant to inhibitors that block the early steps of T cell activation. Compounds that inhibit the progression of T cells to proliferation, however, were inhibitory. Our data provide the first example, to our knowledge, for parasite-mediated JNK activation, and our findings strongly suggest that T. parva not only lifts the requirement for antigenic stimulation but also entirely bypasses early TCR-dependent signal transduction pathways to induce continuous proliferation.
Several intracellular parasites reside in cells of the immune system and pervert the host cells to their own end. For example, Leishmania donovani has been shown to interfere with the activation of macrophage protein kinase C (PKC) (1), and glycoinositolphospholipid-derived ceramide of Trypanosoma cruzi has been demonstrated to inhibit T-cell mitogenesis (2), in all likelihood by suppressing nuclear factors that bind to specific sites on the interleukin-2 (IL-2) enhancer (3). Theileria parva, the causative agent of the lymphoproliferative disease East Coast fever, is transmitted by ticks and infects lymphocytes. The parasite resides free in the host cell cytoplasm (4) and has acquired the ability to stimulate the infected cells into continuous, uncontrolled proliferation (5–7). T. parva-infected cells show several characteristics of tumor cells. They can be cultured indefinitely in vitro and, most notably, they form invasive tumors when injected into nude mice (8). Transformation induced by T. parva is completely reversible, and lymphocytes return to a resting phenotype upon elimination of the parasite by treatment with the parasite-specific drug BW720c (6, 9). Upon infection with T. parva, antigen-specific T-cell clones cease to require either stimulation or exogenous IL-2 to proliferate (10, 11). When cells are cured of the parasite, however, they become dependent again on exogenous IL-2 for their survival (6).
In previous work, we have shown that T. parva induces constitutive NF-κB activation (12) and IL-2 and IL-2 receptor (IL-2R) expression (13, 14) in a parasite-dependent manner. Using anti-IL-2 antibodies and antisense IL-2 RNA, (14–16), it could be demonstrated that proliferation of the T. parva-infected T cell line TpM(803) (further referred to as TpM) is, at least in part, dependent on an autocrine loop.
Under physiological conditions, T cell activation and proliferation results from the specific engagement of the T cell receptor (TCR) by an antigen presented as a peptide associated with major histocompatibilty complex class I or II molecules expressed on the surface of antigen-presenting cells. This results in the tyrosine phosphorylation of the invariant CD3 and TCRζ chains, allowing the docking of SH2-containing proteins that form part of the TCR signal transduction cascades (reviewed in refs. 17, 18). This branches into several different pathways, which then converge in the activation of a number of transcription factors—among them NFAT, NF-κB, and AP-1 (18). AP-1 can be made up of different heterodimers consisting of members of the Fos and Jun family of proteins (19), and both AP-1 and NF-κB-specific binding sites can be found in the promotor region of both the IL-2 and IL-2R genes.
Members of the mitogen-activated protein kinase family function as pivotal components in pathways that control cellular differentiation, proliferation, and apoptosis (reviewed in ref. 20). In T cells, both extracellular signal-related kinase 2 (ERK-2) and Jun NH2-terminal kinase (JNK, also called stress-activated protein kinase) are required for the activation of transcription factors that regulate IL-2 and IL-2R gene expression (reviewed in ref. 18). TCR stimulation induces the activation of the GTP-binding protein p21ras (reviewed in ref. 17). This results in the stimulation of a Ras/Raf-dependent cascade leading to the activation of ERK. ERK, in turn, regulates the transcription of c-fos through phosphorylation of the transcription factors Elk-1/TCF (21) and, via pp90rsk activation, CREB (22). JNK is activated in response to a wide range of stimuli, including some growth factors, cytokines, UV irradiation, heat shock, ceramide, and certain inhibitors of protein synthesis (23, 24–27). In addition, signaling via the receptor for costimulation, CD28, also can activate JNK (28, 29). JNK activates c-Jun by phosphorylating two serine residues (S63 and S73) in the c-Jun NH2-terminal domain. JNK also activates ATF-2 (30), which is related to c-Jun, and both are involved in regulating the expression of the c-Jun gene itself (30, 31).
To address the question whether T. parva uses the normal signaling pathways to induce continuous proliferation, we first examined whether the parasite influences the state of activation of ERK and JNK. We show that JNK is constitutively active in a parasite-dependent manner that differs from that observed in uninfected T cells. In addition, we provide evidence that ERK is not activated and that T. parva bypasses the early TCR signal transduction pathways.
MATERIALS AND METHODS
Cell Culture.
The maintenance and characteristics of the T. parva-infected cell line, TpM(803), and preparation of concanavalin A (ConA)-stimulated lymph node (LN) T cells have been described elsewhere (6, 31). To eliminate the parasite, BW720c was added at 50 ng/ml.
Proliferation Assays.
TpM (105 cells/ml) or LN T cells (5 × 105 cells/ml, with or without 10 μg/ml ConA) were cultured in flat-bottomed 96-well microtiter plates for the indicated length of time with or without inhibitor. Proliferation was monitored by measuring [methyl-3H]thymidine incorporation (0.5 μCi/well, Amersham; 1 Ci = 37 GBq) over a period of 4 hr as described (33).
Stimulation and Inhibition Experiments.
