Abstract
Advances in the field of kidney transplantation have led to a significant increase in the life of renal allograft with 1 - year graft survival rates of 93% to 99%.This increase in early graft survival has made it possible to observe the long-term morbidities that accompany renal transplantation.
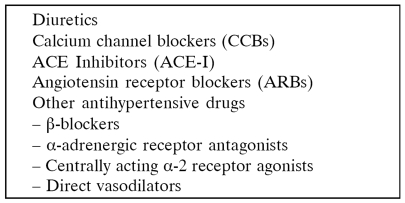
Studies correlating the reduction of arterial blood pressure with patient and graft survival as well as the risk of cardiovascular disease do not exist. The recommendations come from the general population and from comparative studies of hypertensive and normotensive kidney graft recipients. It is known that in the general population hypertension is a risk factor for chronic kidney disease but at the same time a risk factor for death, ischaemic heart disease, chronic heart failure and left ventricular hypertrophy. We must always have in mind that there are many similarities between a kidney graft recipient and a patient with chronic kidney disease. Renal transplant recipients represent a patient population with a very high risk for development of cardiovascular disease which has been identified as the leading cause of death in these patients1. Of 18,482 deaths among renal allograft recipients, 38% had functioning renal allografts 2, 3. Successful renal transplantation (Rt) can result in partial regression of left ventricular hypertrophy (LVH) if it is associated with hypertension (HTN) remission or if HTN is controlled by medications. Frequently post transplant HTN is associated with failure of LVH to regress. Transplant clinicians must choose antihypertensive agents that will provide their patients with maximum benefit from renal allograft and cardiovascular perspective. The target must always be long term patient and graft survival and acceptable quality of life. The antihypertensive drugs usually used after kidney transplantation are diuretics, calcium channel blockers, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers and β – blockers. Most emphasis is given lately to ACEIs/ARBs and β – blockers because of their cardioprotecive effect.
Keywords: kidney transplantation, hypertension, anti - hypertensive agents
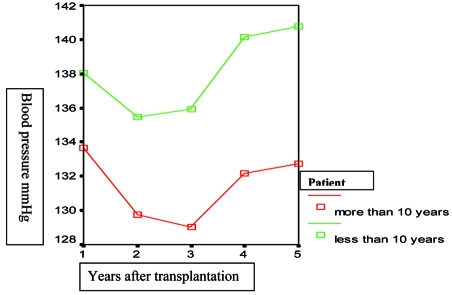
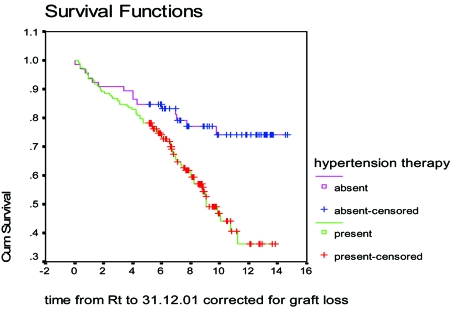
Posttransplant hypertension, perhaps the comorbidity with the greatest concern, occurs in about 70% to 90% of renal transplant recipients (Figure 1) in the cyclosporine era4–6. Systolic blood pressure7 (Figure 2) as well as the pulse pressure (unpublished data) of recipients with graft function longer than 10 years is significantly lower compared with those of patients with graft survival >1 and < 10 years. Posttransplant hypertension has been recognized as an independent risk factor for chronic allograft dysfunction-nephropathy and graft loss8, 9 (Figure 3). Finally hypertension causes cardiac hypertrophy and is associated with increased cardiac morbidity and patient mortality in both the general and transplant populations2, 8, 10–12 (Table 1).
Figure 1. Five year follow up of systolic and diastolic blood pressure after kidney transplantation• 272 patients, period 1987-1995 (normal blood pressure considered to be systolic > 140 mmHg and diastolic > 90 mmHg) • Frequency on 7th pt day: 72.7%, on 5th year:67.6%.
Figure 2. Five year systolic blood pressure of patients with graft survival > 10 years and patients with graft survival <1 and > 10 years graft survival (p: 0.01).
Figure 3. Graft survival in patients with normal blood pressure (red line) and hypertension (green line)9.
Table 1. Cardiovascular disease as leading cause of death2, 3.
Etiology and mechanisms causing hypertension after renal transplantation
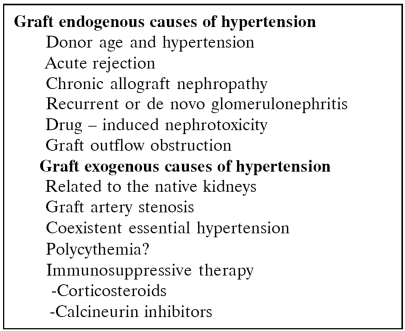
Many factors have been incriminated for the development of hypertension after renal transplantation (Table 2). Donor hypertension and death due to subarachnoid hemorrhage have been connected with higher blood pressure of the recipient. Recurrence of primary renal disease has been considered responsible for hypertension after a renal transplant. Hypertension is common among patients with acute or hyperacute rejection and this is due to impairment of graft excretory function. Renal artery stenosis may cause hypertension not responding to antihypertensive drugs.
