Abstract
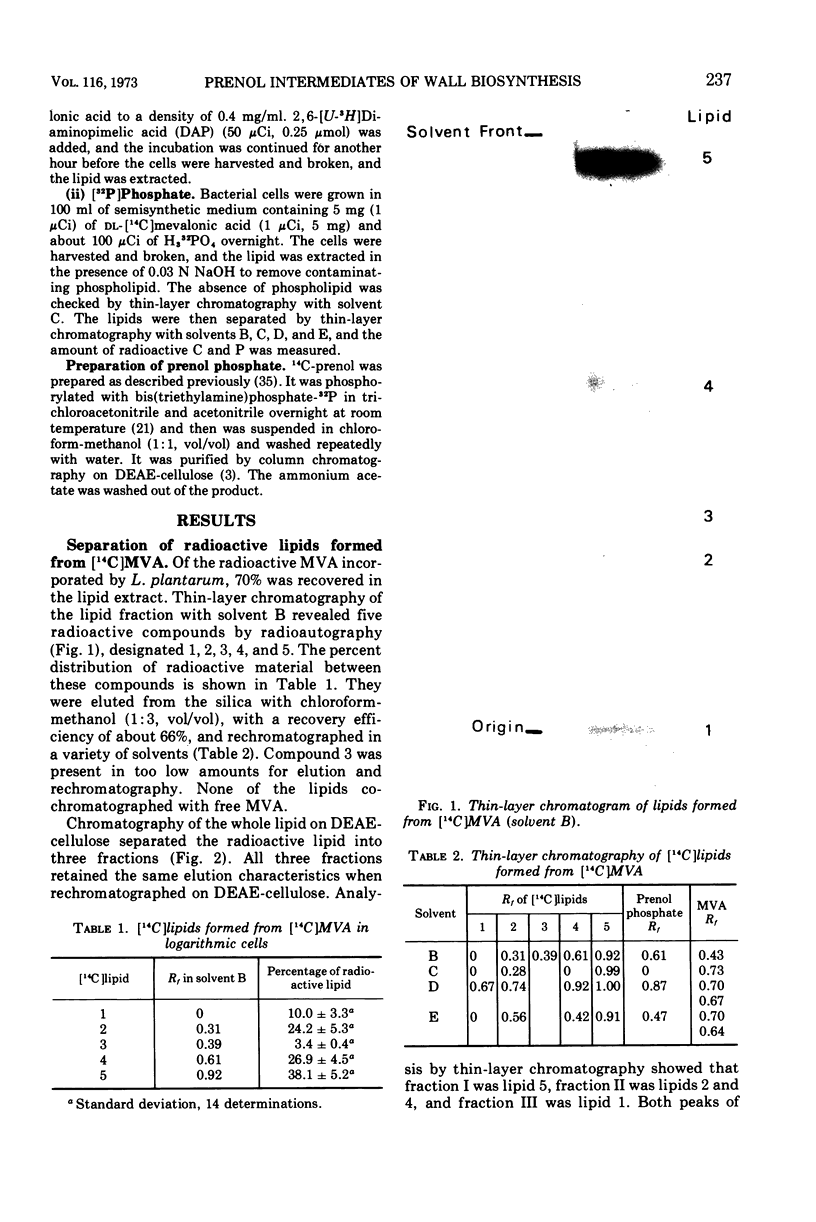
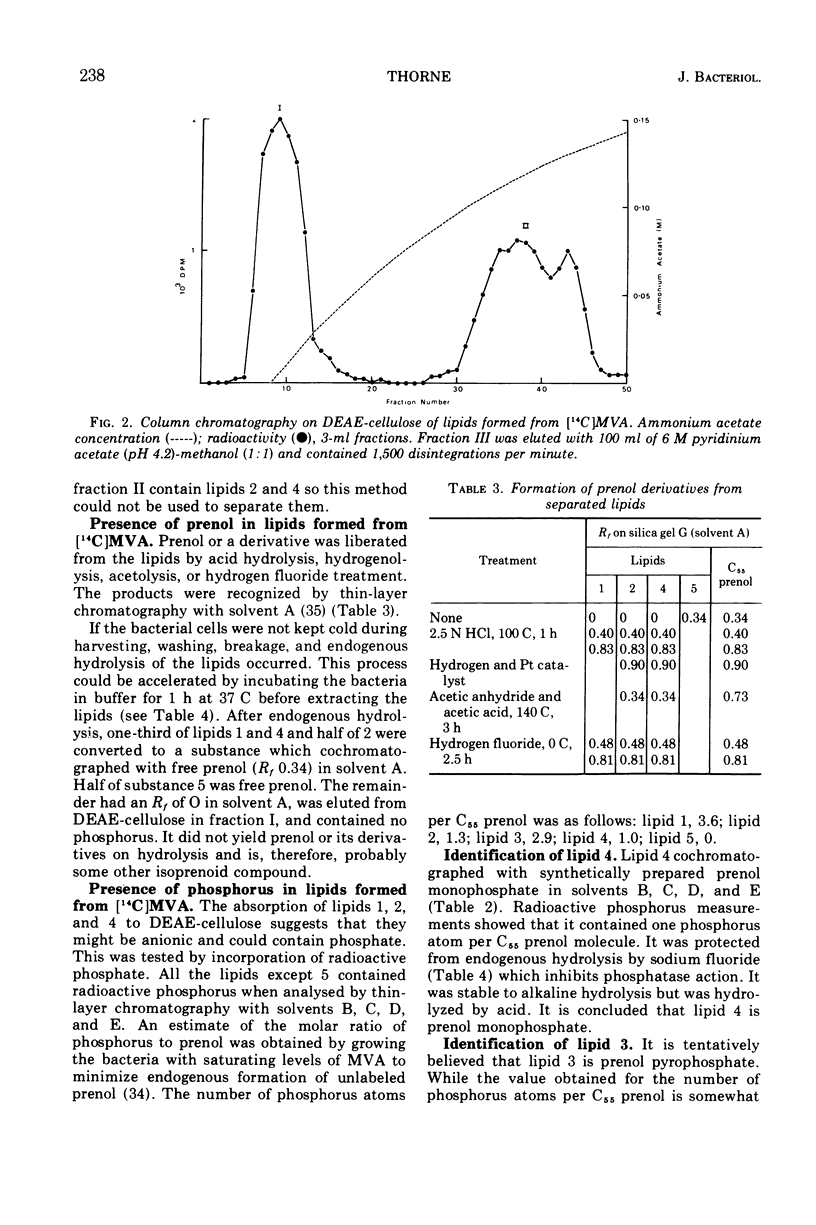
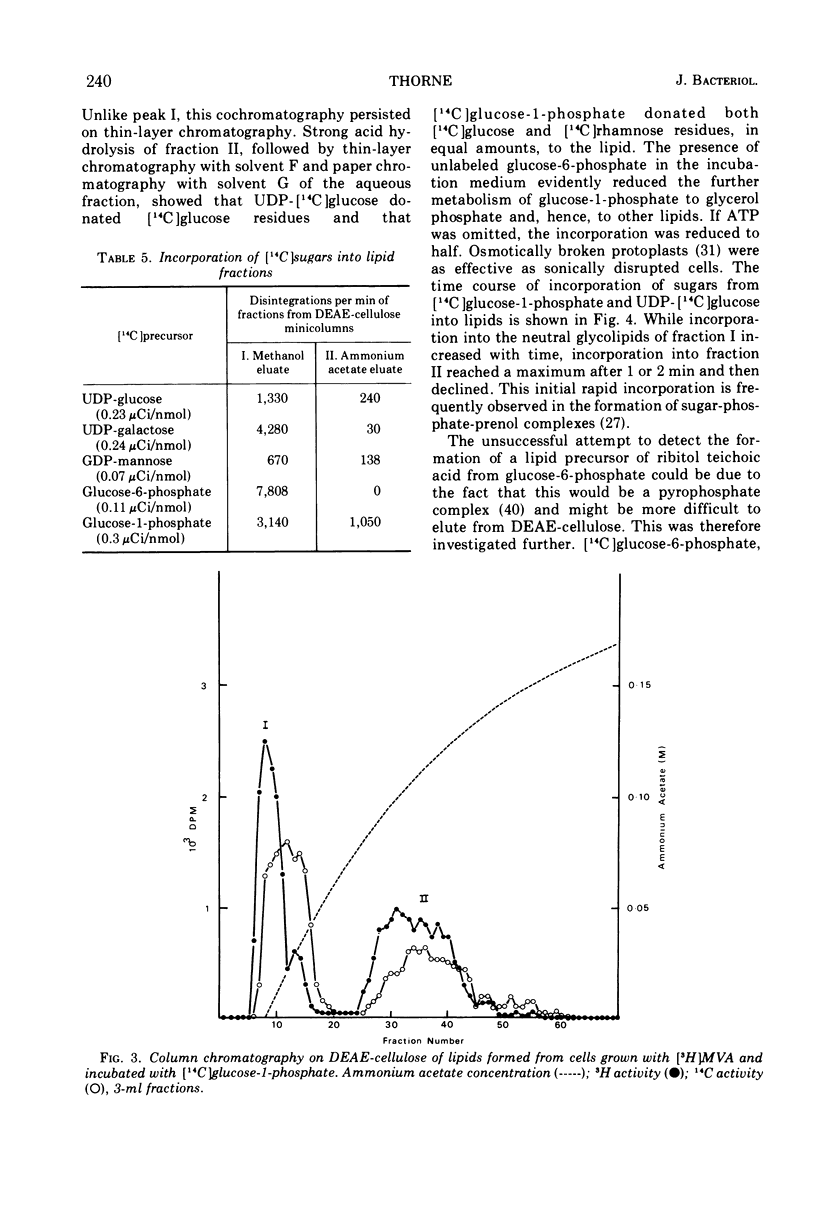
The incorporation of 14C-mevalonic acid by Lactobacillus plantarum predominantly into C55 prenol made it possible to determine the distribution of 14C-prenol between all its derivatives. In logarithmic-phase cells, 25% of the prenol was free, 31% was as monophosphate, 4% as pyrophosphate, 12% as peptidoglycan precursor, and 28% as glyco-phospho-prenol. The glyco-phospho-prenol contained rhamnose, and probably glucose, galactose, and ribitol phosphate, and it may, therefore, be involved in polysaccharide and teichoic acid biosynthesis. The proportion of free prenol increased, up to 73%, as the cell culture aged. Free prenol was also formed when cells were incubated in buffer. The free prenol was readily reutilized when cells were returned to growth medium.
Full text
PDF


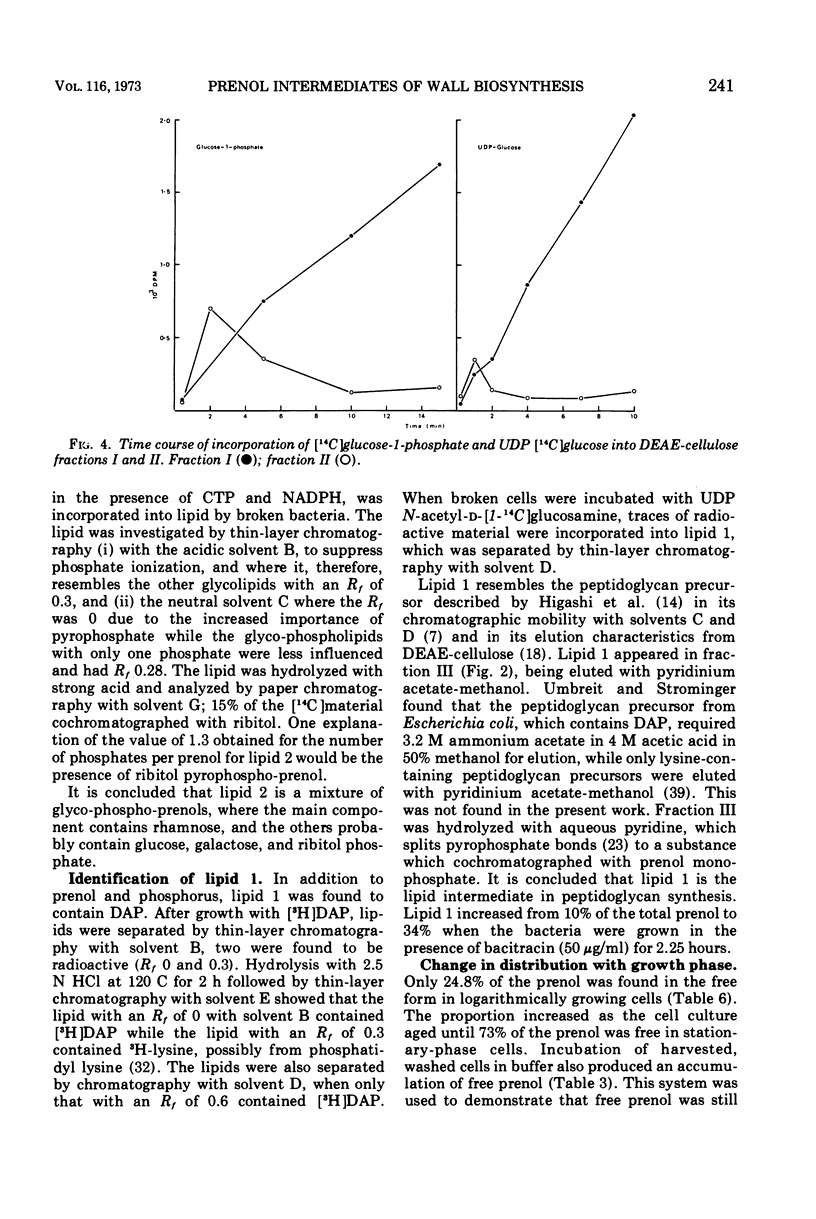
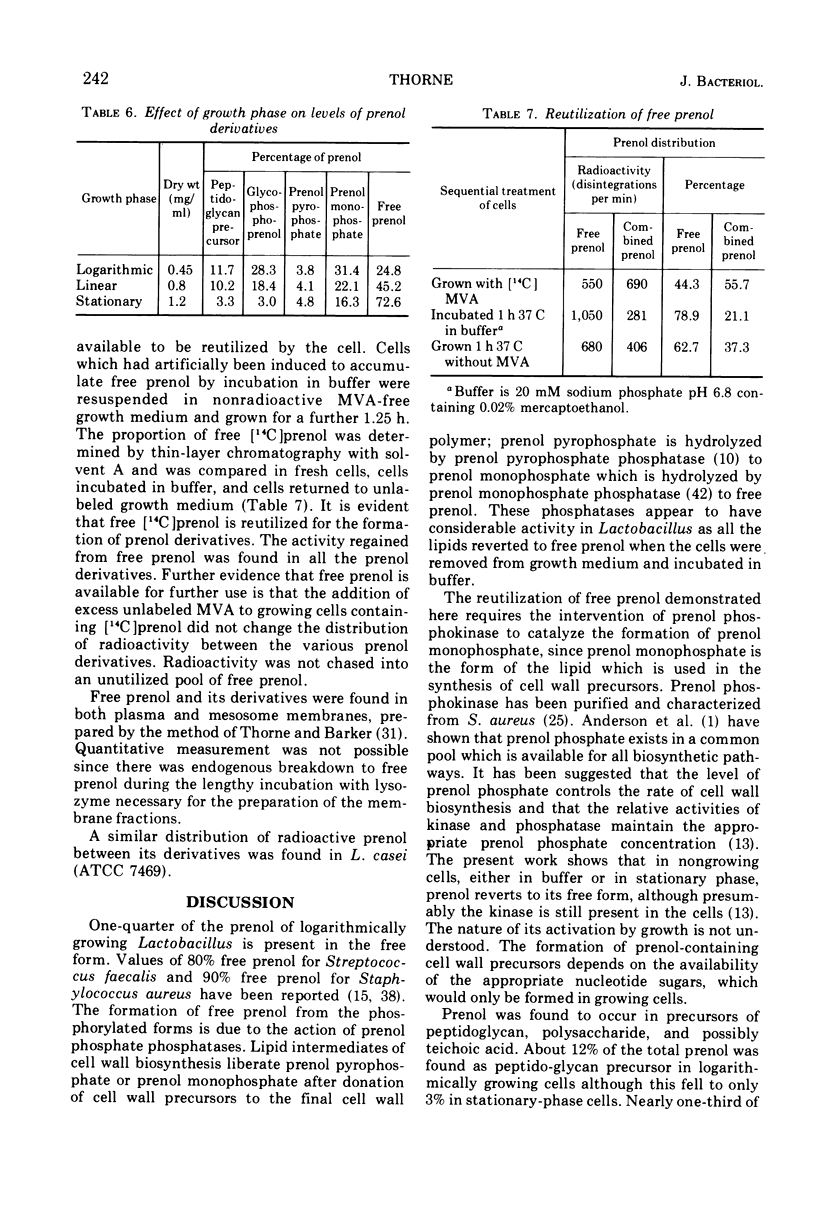


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCHIBALD A. R., BADDILEY J., BUCHANAN J. G. The ribitol teichoic acid from Lactobacillus arabinosus Walls: isolation and structure of ribitol glucosides. Biochem J. 1961 Oct;81:124–134. doi: 10.1042/bj0810124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. G., Hussey H., Baddiley J. The mechanism of wall synthesis in bacteria. The organization of enzymes and isoprenoid phosphates in the membrane. Biochem J. 1972 Mar;127(1):11–25. doi: 