Abstract
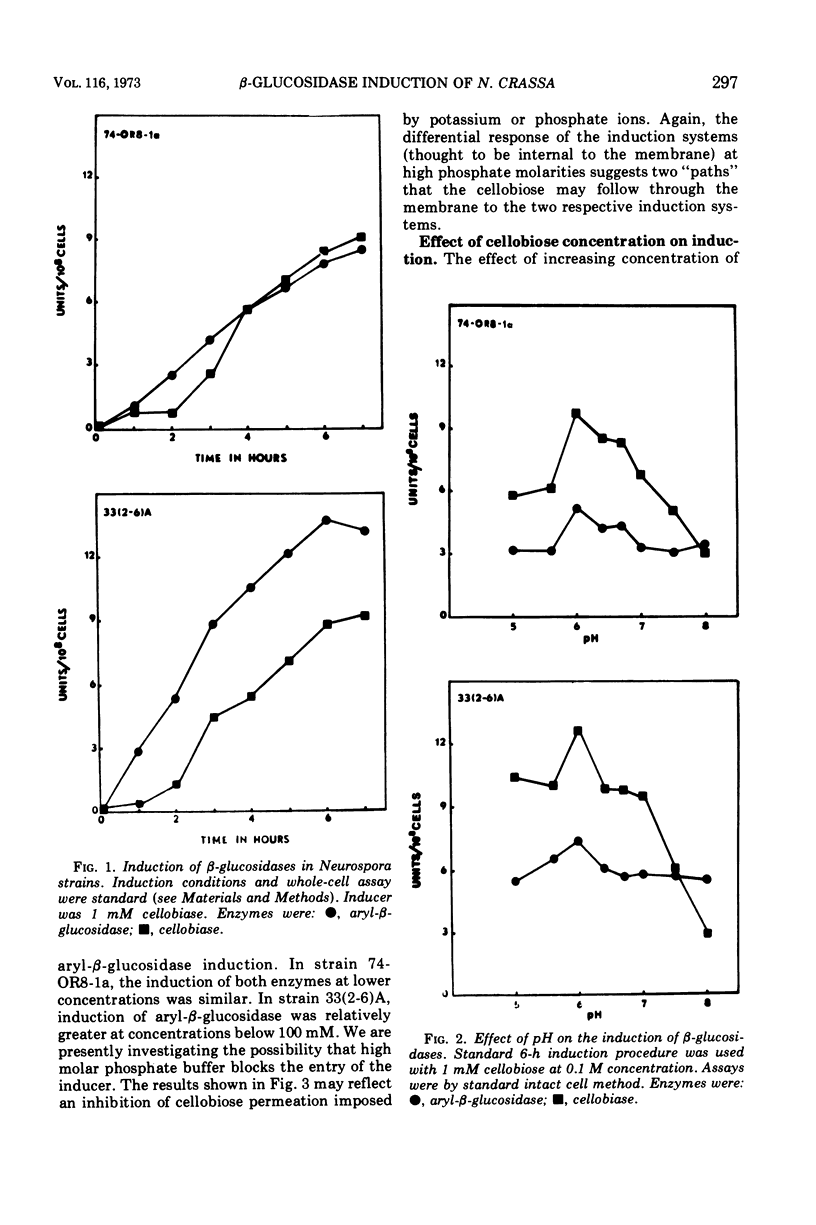
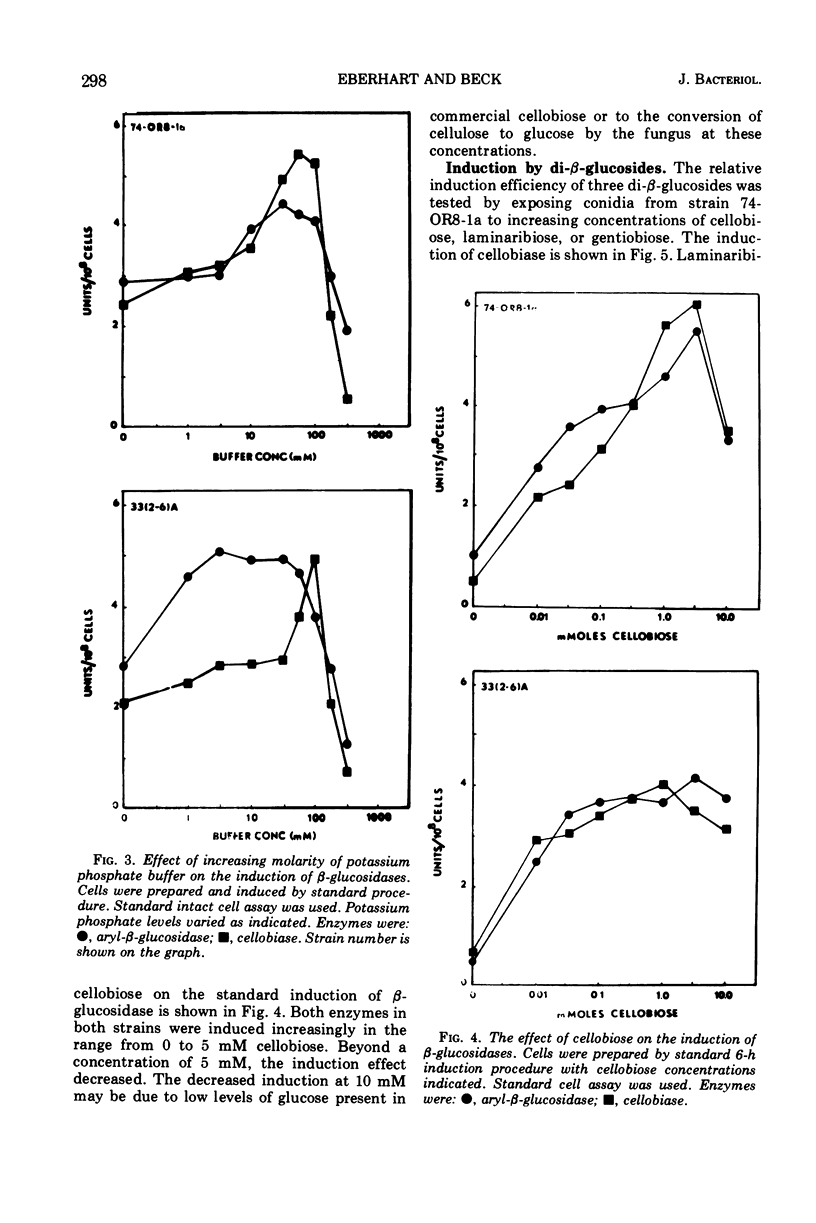
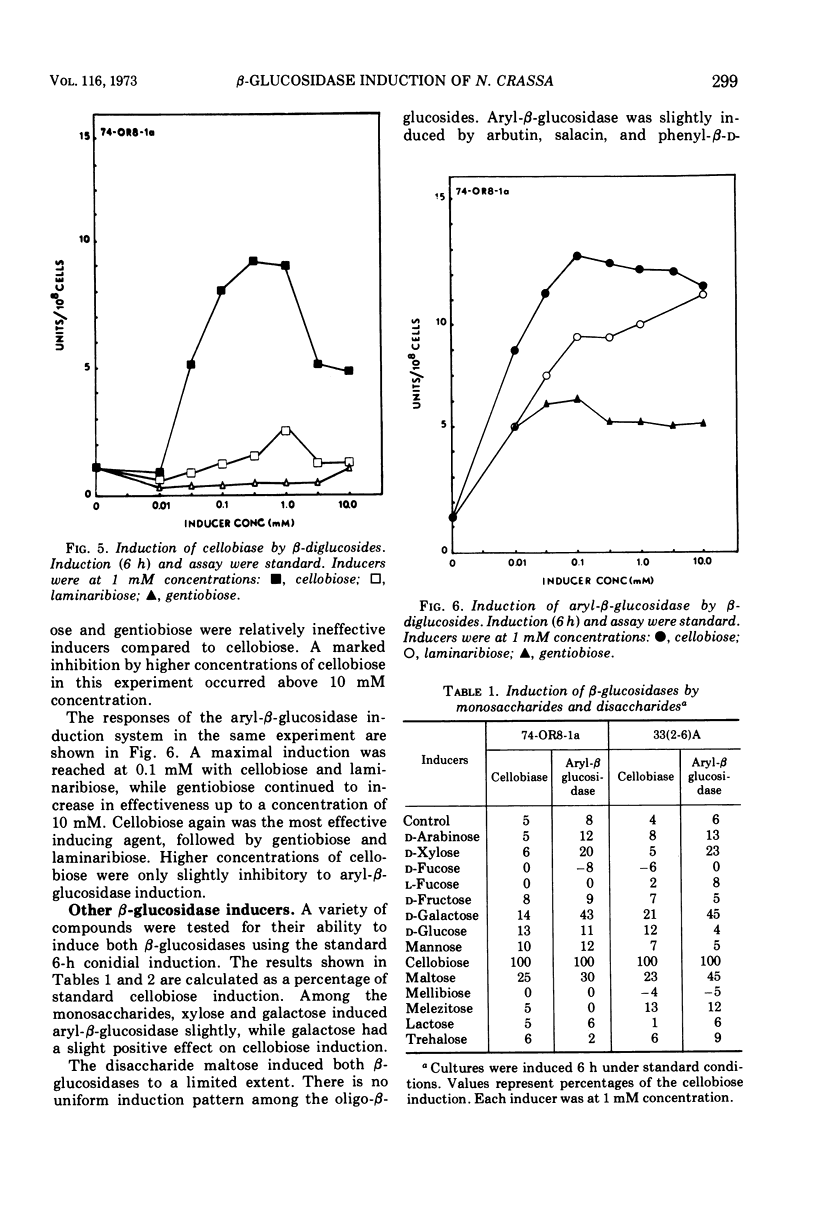
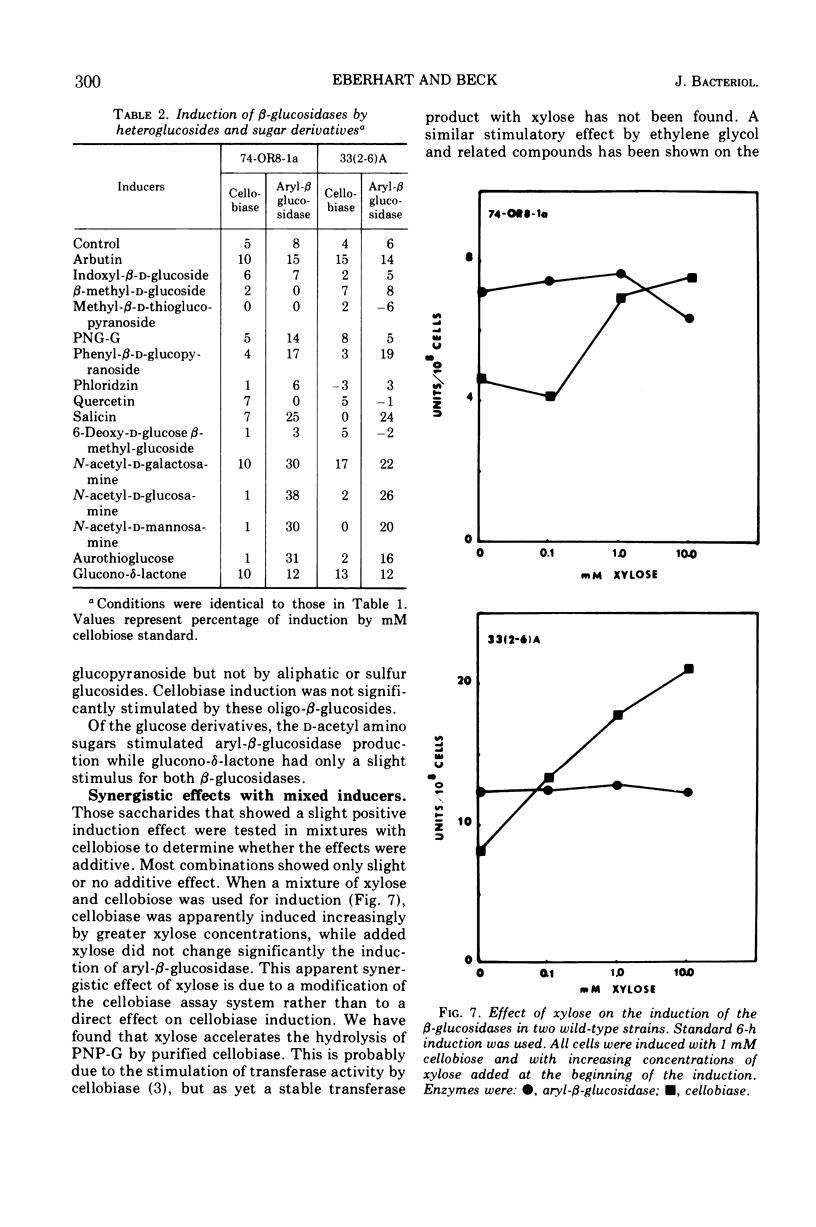
The induction of β-glucosidases (EC 3.2.1.21) was studied in Neurospora crassa. Cellobiase was induced by cellobiose, but other inducers had little effect on this enzyme. Cellobiase activity was very low in all stages of the vegetative life cycle in the absence of di-β-glucoside inducer. Aryl-β-glucosidase was semiconstitutive at late stages of culture growth prior to conidiation. At early stages, aryl-β-glucosidase was induced by cellobiose, laminaribiose, and gentiobiose, and weakly induced by galactose, amino sugars, and aryl-β-glucosides. The induction properties of the β-glucosidases are compared with those of the other disaccharidases of Neurospora. The induction of β-glucosidases was inhibited by glucose, 2-deoxy-d-glucose, and sodium acetate. Sodium phosphate concentrations between 0.01 and 0.1 M stimulated induction of both enzymes, while concentrations above 0.1 M were inhibitory. The optimal condition for induction of both β-glucosidases was pH 6.0. Cellobiase induction was relatively more inhibited than aryl-β-glucosidase in the range of pH 6.0 to 8.0.