Ascomycin (a gift from T. Payne, Novartis, Basel) was dissolved in ethanol; rapamycin (Calbiochem) in methanol; 8Br-cAMP (Boehringer Mannheim) in water; ionomycin (A23187, Calbiochem) in ethanol; phorbol 12-myristate 13-acetate (PMA, Sigma,) and bisindolylmaleimide (BIM, Calbiochem) in dimethyl sulfoxide. For anti-CD3/anti-CD4 stimulation, TpM cells were washed twice in ice-cold PBS and resuspended at a concentration of 107 cells/ml in cold cL15 medium for 10 min. The culture was then supplemented with 100 μg/ml mAb MM1A (anti-CD3, ref. 34) and IL-A11 (anti-CD4, ILRI, Nairobi, Kenya) and incubated on ice for 10 min. Rabbit anti-mouse IgG+IgM (30 μg/ml, Dianova) was added, and cells were incubated for 10 min on ice. To induce signalling, cells were shifted to 37°C for either 2 or 5 min, then washed twice with ice-cold PBS and processed for further analysis.
Immunoprecipitation of the TCR/CD3 Complex.
TpM cells were washed three times with ice-cold PBS, resuspended in lysis buffer [1% Brij96 (Sigma)/20 mM Tris, pH 8.0/150 mM NaCl/10 mM NaF/1 mM Na3VO4/10 μg/ml antipain, pepstatin A, leupeptin, and chemostatin] at 107 cells/ml. Cells were lysed at 4°C for 30 min, and lysates were cleared by centrifugation at 4°C for 30 min at 14,000 × g. The TCR/CD3 complex was immunoprecipitated from 1 ml of lysate (for 4 hr, continuous rotation) using 30 μg of the mAb MM1A (34) coupled to protein G-Sepharose (Pharmacia). Sepharose beads then were washed twice with NET1 buffer (50 mM Tris, pH 8.0/500 mM NaCl/5 mM EDTA/0.1% NaN3/0.5% Triton X-100/1 mg/ml BSA) and twice with NET2 buffer (50 mM Tris, pH 8.0/150 mM NaCl/5 mM EDTA/0.1% NaN3/0.5% Triton X-100). Samples were boiled for 5 min at 100°C in Laemmli buffer, separated by NaDodSO4/PAGE, and blotted onto a nitrocellulose membrane (Schleicher and Schuell). Filters were probed with rabbit anti-human CD3ɛ antibody (Dako, Nr.A452, diluted 1/50) or anti-phosphotyrosine antibody (Transduction Laboratories, Lexington, KY; PY20, Nr. P11120, diluted 1/300). Antibody binding was demonstrated using recombinant protein G-peroxidase (Zymed; dilution 1/3,000) and ECL (Amersham).
ERK Immune Complex Kinase Assay.
To monitor ERK activity, 4 × 107 cells were disrupted in 500 μl of lysis buffer (10 mM Tris, pH 7.0/50 mM NaCl/1% Triton X-100/50 mM NaF/5 μM ZnCl2/30 mM sodium pyrophosphate/0.1 mM Na3VO4/2 mM iodoacetamide), and 3 μg of anti-ERK2 antibody (Santa Cruz, No sc154) were added and rotated overnight at 4°C. Protein A-Sepharose CL-4B (Pharmacia) was added and immune complexes absorbed by rotation for 2 hr at 4°C. Immunoprecipitates were washed twice with lysis buffer and once with 10 mM Tris, pH 8.0, 140 mM NaCl and once with 50 mM Tris, pH 6.8. ERK2 kinase assays were performed as described previously (35). The substrate was myelin basic protein (Sigma), and kinase reactions were done in the presence of 5 μCi of γ-32P-labeled ATP (Amersham) for 30 min at 30°C. Reactions were stopped by adding 5× Laemmli sample buffer and boiling for 5 min. Proteins were separated by NaDodSO4/PAGE and blotted onto nitrocellulose membranes (Schleicher and Schuell). Filters were analyzed by autoradiography and Molecular Imager (GS-363, Bio-Rad) using Molecular Analyst Software Version 2.0.1.
JNK in Vitro and In-Gel Kinase Assays.
Protein extracts for JNK assays were prepared as described for ERK. JNK was isolated from lysates by adsorption to a glutathione S-transferase (GST)-c-Jun fusion protein (30). Routinely, glutathione-agarose beads (Pharmacia) containing 50 μg of GST-c-Jun were added to 1 mg of extract. In-gel and solid kinase assays were performed as in ref. 36. For in-gel kinase assays, GST-c-Jun(1–166) was copolymerized (40 μg/ml) in a NaDodSO4/12.5% polyacrylamide gel. The kinase reaction was performed in 20 ml of in-gel kinase buffer containing 250 μCi of γ-32P-labeled ATP for 1 hr. The gel was washed for 24 hr in 100 ml of 5% trichloroacetic acid and 1% Na4P2O7, at room temperature with several changes followed by drying and autoradiography. Quantitative analysis was performed by phosphoimager. The solid-state Jun kinase assay was performed for 30 min at 30°C in the presence of 5 μCi of γ-32P-labeled ATP. Phosphorylated GST-c-Jun(1–166) was analyzed by gel electrophoresis followed by autoradiography phosphoimager analysis.
RESULTS
JNK, but Not ERK, Is Constitutively Activated in TpM T Cells.