Table 2. Etiology of hypertension after renal transplantation13.
Uncontrolled renin secretion from the native kidneys can be responsible for hypertension in the renal transplant recipient.
Treatment of hypertension after renal transplantation
Treatment of elevated blood pressure in renal transplant recipients significantly reduces morbidity and mortality14, 15. Aggressine treatment of hypertemsion must be voidel the first few days after transplantation. A systolic blood pressure of 140 – 160 mmHg and a diastolic < 90 mmHg is preferred in order to achieve a sufficient blood perfusion of the transplanted kidney. When the systolic blood pressure is > 200 mm Hg and diastolic blood pressure is > 120 mm Hg, hypertension may precipitate a medical emergency requiring rapid reduction of blood pressure to prevent vital organ damage.
The final regulation of blood pressure should be managed on an outpatient basis (Table 3) in a stable transplant recipient. Multiple factors may play important role in the fluctuation of blood pressure. It is important to take into account life style, the associated risk factors and the immunosuppressive regimen. So, the treatment of hypertension is individualized (Table 3, 4).
Table 3. Therapeutic approach of hypertension in renal transplant recipients.
Table 4. Adjustment of immunosuppressive medications.
Immunosuppression manipulation and arterial hypertension
Corticosteroids
During the initial high dose corticosteroid therapy, sodium retention occurs and plasma volume increases substantially (mineralocorticoid action) and leads to an increase of blood pressure and cardiac output16. Other postulated mechanisms of corticosteroids causing hypertension include increased sensitivity to endothelin-1 and angiotensin II leading to increased vascular resistance, increased density of glucocorticoid receptors in the vascular muscle and decreased production of vasodilatory prostaglandins5, 17, 18. The daily dose and cumulative dose of prednisone are related to blood pressure19, 20. High long term maintenance dose may cause or aggravate hypertension. The estimated incidence of arterial hypertension induced by corticosteroids is 15% 21. The conversion from every day to alternate day corticosteroid therapy may cause a reduction in arterial blood pressure. Steroid withdrawal is associated with fall of arterial blood pressure22. The lowest possible corticosteroid dose coupled with low salt diet and judicious use of diuretics is an interesting option.
Calcineurin inhibitors: Cyclosporine - tacrolimus
Both CsA and tacrolimus cause hypertension17. The mechanism is multifactorial, causes reduction in GFR and renal blood flow, and includes increase in sympathetic nervous tone, increase of intra-renal vascular resistance and increased sodium retention, a rise in peripheral vascular resistance and vasoconstriction of the afferent arterioles which produces vessel wall damage and impaired autoregulatory function17, 23, 24. These may be the result of imbalance between vasoconstrictive (endothelin and thromboxane) and vasodilator factors (NO and prostacyclin) in CsA and tacrolimus treated patients25, 26. Endothelin may play an important role in the development of hypertension in these patients27. Calcinurin is present in several tissues including the kidney, vascular smooth muscle and nervous system, all of which are targets for hypertension. Possibly, calcinurin inhibition in these tissues is the cause of the increased sodium and water retention, vasoconstriction and sympathetic activity28. Hypertension in patients taking cyclosporine in combination with corticosteroids has been described in 50% to 80% of cases26. CsA induced hypertension is characterized by nocturnal hypertension25.
Tacrolimus has been associated with less systemic vasoconstriction and less arterial hypertension than CsA in liver transplant patients29. The most recent European multicentre study comparing tacrolimus with CsA microemulsion in kidney transplantation showed that the incidence of new onset or worsening hypertension was less common in the tacrolimus group30. In general, the existing data today suggest that renal transplant patients under tacrolimus based therapy show less arterial hypertension compared with patients under CsA immunosuppression31.
Mycophenolate Mofetil
All the available literature support that MMF is not nephrotoxic and does not have any hypertensive effect, does not cause diabetes or hyperlipidaemia22, 32–34.
Sirolimus
In Phase III studies using sirolimus in combination with CsA, there was a higher frequency of hypertension than control groups. It is possible that sirolimus potentiates the nephrotoxic effect of cyclosporine, which would explain the increase of blood pressure35.
Immunosuppressive protocols
Immunosuppression protocols using MMF or azathioprine with CsA and steroids did not show difference in the incidence of hypertension36. Protocols with steroid low dose and discontinuation present lower incidence of hypertension compared to triple scheme37. These protocols have the drawback of high acute rejection rate38.
The dose reduction or discontinuation of cyclosporine, in protocols with MMF and steroids, results in lower systolic and diastolic blood pressure39.
Protocols based on sirolimus (plus steroid and azathioprine/MMF) show lower systolic and diastolic blood pressure but renal function at two years was lower in sirolimus treated compared to cyclosporine treated patients40.