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGER L. S., EBERHART B. M. Extracellular beta-transglucosidase activity from conidia of Neurospora crassa. Biochem Biophys Res Commun. 1961 Oct 23;6:62–66. doi: 10.1016/0006-291x(61)90186-3. [DOI] [PubMed] [Google Scholar]
- Bates W. K., Hedman S. C., Woodward D. O. Comparative inductive responses of two beta-galactosidases of Neurospora. J Bacteriol. 1967 May;93(5):1631–1637. doi: 10.1128/jb.93.5.1631-1637.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUERKSEN J. D., HALVORSON H. The specificity of induction of beta-glucosidase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1959 Nov;36:47–55. doi: 10.1016/0006-3002(59)90068-x. [DOI] [PubMed] [Google Scholar]
- EBERHART B. M. Exogenous enzymes of Neurospora conidia and mycelia. J Cell Comp Physiol. 1961 Aug;58:11–16. doi: 10.1002/jcp.1030580103. [DOI] [PubMed] [Google Scholar]
- EBERHART B., CROSS D. F., CHASE L. R. BETA-GLUCOSIDASE SYSTEM OF NEUROSPORA CRASSA. I. BETA-GLUCOSIDASE AND CELLULASE ACTIVITIES OF MUTANT AND WILD-TYPE STRAINS. J Bacteriol. 1964 Apr;87:761–770. doi: 10.1128/jb.87.4.761-770.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart B. M., Beck R. S. Localization of the beta-glucosidases in Neurospora crassa. J Bacteriol. 1970 Feb;101(2):408–417. doi: 10.1128/jb.101.2.408-417.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. B., Woodward D. O. The regulation of synthesis of Krebs cycle enzymes in Neurospora by catabolite and end product repression. Eur J Biochem. 1970 Apr;13(3):548–553. doi: 10.1111/j.1432-1033.1970.tb00959.x. [DOI] [PubMed] [Google Scholar]
- Gratzner H., Sheehan D. N. Neurospora mutant exhibiting hyperproduction of amylase and invertase. J Bacteriol. 1969 Feb;97(2):544–549. doi: 10.1128/jb.97.2.544-549.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL E. P., SUSSMAN A. S. DEVELOPMENT OF TREHALASE AND INVERTASE ACTIVITY IN NEUROSPORA. J Bacteriol. 1964 Dec;88:1556–1566. doi: 10.1128/jb.88.6.1556-1566.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks D. L., Sussman A. S. Control of trehalase synthesis in Neurospora crassa. Am J Bot. 1969 Nov-Dec;56(10):1160–1166. [PubMed] [Google Scholar]