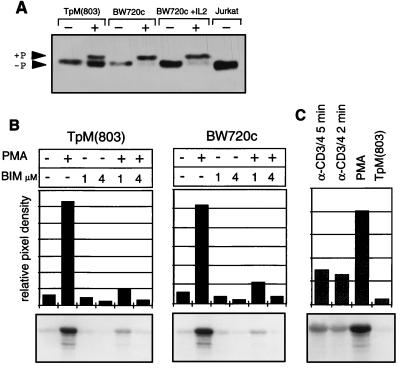
In normal T cells, stimulation via the TCR and the receptor for costimulation results in the activation of ERK and JNK. Because TpM T cells show many features of permanently activated T cells, the state of activation of these enzymes was examined. ERK-2 activation involves its phosphorylation by the dual-specific MAPK kinase, MEK (37), which is reflected by a decrease in electrophoretic mobility in polyacrylamide gels and an increase in enzymatic activity. Western blot analysis shows that ERK is not phosphorylated in TpM T cells, whereas unspecific stimulation with the phorbol ester PMA results in a clear shift in molecular weight (Fig. 1A). Interestingly, in several experiments it could be observed that a considerable proportion of ERK-2 failed to undergo a mobility shift when TpM T-cells were treated with PMA, whereas most of it had converted to the slower migrating form after PMA treatment of BW720c-treated cells.
Figure 1.
ERK-2 is not activated in TpM T cells. (A) Western blot mobility shift analysis of ERK-2. Proteins isolated from TpM T cells or BW720c-treated T cells (cultured with or without IL-2), stimulated for 10 min with PMA (50 ng/ml) as indicated were analyzed by Western blotting using anti-ERK-2. +P indicates the phosphorylated form of ERK-2. Jurkat T cells were used as a control. (B) In vitro ERK-2 kinase assay. TpM and BW720c-treated T cells were stimulated for 15 min with PMA in the presence or absence of BIM (1 μM or 4 μM), as indicated. ERK activity was determined by immune complex kinase assay using myelin basic protein as a substrate. Myelin basic protein phosphorylation was analyzed by autoradiography (Lower) and quantitated by phosphoimager analysis. The signal intensity is presented as relative pixel density (Upper). (C) Activation of ERK activity by anti-CD3/CD4 stimulation. TpM T cells were stimulated either with PMA or with anti-CD3 and anti-CD4 mAbs, and crosslinked by anti-mouse IgG, for the times indicated. ERK activity was determined as above.
To further examine enzymatic activity, ERK-2 was immunoprecipitated and analyzed in an in vitro kinase assay using myelin basic protein as a substrate (Fig. 1B). Consistent with the lack of constitutive phosphorylation, no ERK activity above background levels could be detected in TpM or in BW720c-treated T cells, but stimulation with PMA resulted in a strong induction, confirming that the ERK-2 activation pathway was capable of functioning. PMA-induced ERK-2 activity was markedly suppressed, however, by treatment of the cells with the PKC inhibitor BIM. Identical results were obtained when ERK-2 was immunoprecipitated from uninfected LN cells, with PMA-induced activation also being potently inhibited by BIM (data not shown). Further evidence for an intact ERK pathway was provided by the fact that stimulation with a combination of anti-CD3ɛ and anti-CD4 antibodies also resulted in a significant increase in ERK activation (Fig. 1C). CD3ɛ/CD4-induced ERK-2 activity could be observed within 2 min of stimulation but was invariably lower than the PMA-induced activity.
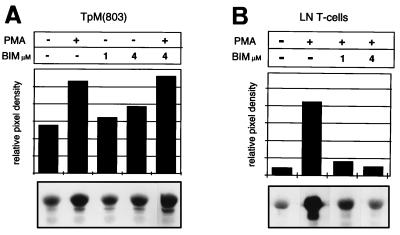
In contrast to ERK, JNK was found to be constitutively activated in TpM T cells (Fig. 2A). Interestingly, treatment with BIM did not inhibit constitutive JNK activity, but consistently resulted in a small, but dose-dependent increase. JNK activity in TpM T cells could be further induced by treatment with PMA, and this PMA-induced JNK activation was also resistant to 4 μM BIM. The constitutive JNK activation observed in TpM T cells differs in several aspects from JNK activation in uninfected LN T cells. As expected, JNK activity is low in freshly isolated LN T cells kept overnight in culture in the absence of stimulation (Fig. 2B). Treatment with PMA results in a potent activation of JNK activity which, unlike that of TpM T cells, however, is strongly inhibited by BIM (Fig. 2B).
Figure 2.
JNK is constitutively activated in TpM T cells. (A) Constitutive and PMA-induced JNK activation are not inhibited by BIM in TpM T cells. TpM T cells were pretreated with BIM for 1 hr as indicated and then stimulated for 15 min with PMA in the presence or absence of BIM. JNK was isolated from 1 mg of extract by absorption to GST-c-Jun(1–166) fusion protein immobilized on glutathione-agarose beads. JNK activity was monitored by measuring the phosphorylation of GST-c-Jun(1–166) in a solid-state kinase assay. Phosphorylated GST-c-Jun(1–166) was visualized by autoradiograph (Lower) and quantitated by phosphoimager analysis (Upper). (B) PMA-induced JNK activation in LN T cells is inhibited by BIM. LN T cells were stimulated with PMA for 15 min in the presence or absence of the inhibitor BIM, as indicated. JNK was isolated from 1 mg of extract, and JNK kinase analysis was performed as in A.