Use of antihypertensive drugs
The selection of antihypertensive therapy in the kidney transplant recipient should be guided, in part, on prior history of success of antihypertensive medications in facilitating blood pressure control before the transplant. In spite the use of new antihypertensive agents, the best treatment of arterial blood pressure after transplantation is not known yet. No single antihypertensive agent (Table 5) has been found to be more efficacious and better tolerated than the others used in the treatment of posttransplant hypertension and the initial antihypertensive therapy must aim at the patient’s risk factors. We diagnose hypertension when the blood pressure is > 130 / 85 mmHg in Rt recipients without proteinuria, or it is > 125 / 75 mmHg in Rt with proteinuria41–43.
Table 5. Pharmacological therapy.
Diuretics (Table 6,7)
Table 6. Diuretics50.
Table 7. Dose, renal effects, dosage adjustment in renal failure of the more commonly used diuretics in renal transplantation.
A careful assessment of blood volume is important in all kidney transplant recipients because they frequently receive corticosteroids and calcinurin inhibitors which may interfere with sodium and water excretion. Depending on the level of the kidney function a thiazide or a loop diuretic may be appropriate.
The mechanism by which diuretics reduce blood pressure is not known. The first period after starting diuretic therapy, the control of blood pressure is by volume depletion. Later possibly there is a vascular hyporesponsiveness to the sympathetic nervous system44. The use of diuretics is mandatory in the immediate post-transplant period in case of hypertension and fluid overload. Loop diuretics are the drugs of choice, alone or in combination with CCBs, especially in cases with urine output less than 50 ml/h and gross haematuria. Sodium load, food-drug interactions and drug-drug interactions may lessen or ablate the effectiveness of diuretic therapy45–47. Loop diuretics can cause prerenal azotemia, hypokalemia, hyperuricemia, hypocalcaemia and exacerbate hyperparathyroidism. A potential problem associated with hypokalemia is interference with insulin release and subsequent impairment of glycemic control. The extended use of diuretics and calcineurin inhibitors may require close electrolyte monitoring to avoid gout and cardiac mortality associated with low magnesium levels48. On the contrary thiazide diuretics may induce hypercalcaemia and potassium sparing agents hyperkalemia. The use of cyclosporine, FK506, ACE inhibitors, b-blockers and cotrimoxazole has been connected with hyperkalemia. Hyperuricaemia and hypomagnessaemia are complications of cyclosporine and tacrolimus therapy49.
Calcium channel blockers (CCBs)
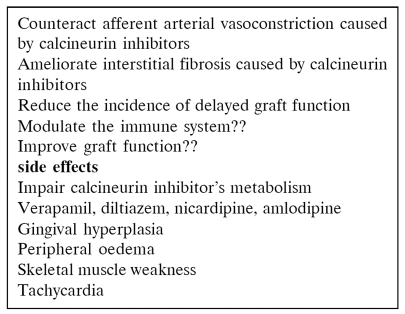
CCBs are usually well tolerated and it has been proved that reduce mean arterial pressure and total renal vascular resistance, provide selective afferent glomerular arterioral dilatation, increase renal blood flow and GFR, reduce cyclosporine toxicity and combat the vasoconstrictive effect of CNIs, cause natriuresis, decrease perfusion injury and the rate of acute tubular necrosis immediately after transplantation because they offer protection to tubular epithelium and may reduce CsA induced hyperuricaemia51–56. The main action of these drugs is the inhibition of entrance of calcium into the smooth muscles of vasoconstricted arterioles through voltage potential-dependent channels (Table 8). For the above reasons, it has been supported that calcium antagonists are the drugs of choice. The use of CCBs after the recovery of cold ischemia time acute tubular necrosis (ATN) and normalization of graft function must target to normal levels of systolic blood pressure (< 130 mmHg) because the afferent arteriolar dilatation conveys the systemic blood pressure into the glomerulus and in long term may result in kidney damage if the systolic blood pressure is not well controlled. However, in a comparative clinical trial, it was found that a calcium antagonist, an ACE-I and an a-blocker were equally effective in reducing blood pressure57 and patient and graft survival did not show difference with the use of b-blockers and / or calcium antagonists in a 5-year follow up58. In a recent longitudinal study it has been suggested that beta blockers as well as ACEIs may have a beneficial effect on cardiovascular mortality and on patient and graft survival over calcium antagonists59.
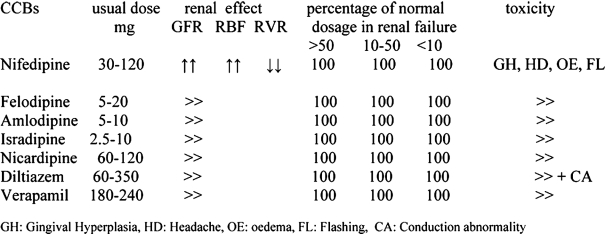
Table 8. Characteristics of CCBs60.
Isradipine (Lomir) and nifedipine (Coracten, Macorel, Adalat) are probably the most effective drugs among calcium channel antagonists and do not cause increase of cyclosporine levels as it happens with diltiazem, verapamile and nicardipine24, 61, 62. These drugs are potent vasodilators and may cause dizziness, flushing, headache, leg oedema and gum hyperplasia63–65.