10.1042/bj1270011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLUMSOM N. L., BADDILEY J. Thymidine diphosphate mannose and thymidine diphosphate rhamnose in Streptomyces grieus. Biochem J. 1961 Oct;81:114–124. doi: 10.1042/bj0810114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr R. M., Hemming F. W. Polyprenol phosphate as an acceptor of mannose from guanosine diphosphate mannose in Aspergillus niger. Biochem J. 1972 Mar;126(5):1203–1208. doi: 10.1042/bj1261203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundish D. E., Shaw N., Baddiley J. Bacterial glycolipids. Glycosyl diglycerides in gram-positive bacteria. Biochem J. 1966 Jun;99(3):546–549. doi: 10.1042/bj0990546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankert M., Wright A., Kelley W. S., Robbins P. W. Isolation, purification, and properties of the lipid-linked intermediates of O-antigen biosynthesis. Arch Biochem Biophys. 1966 Sep 26;116(1):425–435. doi: 10.1016/0003-9861(66)90049-x. [DOI] [PubMed] [Google Scholar]
- Dietrich C. P., Colucci A. V., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. V. Separation of protein and lipid components of the particulate enzyme from Micrococcus lysodeikticus and purification of the endogenous lipid acceptors. J Biol Chem. 1967 Jul 10;242(13):3218–3225. [PubMed] [Google Scholar]
- Douglas L. J., Wolin M. J. Cell wall polymers and phage lysis of Lactobacillus plantarum. Biochemistry. 1971 Apr 27;10(9):1551–1555. doi: 10.1021/bi00785a007. [DOI] [PubMed] [Google Scholar]