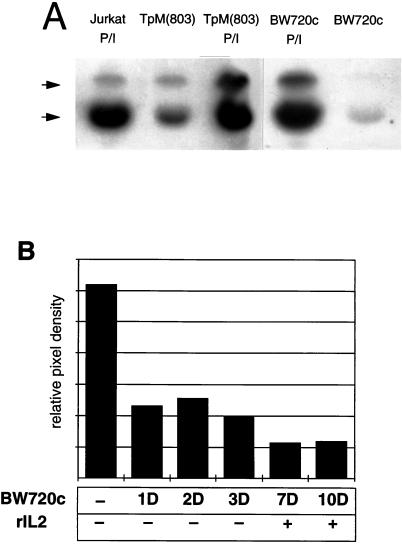
Constitutive JNK Activation Depends on the Presence of Viable Parasite.
There are two forms of JNK, JNK-1 and JNK-2, which are 40% identical at the amino acid level (38). Kinase assays were performed to determine whether both forms of JNK were activated. JNK was first isolated by absorption to a GST-c-Jun fusion protein and its activity tested in an in-gel kinase assay in which the same fusion protein, copolymerized in the gel, acted as a substrate. This revealed that both JNK-1 and JNK-2 are constitutively activated in TpM T cells (Fig. 3A). Elimination of the parasite by treatment with BW720c, resulted in a pronounced reduction in both JNK-1 and JNK-2 activity. JNK activity could again be further induced by treatment of the cells with PMA and Ca2+-ionophore under conditions similar to those described for human Jurkat T-cells (29). Likewise, in BW720c-treated cells, JNK also could be reactivated by addition of PMA and Ca2+-ionophore to the culture medium. A time-course experiment also was performed in which TpM cells were treated with BW720c for varying lengths of time. JNK activity was reduced to approximately 30% within 24 hr (Fig. 3B) and was further reduced after several more days in culture. We previously have shown that by adding recombinant IL-2 to the culture medium, it is possible to maintain BW720c-cured T cells in culture for some time (6), but this is insufficient to restore JNK activity (Fig. 3B).
Figure 3.
Constitutive JNK activation is parasite-dependent. (A) In-gel kinase assay of JNK purified from TpM, BW720c-treated T cells, or control Jurkat T cells. JNK was affinity-purified from TpM T cells, cells treated with BW720c for 4 days, or Jurkat T cells stimulated as indicated with PMA/Ca2+-ionophore (P/I), using GST-c-Jun(1–166) fusion protein attached to agarose beads. JNK activity was tested by in-gel kinase assay using GST-c-Jun(1–166), copolymerised in the gel, as a substrate. Phosphorylated substrate was visualized by autoradiography. The positions of JNK1 (lower) and JNK2 (upper) are indicated. (B) JNK activity in BW720c-treated cells is not restored by the addition of recombinant IL-2 (rIL2). TpM T cells were treated with BW720c for different times. rIL2 was added to the BW720c-treated cells from day 4 onward. JNK activity was analyzed at different time points (marked in days: 1D, 2D, 3D, 7D, and 10D) by solid-state kinase assay and GST-c-Jun(1–166) phosphorylation was quantitated directly by phosphoimager analysis. Data are presented as relative pixel density.
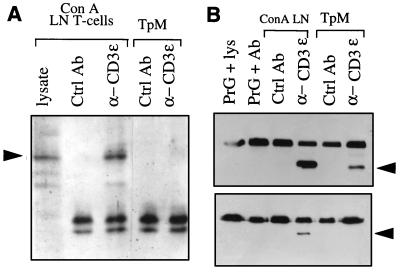
The CD3ɛ and TCRζ Chains Are Not Tyrosine-Phosphorylated in TpM T Cells.
Considering the permanently activated phenotype of TpM T cells it was unexpected to find ERK-2 in its inactive form. This prompted us to examine other components of the TCR-dependent signaling pathways in more detail. Activation of the TCR/CD3 complex results in tyrosine phosphorylation of the TCRζ-chain, and it has been shown that ζ-chain also is involved in CD2-induced T cell activation (39). We therefore investigated if the presence of the parasite influences the state of phosphorylation of the ζ-chain. An antibovine CD3ɛ mAb was used to immunoprecipitate the TCR/CD3 complex from TpM T cells or from ConA-stimulated LN T cells. The different components of the immunoprecipitated TCR/CD3 complex were separated by NaDodSO4/PAGE and subjected to phosphotyrosine immunoblot analysis. Whereas phosphorylation of the TCRζ-chain could clearly be observed in ConA-stimulated LN T cells, no phosphorylation of the ζ-chain was found in TpM T cells (Fig. 4A). After phosphotyrosine immunoblot analysis, the filter was reprobed with an antibovine TCRζ-chain antibody to confirm the identity of the tyrosine-phosphorylated band (data not shown). Likewise, when TCRζ-chains were directly immunoprecipitated using a specific antibovine TCRζ-chain antibody, tyrosine phosphorylation could be observed only in ConA-stimulated LN T cells (data not shown).
Figure 4.