These adverse effects (Table 9) can be minimised by the use of slow release formulations or with agents of a slow onset of action. It has been reported that short – acting calcium antagonists, given in non-transplanted patients, may increase the mortality in those with a recent history of acute myocardial infarction or coronary heart disease66. Recently, safety has proved not to be a problem with calcium channel blockers in hypertensives treated in the ALLHAT study even in those with chronic renal failure67. CCBs present robust antihypertensive action even in the presence of concomitant salt consumption. Clinical trials have shown that nondihydropyridine CCBs are more effective in reducing proteinuria compared with dihydropyridines (such as nifedipine). The combination of CCBs with an ARB or ACE-I reduces further the proteinuria68, 69.
Table 9. Dose, renal effects, dosage adjustment and toxicity of the more commonly used CCBs in renal transplantation.
GH: Gingival Hyperplasia, HD: Headache, OE: oedema, FL: Flashing, CA: Conduction abnormality
Angiotensin converting enzyme inhibitors (ACE-I)
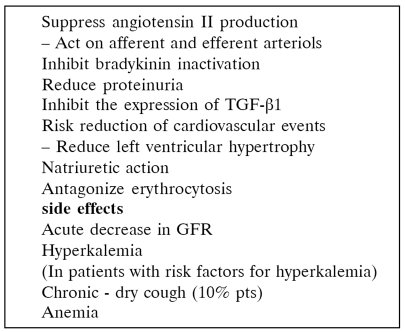
The basic haemodynamic effect of ACE-I is vasodilatory via suppression of angiotensin II production and inhibition of bradikinin inactivation (Table 10). Also, it has been suggested that ACE inhibitors retard the evolution of glomerulosclerosis and chronic decline of renal function, controlling arterial blood pressure and possibly normalizing intraglomerular hemodynamics70, 71.
Table 10. Characterists of ACE-I72,73.
The use of ACE-Is in post-transplant hypertension had been a matter of debate for a long time. In recent clinical studies with a long term follow up in renal transplant patients, ACE-Is have been shown to be effective in the treatment of post-transplant hypertension. There was no difference between ACE-Is’ and calcium antagonists’ antihypertensive effect. Also adrenal plasma flow and GFR were similar in both groups of patients70, 74. Mourard compared nifedipine plus atenolol with lisinopril plus furosemide and found no differences between the two groups as far as the antihypertensive efficacy, the adverse drug reaction profile, the effect on plasma renal flow and GFR. It has been shown that ACE-Is reduce significantly the proteinuria of transplanted patients with chronic allograft nephropathy75.
The decline of renal function (20% - 30%) is due to foll of glomerular capillary pressure and GFR. If there is a more than 30% decrease after treatment with ACE inhibitors it is possibly related to the existence of renal artery stenosis or it may happen in grafts with normal parenchyma when the patient is dehydrated76–78. The use of these drugs has been connected with hyperkalemia, especially in diabetics, and anemia79 (Table 11).
Table 11. Dose, renal effects, dosage adjustment in renal failure and toxicity of most commonly used ACE-Is in renal transplantation.
CA:conduction abnormality, C:cough, A:angioedema, H:hyperkalaemia, An:anaemia
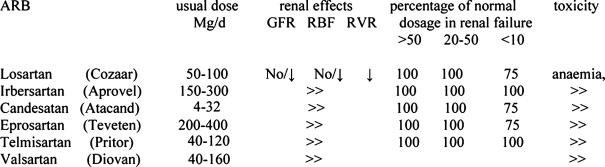
Angiotensin II receptor antagonists (ARBs)
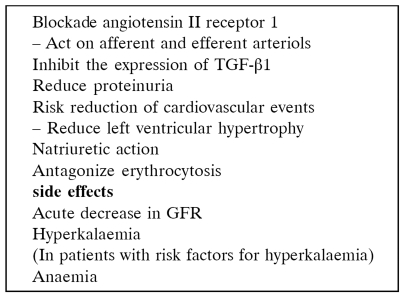
ARBs reduce the capillary pressure, the GFR and the systemic blood pressure by blocking the physiological response to angiotensin II80, 81 (Table 12). It is best to start these drugs during the posttransplant period when kidney function and immunosuppression is stable.
Table 12. Angiotensin receptor blockers.
The recent successful use of angiotensin II receptor antagonists type I has added a new group of drugs in the treatment of renal transplant hypertension71, 82, 83.
These agents have a significant control on the blood pressure, reduce the need for other antihypertensive agents82, 83 and reduce statistically significantly proteinuria (Figure 4, Table 13) of patients with chronic allograft disease84. These agents do not seem to interfere with any of the immunosuppressive agents.
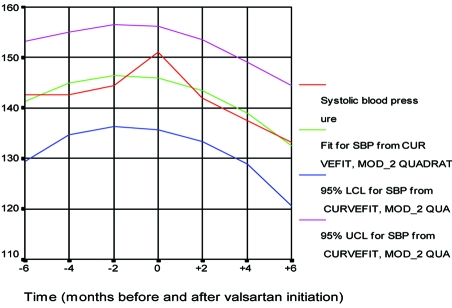
Figure 4. The systolic blood pressure six months before and six months after valsartan treatment Vergoulas G, et al. Hippokratia 2001; 5:61-68.
Table 13. Proteinuria and number of antihypertensive drugs at baseline and six months after treatment with ARBs84.