- Goldman R., Strominger J. L. Purification and properties of C 55 -isoprenylpyrophosphate phosphatase from Micrococcus lysodeikticus. J Biol Chem. 1972 Aug 25;247(16):5116–5122. [PubMed] [Google Scholar]
- Gough D. P., Kirby A. L., Richards J. B., Hemming F. W. The characterization of undecaprenol of Lactobacillus plantarum. Biochem J. 1970 Jun;118(1):167–170. doi: 10.1042/bj1180167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock I. C., Baddiley J. Biosynthesis of the wall teichoic acid in Bacillus licheniformis. Biochem J. 1972 Mar;127(1):27–37. doi: 10.1042/bj1270027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y., Siewert G., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XIX. Isoprenoid alcohol phosphokinase. J Biol Chem. 1970 Jul 25;245(14):3683–3690. [PubMed] [Google Scholar]
- Higashi Y., Strominger J. L., Sweeley C. C. Biosynthesis of the peptidoglycan of bacterial cell walls. XXI. Isolation of free C55-isoprenoid alcohol and of lipid intermediates in peptidoglycan synthesis from Staphylococcus aureus. J Biol Chem. 1970 Jul 25;245(14):3697–3702. [PubMed] [Google Scholar]
- Higashi Y., Strominger J. L., Sweeley C. C. Structure of a lipid intermediate in cell wall peptidoglycan synthesis: a derivative of a C55 isoprenoid alcohol. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1878–1884. doi: 10.1073/pnas.57.