- Hanks D. L., Sussman A. S. The relation between growth, conidiation and trehalase activity in Neurospora crassa. Am J Bot. 1969 Nov-Dec;56(10):1152–1159. [PubMed] [Google Scholar]
- LANDMAN O. E. Neurospora lactase. II. Enzyme formation in the standard strain. Arch Biochem Biophys. 1954 Sep;52(1):93–109. doi: 10.1016/0003-9861(54)90092-2. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MAHADEVAN P. R., EBERHART B. THE BETA-GLUCOSIDASE SYSTEM OF NEUROSPORA CRASSA. 3. FURTHER STUDIES ON AN ARYL BETA-GLUCOSIDASE MUTANT. Arch Biochem Biophys. 1964 Oct;108:30–35. doi: 10.1016/0003-9861(64)90351-0. [DOI] [PubMed] [Google Scholar]
- MAHADEVAN P. R., EBERHART B. THE BETA-GLUCOSIDASE SYSTEM OF NEUROSPORA CRASSA. II. PURIFICATION AND CHARACTERIZATION OF ARYL BETA-GLUCOSIDASE. Arch Biochem Biophys. 1964 Oct;108:22–29. doi: 10.1016/0003-9861(64)90350-9. [DOI] [PubMed] [Google Scholar]
- MANDELS M., REESE E. T. Induction of cellulase in fungi by cellobiose. J Bacteriol. 1960 Jun;79:816–826. doi: 10.1128/jb.79.6.816-826.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METZENBERG R. L. A gene affecting the repression of invertase and trehalase in Neurospora. Arch Biochem Biophys. 1962 Mar;96:468–474. doi: 10.1016/0003-9861(62)90322-3. [DOI] [PubMed] [Google Scholar]
- Metzenberg R. L. Genetic regulatory systems in Neurospora. Annu Rev Genet. 1972;6:111–132. doi: 10.1146/annurev.ge.06.120172.000551. [DOI] [PubMed] [Google Scholar]
- Myers M. G., Eberhart B. Regulation of cellulase and cellobiase in Neurospora crassa. Biochem Biophys Res Commun. 1966 Sep 8;24(5):782–785. doi: 10.1016/0006-291x(66)90394-9. [DOI] [PubMed] [Google Scholar]
- Neville M. M., Suskind S. R., Roseman S. A derepressible active transport system for glucose in Neurospora crassa. J Biol Chem. 1971 Mar 10;246(5):1294–1301. [PubMed] [Google Scholar]
- Scott W. A., Metzenberg R. L. Location of Aryl Sulfatase in Conidia and Young Mycelia of Neurospora crassa. J Bacteriol. 1970 Dec;104(3):1254–1265. doi: 10.1128/jb.104.3.1254-1265.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