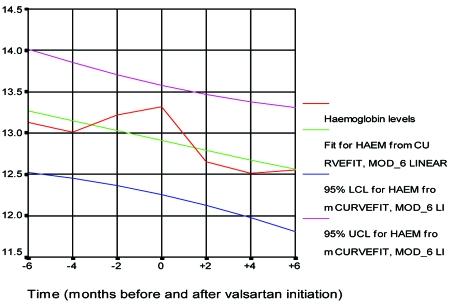
Stimulation of AT1 receptors of erythroid progenitor cells by Ag II is believed to increase red blood cell mass independently from circulating erythropoietin. AT1 blockers cause statistically significant fall of Ht and Hb (Figure 5) and are very useful in hypertensive patients with erythremia82, 83. It is believed that the blockade of AT1 receptors results in a decrease of red blood-cell mass independently of erythropoietin and initial haemoglobin levels85, 86.
Figure 5. Hb levels six months before and six months after valsartan treatment. Vergoulas G, et al. Hippokratia 2001; 5:61-68.
In some patients supplementation with erythropoietin may be required and some may consider this as an inappropriate trade-off. However the opportunity to reduce both kidney and cardiovascular risks simultaneously in patients with greater cardiovascular burden may be an important consideration.
These drugs have the ability to lower significantly the levels of TGF-β1 in the plasma of transplanted patients with chronic allograft nephropathy87. Usually, they do not cause hyperkalemia or hyperuricaemia and cause a slight but significant rise of serum creatinine level88 (Table 14). A rise of serum creatinine of 20-30% is acceptable but always must be kept in mind the possibility that the rise is due to rejection. The reduction of GFR correlates with stabilization of kidney function over time possibly due to an anatomical protection of kidney89. Serum creatinine level increase by more than 30% could indicate diminished effective arterial blood volume because of diuretic use or renal artery stenosis. From the first four drugs of this class of antihypertensives (losartan, valsartan, irbersartan, candesartan) it seems that losartan has the weaker antihypertensive effect and candesartan the stronger90–92.
Table 14. Dose, renal effects, dosage adjustment in renal failure and toxicity of ARBs in renal transplantation.
The drugs of this category could be proved valuable not only for the treatment of hypertension of renal transplant recipients but also for the intervention in the evolution of chronic allograft nephropathy by interfering with profibrogenic and scarring processes within the kidney93–95 and this possibly is the great difference between ARBs and ACE-Is because much production of angiotensin II takes place by non-ACE pathways both systemically and at the tissue level. These alternative pathways include direct formation from angiotensinogen, cathepsin G, and tissue plasminogen activation. Angiotensin I also can be converted to angiotensin II at the tissue level by chymase and cathepsin G. These local pathways are very important because tissue levels of angiotensin II are nearly 1,000 times greater than the levels in the circulation. Angiotensin II converting enzyme inhibitors have no effect on angiotensin II formed by these alternate pathways96–100. Another point in favour of ARBs is the high percentage of patients that can not tolerate ACE-I101, 102.
There have been some concerns about the early administration of ACEIs and ARBs after transplantation because of the fear of ATN development or DGF. Recently it has been supported that the early administration of ACEis/ARBs after transplantation is associated with faster decrease of serum creatinine levels, faster recovery time from delated graft function (DGF) and lower proteinuria103.
It must be noticed that ACEIs/ARBs improve left ventricular function and reduce left ventricular hypertrophy due to hypertension in general population and possibly they have the same effect on the kidney transplant recipients with hypertension104–106.
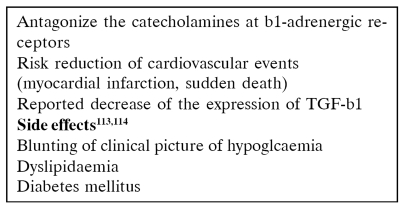
B - blockers (Table 15)
Table 15. β-Blockers111,112.
The precise mode of action of β-blockers in reducing blood pressure is not known. In a small study of the effect of β-blockers on hypertensive renal transplant recipients, a blood pressure reduction was noticed only in hypertensive patients with their native kidneys in situ107. Native kidneys may play an important role in activation of renin - angiotensin system mediated by activation of the sympathetic nervous system. In a randomized study renal transplant recipients received atenolol or quinapril. In both groups blood pressure control was achieved. However quinapril lowered significantly albumin excretion108.
β-Blockers may mask the symptoms of hypoglycaemia and thyrotoxicosis and cause sexual dysfunction, muscle weakness, tiredness and fatigue. Abrupt discontinuation of β-blockers may exacerbate rebound hypertension and so the dosage should be reduced slowly over a 1- to 2-week period.
They can also complicate the lipid profile in renal transplant recipients. The beneficial effect of β-blockers on transplant recipients, with a history of MI, CHD, hypertrophic cardiomyopathy or systolic heart failure outweigh the risk of their adverse effects109.
The use of mTOR inhibitors may complicate further the problem of hyperlipidemia. Newer third generation β-blockers such as carvedilol (Dilatrend®) may solve the problem since they have neutral or positive effect on dyslipidemia and insulin resistance110. β-Blockers should be considered first choice treatment together with the ACI / ARBs for patients with a renal transplant and a history of coronary heart disease.