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey H., Baddiley J. Lipid intermediates in the biosynthesis of the wall teichoic acid in Staphylococcus lactis 13. Biochem J. 1972 Mar;127(1):39–50. doi: 10.1042/bj1270039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanegasaki S., Wright A. Mechanism of polymerization of the Salmonella O-antigen: utilization of lipid-linked intermediates. Proc Natl Acad Sci U S A. 1970 Oct;67(2):951–958. doi: 10.1073/pnas.67.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz W., Matsuhashi M., Dietrich C. P., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. IV. Incorporation of glycine in Micrococcus lysodeikticus. J Biol Chem. 1967 Jul 10;242(13):3207–3217. [PubMed] [Google Scholar]
- Lahav M., Chiu T. H., Lennarz W. J. Studies on the biosynthesis of mannan in Micrococcus lysodeikticus. II. The enzymatic synthesis of mannosyl-l-phosphoryl-undecaprenol. J Biol Chem. 1969 Nov 10;244(21):5890–5898. [PubMed] [Google Scholar]
- Nikaido K., Nikaido H. Glucosylation of lipopolysaccharide in Salmonella: biosynthesis nof O antigen factor n12 2 . II. Structure of the lipid intermediate. J Biol Chem. 1971 Jun 25;246(12):3912–3919. [PubMed] [Google Scholar]
- POPJAK G., CORNFORTH J. W., CORNFORTH R. H., RYHAGE R., GOODMAN D. S. Studies on the biosynthesis of cholesterol. XVI. Chemical synthesis of 1-H2-3-2-C-14- and 1-D2-2-C-14-trans-trans-farnesyl pyrophosphate and their utilization in squalene biosynthesis. J Biol Chem. 1962 Jan;237:56–61. [PubMed] [Google Scholar]
- RENKONEN O. INDIVIDUAL MOLECULAR SPECIES OF DIFFERENT PHOSPHOLIPID CLASSES. II. A METHOD OF ANALYSIS. J Am Oil Chem Soc. 1965 Apr;42:298–304. doi: 10.1007/BF02540133. [DOI] [PubMed] [Google Scholar]