The TCRζ-chain and CD3ɛ are not tyrosine-phosphorylated in TpM T cells. (A) The TCRζ-chain is not tyrosine-phosphorylated in TpM T cells. The TCR/CD3 complex was immunoprecipitated from Brij96 lysates prepared from TpM or ConA-stimulated LN T cells, using an anti-CD3ɛ mAb. Control immunoprecipitations were carried out using a mAb directed against CD8 (Ctrl Ab). Immunoprecipitated proteins were separated by electrophoresis under nonreducing conditions and analyzed by Western blot using an antiphosphotyrosine mAb (PY20). Whole cell lysate from ConA-stimulated LN T cells was loaded as a control. The position of the 34-kDa TCRζ-chain dimer is indicated by an arrow. The doublets are protein G. (B) The CD3ɛ chain of TpM T cells is not tyrosine-phosphorylated. CD3ɛ was immunoprecipitated directly from TrX-100 lysates of TpM or ConA-stimulated LN T cells using either an anti-CD3ɛ mAb (α-CD3) or a control mAb directed against bovine γδ TCR (Ctrl Ab). Further controls included protein G-Sepharose incubated with anti-CD3ɛ mAb alone (PrG + Ab) or protein G-Sepharose incubated with lysate of ConA-stimulated LN T cells in the absence of antibody (PrG + lys). Immunoprecipitated proteins were divided into two equal fractions and analysis by Western blot using anti-human CD3ɛ, recognizing the cytoplasmic region of the bovine CD3ɛ (Upper), or anti-phosphotyrosine antibody PY20 (α-PY, Lower). The position of the 24-kDa CD3ɛ is indicated by arrows. The top bands are protein G.
Because it has been shown that in addition to the TCRζ-chain, the CD3ɛ chain can mediate TCR function (40), CD3ɛ tyrosine phosphorylation also was analyzed. As was the case for the TCRζ-chain, CD3ɛ isolated from TpM T cells was not tyrosine-phosphorylated, whereas CD3ɛ immunoprecipitated from ConA-stimulated LN T cells reacted clearly with antiphosphotyrosine antibodies (Fig. 4B).
TpM T Cells Are Insensitive to Inhibitors of the Early Steps of T Cell Stimulation, but Are Inhibited by Compounds That Block T Cell Progression to Proliferation.
One of the early events after TCR stimulation is the activation of phospholipase C-γ1 resulting in the generation of inositol 1,4,5-trisphosphate and diacylglycerol. Inositol 1,4,5-trisphosphate is responsible for the mobilization of intracellular calcium and activates a calcium/calmodulin-dependent pathway that involves the phosphatase calcineurin. Calcineurin is the main target of immunosuppressive drugs such as cyclosporin A and FK506 or its derivative ascomycin. Diacylglycerol, on the other hand, activates PKC-dependent signaling pathways in T cells, predominantly via the PKC α and β isoforms. The latter can be inhibited by the PKC inhibitor BIM.
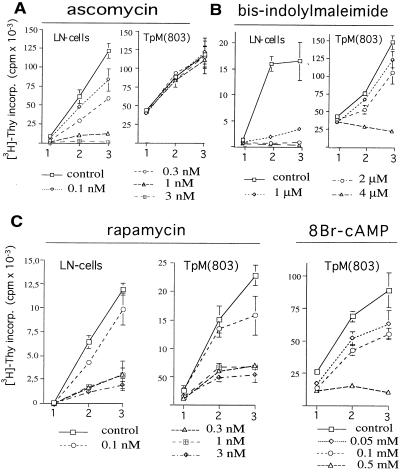
Phosphotyrosine immunoblot analysis of immunoprecipitated phospholipase C-γ1 suggests that it is activated in TpM T cells (data not shown). Although phospholipase C-γ1 can become activated upon stimulation of a variety of surface receptors, it is conceivable that T. parva engages a TCR-specific signal transduction pathway at a point distal to the TCR/CD3 complex, but proximal to calcineurin or PKC. To examine if calcineurin activity is essential for continuous proliferation, the effect of ascomycin on the proliferation of TpM T cells was tested. Fig. 5A shows that the growth of ConA-stimulated LN T cells was clearly inhibited by ascomycin in a dose-dependent manner, whereas TpM T cells were not affected. In some experiments, a moderate inhibition of ≤20% could be observed at the highest dose (3 nM). The lack of inhibition by ascomycin is in agreement with the previously reported insensitivity of TpM T cells to inhibition with cyclosporin A (33).
Figure 5.
Effect of ascomycin, BIM, rapamycin, and 8Br-cAMP on the proliferation of TpM T cells. (A) TpM T cells are resistant to ascomycin. TpM or ConA-stimulated LN T cells were cultured in the presence or absence of different concentrations of ascomycin. Proliferation was monitored for 3 days by measuring [3H]thymidine incorporation. Error bars represent 1 SD of quadruple samples. (B) TpM T cells are less sensitive than ConA-stimulated LN T cells to growth inhibition by BIM. Cells were cultured in the absence (control) or presence of different concentrations of BIM and proliferation monitored for three days as in A. (C) TpM and LN T cells are equally sensitive to rapamycin- and cAMP-mediated growth inhibition. TpM or ConA-stimulated LN T cells were cultured in the presence or absence of different concentrations of rapamycin or 8Br-cAMP as indicated. Proliferation was monitored for 3 days as in A. The profile of the 8Br-cAMP-induced inhibition of control LN T cells was similar to that for rapamycin and is not shown.