References
- 1.U.S. Renal Data Systems. Bethesda, MD: The National Institutes of Health- National Institute of Diabetes, Digestive and Kidney Diseases; 1998. USRDS 1998 annual report. [Google Scholar]
- 2.Ojo AO, Hunson GA, Wolfe RA, et al. Long – term survival in renal transplant recipients with graft function. Kidney Int. 2000;57:307–313. doi: 10.1046/j.1523-1755.2000.00816.x. [DOI] [PubMed] [Google Scholar]
- 3.Riggato C, Folley RN, Kent GM, Guttman R, Parfrey PS. Long-term changes in left ventricular hypertrophy after renal transplantation. Transplantation. 2000;70:570–575. doi: 10.1097/00007890-200008270-00006. [DOI] [PubMed] [Google Scholar]
- 4.Jarowenko MV, Flechner SM, Van Buren CT, et al. Influence of cyclosporine on posttransplant blood pressure response. Am J Kidney Dis. 1987;10:98–103. doi: 10.1016/s0272-6386(87)80039-2. [DOI] [PubMed] [Google Scholar]
- 5.Zeier M, Mandelbaum A, Ritz E. Hypertension in the transplant patient. Nephron. 1998;80:257–268. doi: 10.1159/000045184. [DOI] [PubMed] [Google Scholar]
- 6.Vergoulas G, Miserlis Gr, Karasavvidou F, et al. The blood pressure after renal transplantation. A single centre experience. Hippokratia. 2002;6:62–70. [Google Scholar]
- 7.Vergoulas G, Tasiopoulou C, Angelou A, et al. Risk factors affecting ten year kidney graft survival. 2nd World Congress on "Quality in Clinical Practice"; 3-6 June 2004; Porto Carras, Chalkidiki. 1985. p. 108. (poster) [Google Scholar]
- 8.Opelz G, Wujciak T, Ritz E. Association of chronic kidney graft failure with recipient blood pressure. Kidney Int. 1998;53:217–222. doi: 10.1046/j.1523-1755.1998.00744.x. [DOI] [PubMed] [Google Scholar]
- 9.Vergoulas G, Miserlis Gr, Atmatzidis E, et al. Clinical risk factors on patient and graft survival after kidney transplantation. BANTAO Journal. 2003;1:261–263. [Google Scholar]
- 10.Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first line agents: a systematic review and meta - analysis. JAMA. 1997;277:739–745. [PubMed] [Google Scholar]
- 11.Howard RJ, Patton PR, Reed AI, et al. The changing causes of grft loss and health after kidney transplantation. Transplantation. 2002;73:1923–1928. doi: 10.1097/00007890-200206270-00013. [DOI] [PubMed] [Google Scholar]
- 12.Mange KC, Cizman B, Joffe M, Feldman HI. Arterial hypertension and renal allograft survival. JAMA. 2000;283:633–638. doi: 10.1001/jama.283.5.633. [DOI] [PubMed] [Google Scholar]
- 13.Vergoulas G, Miserlis Gr, Karasavvidou F, et al. The blood pressure after renal transplantation. A single centre experience. Hippokratia. 2001;6:62–70. [Google Scholar]
- 14.Kokado Y, Takahara S, Kameoka H, et al. Hypertension in renal transplant recipients and its effect on long term allograft survival. Transplant Proc. 1996;28:1600–1602. [PubMed] [Google Scholar]
- 15.Cosio FG, Falkenhain ME, Pesavento TE, et al. Relationships arterial hypertension and renal allograft survival in African-American patients. Am J Kidney Dis. 1997;29:419–417. doi: 10.1016/s0272-6386(97)90204-3. [DOI] [PubMed] [Google Scholar]
- 16.Whitworth JA. Mechanisms of glucocorticoid - induced hypertension. Kidney Int. 1987;31:1213–1224. doi: 10.1038/ki.1987.131. [DOI] [PubMed] [Google Scholar]
- 17.Kutkuhn B, Hollenbeck M, Weskoff A, et al. Renin secretion and captopril stimulation in hypertensive renal transplant recipients. Urol Int. 1994;52:82–86. doi: 10.1159/000282579. [DOI] [PubMed] [Google Scholar]
- 18.First MR, Neylan JF, Rocher LL, Tejani A. Hypertension after renal transplantation. J Am Soc Nephrol. 1994;4(Suppl 1):S30–S38. doi: 10.1681/ASN.V48s30. [DOI] [PubMed] [Google Scholar]
- 19.Taler SJ, Textor SC, Canzanello VJ, et al. Role of steroid dose in hypertension early after liver transplantation with tacrolimus and cyclosporine. Transplantation. 1996;62:1588–1592. doi: 10.1097/00007890-199612150-00011. [DOI] [PubMed] [Google Scholar]
- 20.Fryer JP, Granger DK, Leventhal JR, et al. Steroid related complications in the cyclosporine era. Clin Transplant. 1994;8:224–228. [PubMed] [Google Scholar]
- 21.Veenstra DL, Best JH, Hornberger J, et al. Incidence and long term cost of steroid related side effects after renal transplantation. Am J Kidney Dis. 1999;33:829–833. doi: 10.1016/s0272-6386(99)70414-2. [DOI] [PubMed] [Google Scholar]