- Rutherford G., Morgan A. R. The specific chemical cleavage of pyrophosphate diesters. Can J Biochem. 1972 Mar;50(3):287–291. doi: 10.1139/o72-040. [DOI] [PubMed] [Google Scholar]
- SAITO K., HANAHAN D. J. A study of the purification and properties of the phospholipase A of Crotalus admanteus venom. Biochemistry. 1962 May 25;1:521–532. doi: 10.1021/bi00909a025. [DOI] [PubMed] [Google Scholar]
- Sandermann H., Jr, Strominger J. L. Purification and properties of C 55 -isoprenoid alcohol phosphokinase from Staphylococcus aureus. J Biol Chem. 1972 Aug 25;247(16):5123–5131. [PubMed] [Google Scholar]
- Scher M., Lennarz W. J. Studies on the biosynthesis of mannan in Micrococcus lysodeikticus. I. Characterization of mannan-14C formed enzymatically from mannosyl-1-phosphoryl-undecaprenol. J Biol Chem. 1969 May 25;244(10):2777–2789. [PubMed] [Google Scholar]
- Scher M., Lennarz W. J., Sweeley C. C. The biosynthesis of mannosyl-1-phosphoryl-polyisoprenol in Micrococcus lysodeikticus and its role in mannan synthesis. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1313–1320. doi: 10.1073/pnas.59.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw N., Smith P. F., Verheij H. M. The structure of a glycerylphosphoryldiglucosyl diglyceride from the lipids of Acholeplasma laidlawii strain B. Biochem J. 1972 Aug;129(1):167–173. doi: 10.1042/bj1290167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw N. The detection of lipids on thin-layer chromatograms with the periodate-Schiff reagents. Biochim Biophys Acta. 1968 Oct 22;164(2):435–436. doi: 10.1016/0005-2760(68)90171-9. [DOI] [PubMed] [Google Scholar]