PKC also plays an important role in the early stages of T cell activation, and both PKC inhibitors or PKC-down-regulation induced by treatment with high doses of phorbol esters before stimulation effectively block T cell activation (32, 41). In vitro proliferation studies showed that TpM T cells are resistant to treatment with BIM (Fig. 5B). TpM continued to proliferate in the presence of 1 μM BIM, a concentration that inhibits PKC α, β, and γ isoforms, whereas ConA-stimulated LN T cells were strongly inhibited. At 2 μM, proliferation of TpM T cells was only partly reduced compared with the complete inhibition observed in uninfected LN T cells. At the high-concentration 4 μM BIM, however, both TpM and ConA-stimulated LN T cells were inhibited.
Rapamycin also is used as an immunosuppressive compound, and although it also associates with members of the FKBP family, the target of inhibition in T cells is different from that of CsA, FK506, or ascomycin. Instead of inhibiting the early steps of T cell activation, rapamycin inhibits mitogenic stimuli delivered by IL-2 binding to the IL-2R (42) and interferes with CD28-dependent signaling (43). Likewise, increases in the level of intracellular cAMP have been shown to inhibit the progression of activated T cells to the proliferation stage (44) rather than the early events. In contrast to ascomycin and BIM, both rapamycin and 8Br-cAMP strongly inhibited the proliferation of TpM T cells (Fig. 5C).
DISCUSSION
Given the central role of mitogen-activated protein kinases in the regulation of T-cell differentiation and proliferation in general and their known participation in T cell activation pathways we examined the state of activation of ERK-2, JNK-1, and JNK-2 in T. parva-transformed T cells. JNK-1 and JNK-2 were found to be constitutively activated in a parasite-dependent manner, whereas ERK-2 was silent.
In several systems, ERK has been described as a key component in the AP-1 activation pathway, which in normal T cells regulates IL-2 and IL-2R gene expression (17). Constitutively active ERK also has been associated with cellular transformation (45). Considering that both the IL-2 and IL-2R genes are constitutively expressed and that TpM cells are transformed, the absence of ERK activity was surprising. The ERK-activation pathways were shown to be functional, however, because either treatment with phorbol ester or antibody-mediated CD3/CD4 stimulation of TpM T cells resulted in a strong burst of ERK activity.
In contrast to PMA-induced JNK activation in uninfected LN T cells, constitutive JNK activation in TpM T cells was unaffected by high doses of BIM. Interestingly, whereas PMA-induced JNK activation was completely blocked by BIM in uninfected LN cells, PMA-induced JNK activation in TpM T cells could not be inhibited by 4 μM BIM, suggesting that PMA triggers JNK activation via different, PKC-independent pathways.
JNK activation can result from any of a wide range of extracellular inducers, including UV radiation, genotoxic agents, and the engagement of several surface receptors. To our knowledge, this is the first report of constitutive JNK activation caused by the presence of an intracellular parasite in the host cell cytoplasm. As mentioned before, NF-κB is also constitutively active in TpM T cells, and it is worth noting that the range of stimuli that induces NF-κB overlaps considerably with those inducing JNK, including TNF (26), CD28 (46), and UV irradiation (47). The nature of the stimulus that induces constitutive JNK or NF-κB activation in TpM T cells is still unknown, and it is not yet clear whether a parasite-derived product interferes directly with an activation cascade or indirectly activates one or more of the cellular pathways that includes these molecules. JNKs are also called stress-activated protein kinases. It is conceivable that the Theileria schizont, which resides free in the host cell cytoplasm, exerts a direct, perturbing effect on the T cell, resulting in JNK activation. Parasite-encoded proteins released into the host cell cytoplasm, or exposed on the surface of the schizont, could interfere with signaling pathways. It also cannot be excluded that other parasite-derived substances, such as lipids, function as second messengers in host cell pathways.
Whatever the path of activation, parasite-induced constitutive JNK activation can be expected to have important biological consequences for the T cell. It also has recently been shown that JNK can phosphorylate TCF/Elk-1, normally considered to be a substrate of ERK, resulting in the induction of c-fos expression (48). This way, JNK may compensate for the lack of ERK activation in TpM T cells. An interesting consequence of constitutive JNK activity may be the permanent activation of the transcription factor ATF-2 (30). ATF-2 associates with c-Jun to form a heterodimer that regulates c-Jun expression (38), and ATF-2 also has been shown to interact with NF-κB (49, 50). Interestingly, activated ATF-2/c-Jun has been implicated in oncogenic transformation (51), and ATF-2 binding to the tumor suppressor gene product Rb in addition to several viral proteins, including E1A and HTLV-I Tax, also has been demonstrated.
It would have been reasonable to assume that one of the possible mechanisms by which T. parva induces transformation could be by constitutively activating antigen receptor-mediated signaling pathways. In TpM T cells, however, no tyrosine phosphorylation of the TCRζ and CD3ɛ-chain could be detected and proliferation was not inhibited by compounds that block early TCR-dependent signaling pathways. Our findings thus show that not only the requirement for antigen-specific triggering (10, 11), but also the downstream components of the TCR-dependent signal transduction pathways are bypassed by the parasite. Additional observations indirectly support our findings. Stimulation of T cells normally is accompanied by the internalization of the TCR/CD3 complex (52). In TpM T cells, expression of the TCR/CD3 complex was found to be stable (Y.G. and D.D., unpublished observations), whereas the IL-2R displayed the typical cycling pattern of a constitutively activated receptor (13).