- 22.Κasiske BL, Ballantyne CM. Cardiovascular risk associated with immunosuppression in renal transplantation. Transplant Rev. 2002;16:1–21. [Google Scholar]
- 23.van den Dorpel MA, van der Meiracker AH, Lameris TW, et al. Cyclosporine A impairs the nocturnal blood pressure fall in renal transplant recipients. Hypertension. 1996;28:304–307. doi: 10.1161/01.hyp.28.2.304. [DOI] [PubMed] [Google Scholar]
- 24.de Mattos AM, Olyaei AJ, Bennett WM. Pharmacology of immunosuppressive medications used in renal diseases and transplantation. Am J Kidney Dis. 1996;28:631–637. doi: 10.1016/s0272-6386(96)90246-2. [DOI] [PubMed] [Google Scholar]
- 25.Taler SJ, Textor SC, Canzanello VJ, et al. Cyclosporine–induced hypertension: Incidence, pathogenesis and management. Drug Saf. 1999;20:437–457. doi: 10.2165/00002018-199920050-00004. [DOI] [PubMed] [Google Scholar]
- 26.Haas M, Mayer G. Cyclosporine A – associated hypertension. Pathomechanisms and clinical consequences. Nephrol Dial Transplant. 1997;12:395–397. doi: 10.1093/oxfordjournals.ndt.a027761. [DOI] [PubMed] [Google Scholar]
- 27.Takeda Y, Mitamori I, Wu P, et al. Effects of an endothelin receptor antagonist in rats with cyclosporine induced hypertension. Hypertension. 1995;26:932–937. doi: 10.1161/01.hyp.26.6.932. [DOI] [PubMed] [Google Scholar]
- 28.Sander M, Lyson T, Thomas GD, et al. Sympathetic neural mechanisms of cyclosporine induced hypertension. Am J Hypertens. 1996;9(Suppl):S124–S127. doi: 10.1016/0895-7061(96)00288-9. [DOI] [PubMed] [Google Scholar]
- 29.Textor SC, Weisner R, Wilson DJ, et al. Systemic and renal hemodynamic differences between FK506 and cyclosporine in liver transplant recipients. Transplantation. 1993;55:1332–1337. doi: 10.1097/00007890-199306000-00023. [DOI] [PubMed] [Google Scholar]
- 30.Margreiter R. Efficacy and safety of tacrolimus compared with cyclosporine microemulsion in renal transplantation. A randomized multicentre study. The European Tacrolimus vs Ciclosprine micoeulsion Renal Transplantation Study Group. Lancet. 2002;359:741–746. doi: 10.1016/S0140-6736(02)07875-3. [DOI] [PubMed] [Google Scholar]
- 31.Morales JM. Influence of the new immunosuppressive combinations on arterial hypertension after renal transplantation. Kidney Int. 2002;62(Supp l82):S81–S87. doi: 10.1046/j.1523-1755.62.s82.16.x. [DOI] [PubMed] [Google Scholar]
- 32.Platz DP, Sollinger HW, Hullet DA, et al. RS-61443, a new potent immunosuppressive agent. Transplantation. 1991;51:27–31. doi: 10.1097/00007890-199101000-00003. [DOI] [PubMed] [Google Scholar]
- 33.European Mycophenolate Mofetil Cooperative Study Group. Placebo-controlled study of mycophenolate mofetil combined with cyclosporine and corticosteroids for prevention of acute rejection. Lancet. 1995;345:131–1325. [PubMed] [Google Scholar]
- 34.Ojo AO, Meier–Kriesche HU, Hanson JA, et al. Mycophenolate mofetil reduces late renal allograft loss independently of acute rejection. Transplantation. 2000;69:2405–2409. doi: 10.1097/00007890-200006150-00033. [DOI] [PubMed] [Google Scholar]
- 35.Kahan BD, Camardo JS. Rapamycin: Clinical results and future opportunities. Transplantation. 2001;72:1181–1193. doi: 10.1097/00007890-200110150-00001. [DOI] [PubMed] [Google Scholar]
- 36.Sollinger HW. U.S. Renal Mycophenolate Mofetil Study Group. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. Transplantation. 1995;60:225–232. doi: 10.1097/00007890-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Vanrenterghem Y, Lebranchu Y, Hene R, et al. Double blind comparison of two cortical regimens plus mycophenolate ofetil and cyclosporine for prevention of acute renal allograft rejection. The Steroid Dosing Study Group. Transplantation. 2000;70:1352–1359. doi: 10.1097/00007890-200011150-00015. [DOI] [PubMed] [Google Scholar]
- 38.Steroid Withdrawal Study Group. Prednisone withdrawal in kidney transplant recipients on cyclosporine and mycophenolate mofetil. A prospective randomised trial. Transplantation. 1999;68:1865–1874. doi: 10.1097/00007890-199912270-00009. [DOI] [PubMed] [Google Scholar]