- Stone K. J., Strominger J. L. Isolation of C 55 -isoprenylpyrophosphate from micrococcus lysodeikticus. J Biol Chem. 1972 Aug 25;247(16):5107–5112. [PubMed] [Google Scholar]
- THORNE K. J., KODICEK E. The metabolism of acetate and mevalonic acid by lactobacilli. I. The effect of acetate and mevalonic acid on growth. Biochim Biophys Acta. 1962 May 21;59:273–279. doi: 10.1016/0006-3002(62)90175-0. [DOI] [PubMed] [Google Scholar]
- THORNE K. J., KODICEK E. The metabolism of acetate and mevalonic acid by lactobacilli. II. The incorporation of [14C]acetate and [14C]mevalonic acid into the bacterial lipids. Biochim Biophys Acta. 1962 May 21;59:280–294. doi: 10.1016/0006-3002(62)90176-2. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Thorne K. J., Barker D. C. The occurrence of bactoprenol in the mesosome and plasma membranes of Lactobacillus casei and Lactobacillus plantarum. J Gen Microbiol. 1972 Apr;70(1):87–98. doi: 10.1099/00221287-70-1-87. [DOI] [PubMed] [Google Scholar]
- Thorne K. J., Constable B. J., Day K. C. Technicon autoanalysis of the ninhydrin-positive phospholipids of Lactobacillus casei. Nature. 1965 Jun 12;206(989):1156–1157. doi: 10.1038/2061156b0. [DOI] [PubMed] [Google Scholar]
- Thorne K. J., Kodicek E. The structure of bactoprenol, a lipid formed by lactobacilli from mevalonic acid. Biochem J. 1966 Apr;99(1):123–127. doi: 10.1042/bj0990123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbreit J. N., Stone K. J., Strominger J. L. Isolation of polyisoprenyl alcohols from Streptococcus faecalis. J Bacteriol. 