TpM T cells were inhibited by compounds that block the progression, but not the initiation, of T cell proliferation. Rapamycin and membrane-permeant cAMP both interfere with signaling through the p70S6K protein kinase in response to IL-2 stimulation (53, 54) and block the progression of T cells from G1 into S by inhibiting the IL-2-induced elimination of the cyclin-dependent kinase inhibitor, p27Kip1 (42, 55). It is reasonable to assume that growth inhibition of TpM T cells also occurs via this pathway.
Finally, it has been proposed that a balance between ERK and JNK activation pathways may be important in determining whether a cell survives or undergoes apoptosis (56), with JNK activation directing the cell toward apoptosis. Clearly, in TpM T cells, constitutive JNK activity in the absence of ERK activation appears to be associated with continuous proliferation rather than apoptosis.
Acknowledgments
We thank I. Roditi, G. Palmer, and E. Mueller for reading the manuscript and helpful suggestions, P. Angel for the GST-c-Jun(1–166) plasmid, and T. Payne for ascomycin. We are grateful to T. Périnat for excellent technical help. This work was supported by a Swiss National Science Foundation grant (No. 31.32617.91) to D.D. and a United States Department of Agriculture grant (No. 89–37265-4537) to W.D.
ABBREVIATIONS
- BIM
bisindolylmaleimide
- ConA
concanavalin A
- ERK
extracellular signal-regulated kinase
- GST
glutathione S-transferase
- IL-2
interleukin-2
- IL-2R
IL-2 receptor
- JNK
jun NH2-terminal kinase
- LN
lymph node
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- TCR
T cell receptor
References
- 1.Descoteaux A, Matlashewski G, Turco S J. J Immunol. 1992;149:3008–3015. [PubMed] [Google Scholar]
- 2.Gomes N A, Previato J O, Zingales B, Mendonca-Previato L, DosReis G A. J Immunol. 1996;156:628–635. [PubMed] [Google Scholar]
- 3.Soong L, Tarleton R L. Eur J Immunol. 1994;24:16–23. doi: 10.1002/eji.1830240104. [DOI] [PubMed] [Google Scholar]
- 4.Fawcett D W, Doxsey S, Stagg D A, Young A S. Eur J Cell Biol. 1982;27:10–21. [PubMed] [Google Scholar]
- 5.Brown C G D, Stagg D A, Purnell R E, Kanhai G K, Payne R C. Nature (London) 1973;245:101–103. doi: 10.1038/245101a0. [DOI] [PubMed] [Google Scholar]
- 6.Dobbelaere D A E, Coquerelle T M, Roditi I J, Eichhorn M, Williams R O. Proc Natl Acad Sci USA. 1988;85:4730–4734. doi: 10.1073/pnas.85.13.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eichhorn M, Dobbelaere D A E. Parasitol Today. 1994;10:469–472. doi: 10.1016/0169-4758(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 8.Irvin A D, Brown C G D, Kanhai G K, Stagg D A. Nature (London) 1975;255:713–714. doi: 10.1038/255713a0. [DOI] [PubMed] [Google Scholar]
- 9.Hudson A T, Randall A W, Fry M, Ginger C D, Hill B, Latter V S, McHardy N, Williams R B. Parasitology. 1985;90:45–55. doi: 10.1017/s0031182000049003. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin C L, Teale A J. Eur J Immunol. 1987;17:1859–1862. doi: 10.1002/eji.1830171230. [DOI] [PubMed] [Google Scholar]
- 11.Brown W C, Logan K S. Paras Immunol. 1986;8:189–192. doi: 10.1111/j.1365-3024.1986.tb00844.x. [DOI] [PubMed] [Google Scholar]
- 12.Ivanov V, Stein B, Baumann I, Dobbelaere D A E, Herrlich P, Williams R O. Mol Cell Biol. 1989;11:4677–4686. doi: 10.1128/mcb.9.11.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coquerelle T M, Eichhorn M, Magnuson N S, Reeves R, Williams R O, Dobbelaere D A E. Eur J Immunol. 1989;19:655–659. doi: 10.1002/eji.1830190413. [DOI] [PubMed] [Google Scholar]
- 14.Heussler V T, Eichhorn M, Reeves R, Magnuson N S, Williams R O, Dobbelaere D A E. J Immunol. 1992;149:562–567. [PubMed] [Google Scholar]
- 15.Dobbelaere D A E, Roditi I J, Coquerelle T M, Kelke C, Eichhorn M, Williams R O. Eur J Immunol. 1991;21:89–95. doi: 10.1002/eji.1830210114. [DOI] [PubMed] [Google Scholar]
- 16.Eichhorn M, Dobbelaere D A E. Res Immunol. 1995;146:89–99. doi: 10.1016/0923-2494(96)80242-2. [DOI] [PubMed] [Google Scholar]
- 17.Cantrell D. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 18.Jain J, Loh C, Rao A. Curr Opin Immunol. 1995;7:333–342. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 19.Angel P, Karin M. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 20.Cano E, Mahadevan L C. Trends Biochem Sci. 1995;20:117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- 21.Gille H, Sharrocks A, Shaw P. Nature (London) 1992;358:414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- 22.Xing J, Ginty D D, Greenberg M E. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- 23.Dérijard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis R J. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 24.Cano E, Hazzalin C A, Mahadevan L C. Mol Cell Biol. 1994;14:7352–7362. doi: 10.1128/mcb.14.11.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kallunki T, Su B, Tsigelny I, Sluss H K, Dérijard B, Moore G, Davis R, Karin M. Genes Dev. 1994;8:2996–3007. doi: 10.1101/gad.8.24.2996. [DOI] [PubMed] [Google Scholar]