1972 Dec;112(3):1302–1305. doi: 10.1128/jb.112.3.1302-1305.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbreit J. N., Strominger J. L. Isolation of the lipid intermediate in peptidoglycan biosynthesis from Escherichia coli. J Bacteriol. 1972 Dec;112(3):1306–1309. doi: 10.1128/jb.112.3.1306-1309.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkinson R. J., Hussey H., Baddiley J. Shared lipid phosphate carrier in the biosynthesis of teichoic acid and peptidoglycan. Nat New Biol. 1971 Jan 13;229(2):57–59. doi: 10.1038/newbio229057a0. [DOI] [PubMed] [Google Scholar]
- Weiss N., Plapp R., Kandler O. Die Aminosäuresequenz des DAP-haltigen Mureins von Lactobacillus plantarum und Lactobacillus inulinus. Arch Mikrobiol. 1967;58(4):313–323. [PubMed] [Google Scholar]
- Willoughby E., Highasi Y., Strominger J. L. Enzymatic dephosphorylation of C 55 -isoprenylphosphate. J Biol Chem. 1972 Aug 25;247(16):5113–5115. [PubMed] [Google Scholar]
- Wright A., Dankert M., Fennessey P., Robbins P. W. Characterization of a polyisoprenoid compound functional in O-antigen biosynthesis. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1798–1803. doi: 10.1073/pnas.57.6.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A. Mechanism of conversion of the salmonella O antigen by bacteriophageepsilon 34. J Bacteriol. 1971 Mar;105(3):927–936. doi: 10.1128/jb.105.3.927-936.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]