- 26.Sluss H K, Barrett T, Dérijard B, Davis R J. Mol Cell Biol. 1994;14:8376–8384. doi: 10.1128/mcb.14.12.8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, Haimovitz-Friedman A, Fuks Z, Kolesnick R N. Nature (London) 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 28.Rincón M, Flavell R A. EMBO J. 1994;13:4370–4381. doi: 10.1002/j.1460-2075.1994.tb06757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 30.Van Dam H, Wilhelm D, Steffen A, Herrlich P, Angel P. EMBO J. 1995;14:1798–1811. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Dam H, Duyndam M, Rottier R, Bosch A, de Vries-Smits L, Herrlich P, Zantema A, Angel P, van der Eb A J. EMBO J. 1993;12:479–487. doi: 10.1002/j.1460-2075.1993.tb05680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mastro A M, Pepin K G. Cancer Res. 1980;40:3307–3312. [PubMed] [Google Scholar]
- 33.Eichhorn M, Magnuson N S, Reeves R, Williams R O, Dobbelaere D A E. J Immunol. 1990;144:691–698. [PubMed] [Google Scholar]
- 34.Davis W C, Hamilton M J, Park Y-H, Larsen R A, Wyatt C R. In: Monographs in Animal Immunology. Barta O, editor. Balskburg, VA: BAR-LAB; 1990. pp. 47–70. [Google Scholar]
- 35.Sonntag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 36.Hibi M, Lin A, Smeal T, Minden A, Karin M. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 37.Crews C M, Alessandrin A, Erikson R L. Science. 1992;258:478–480. doi: 10.1126/science.1411546. [DOI] [PubMed] [Google Scholar]
- 38.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. Nature (London) 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 39.Howard F D, Moingeon P, Moebius U, McConkey D J, Yandava B, Gennert T E, Reinherz E L. J Exp Med. 1992;176:139–145. doi: 10.1084/jem.176.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Letourneur F, Klausner R D. Science. 1992;255:79–82. doi: 10.1126/science.1532456. [DOI] [PubMed] [Google Scholar]
- 41.Atluru D, Polam S, Atluru S, Woloschak G E. Cell Immunol. 1990;129:310–320. doi: 10.1016/0008-8749(90)90207-8. [DOI] [PubMed] [Google Scholar]
- 42.Nourse J, Firpo E, Flanagan W M, Coats S, Polyak K, Lee M-H, Massague J, Crabtree G R, Roberts J. Nature (London) 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 43.Luo H, Chen H, Daloze P, St-Louis G, Wu J. Clin Exp Immunol. 1993;94:371–376. doi: 10.1111/j.1365-2249.1993.tb03459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lingk D S, Chan M A, Gelfand E W. J Immunol. 1990;145:449–455. [PubMed] [Google Scholar]
- 45.Mansour, S. J., Matten, W. T., Hermann, A. S., Candia, J. M., Rong, S., Fukasawa, K., Vande Woude, G. F. & Ahn, N. G. Science 265, 966–970. [DOI] [PubMed]
- 46.Tong-Starksen S E, Luciw P A, Peterlin B M. J Immunol. 1989;142:702–707. [PubMed] [Google Scholar]
- 47.Devary Y, Rosette C, DiDonato J A, Karin M. Science. 1993;261:1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- 48.Cavigelli M, Dolfi F, Claret F-X, Karin M. EMBO J. 1995;14:5957–5964. doi: 10.1002/j.1460-2075.1995.tb00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du W, Thanos D, Maniatis T. Cell. 1993;74:887–898. doi: 10.1016/0092-8674(93)90468-6. [DOI] [PubMed] [Google Scholar]
- 50.Gupta S, Campbell D, Dérijard B, Davis R J. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 51.Hagmeyer B M, Koenig H, Herr I, Offringa R, Zantema A, Van der Eb A J, Herrlich P, Angel P. EMBO J. 1993;12:3559–3572. doi: 10.1002/j.1460-2075.1993.tb06030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Telerman A, Amson R B, Romasco F, Wybran J, Galand P, Mosselmans R. Eur J Immunol. 1987;17:991–997. doi: 10.1002/eji.1830170715. [DOI] [PubMed] [Google Scholar]
- 53.Chung J, Kuo C J, Crabtree G R, Blenis J. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- 54.Monfar M, Lemon K P, Grammer T C, Cheatham L, Chung J, Vlahos C J, Blenis J. Mol Cell Biol. 1995;15:326–337. doi: 10.1128/mcb.15.1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kato J Y, Matsuoka M, Polyak K, Massague J, Sherr C J. Cell. 1994;79:487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 56.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]