- 39.Schnuelle P, Van DerHeide JH, Tegzess A, et al. Open randomized trial comparing early withdrawal of either cyclosporine or mycophenolate mofetil in stable renal transplant recipients initially treated with a triple drug regimen. J Am Soc Nephrol. 2002;13:536–543. doi: 10.1681/ASN.V132536. [DOI] [PubMed] [Google Scholar]
- 40.Morales JM, Wramner L, Kreis H, et al. Sirolimus does not exhibit nephrotoxicity compared to cyclosporine in renal transplant recipients. Am J Transplant. 2002;2:436–442. doi: 10.1034/j.1600-6143.2002.20507.x. [DOI] [PubMed] [Google Scholar]
- 41.National Kidney Foundation k/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(suppl):176–182. [PubMed] [Google Scholar]
- 42.Mailloux LU, Levey AS. Hypertension in patients with chronic renal disease. Am J Kidney Dis. 1998;32(Suppl 5):S120–S141. doi: 10.1053/ajkd.1998.v32.pm9820471. [DOI] [PubMed] [Google Scholar]
- 43.European best practice guidelines. Nephrol Dial Transpalnt. 2002;17(Suppl 4):S25–S26. [Google Scholar]
- 44.Neutel JM. Metabolic manifestations of low-dose diuretics. Am J Med. 1996;101:71S–82S. doi: 10.1016/s0002-9343(96)00270-7. [DOI] [PubMed] [Google Scholar]
- 45.Johnson AG, Nguyen TV, Day RO. Do non steroidal antiinflammatory drugs affect blood pressure? A meta-analysis. Ann Intern Med. 1994;121:289–300. doi: 10.7326/0003-4819-121-4-199408150-00011. [DOI] [PubMed] [Google Scholar]
- 46.Swan SK. Diuretic strategies in patients with renal failure. Drugs. 1994;48:380–385. doi: 10.2165/00003495-199448030-00005. [DOI] [PubMed] [Google Scholar]
- 47.Ellison DH. The physiologic basis of diuretic synergism: its role in treating diuretic resistance. Ann Intern Med. 1991;114:886–894. doi: 10.7326/0003-4819-114-10-886. [DOI] [PubMed] [Google Scholar]
- 48.Hoes AW, Grobbee DE, Lubsen J, et al. Diuretics, beta blockers and the risk for sudden cardiac death in hypertensive patients. Ann Intern Med. 1995;123:481–487. doi: 10.7326/0003-4819-123-7-199510010-00001. [DOI] [PubMed] [Google Scholar]
- 49.West C, Carpenter BJ, Hakala TR. The incidence of gout in renal transplant recipients. Am J Kidney Dis. 1995;123:481–487. doi: 10.1016/s0272-6386(87)80103-8. [DOI] [PubMed] [Google Scholar]
- 50.Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36:646–661. doi: 10.1053/ajkd.2000.16225. [DOI] [PubMed] [Google Scholar]
- 51.Dawidson I, Rooth P. Improvement of cadaver renal transplantation outcomes with verapamil. A review. Am J Med. 1991;90:37S. doi: 10.1016/0002-9343(91)90484-f. [DOI] [PubMed] [Google Scholar]
- 52.Bia MJ, Tyler K. Evidence that calcium channel blockade prevents cyclosporine-induced exacerbation of renal ischemic injury. Transplantation. 1991;51:293–295. doi: 10.1097/00007890-199102000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Dawidson I, Rooth P, Always C, et al. Verapamil prevents post–transplant delayed function and cyclosporine A nephrotoxicity. Transplant Proc. 1990;22:1379–1380. [PubMed] [Google Scholar]
- 54.Dawidson I, Rooth P, Lu C, et al. Verapamil improves the outcome after cadaver renal transplantation. J Am Soc Nephrol. 1991;2:983–990. doi: 10.1681/ASN.V25983. [DOI] [PubMed] [Google Scholar]
- 55.Romero JC, Raij L, Granger JP, et al. Multiple effects of calcium entry blockers on renal function in hypertension. Hypertension. 1987;10:140–151. doi: 10.1161/01.hyp.10.2.140. [DOI] [PubMed] [Google Scholar]
- 56.Chanard J, Toupance O, Lavaud S, et al. Amlodipine reduces cyclosporine – induced hyperuricaemia in hypertensive renal transplant recipients. Nephrol Dial Transplant. 2003;18:2147–2153. doi: 10.1093/ndt/gfg341. [DOI] [PubMed] [Google Scholar]
- 57.Martinez–Castelao A, Hueso M, Sanz V, et al. Treatment of hypertension after renal transplantation: long-term efficacy of verapamil, enalapril and deoxazocin. Kidney Int. 1998;68(Suppl):S130–S134. doi: 10.1046/j.1523-1755.1998.06826.x. [DOI] [PubMed] [Google Scholar]
- 58.Morales JM, Rondriguez-Paternina E, Araque A, et al. Long–term protective effect of a calcium antagonist on renal function in hypertensive renal transplant patients on cyclosporine therapy: a 5–year prospective randomized study. Transplant Proc. 1994;26:2598–2599. [PubMed] [Google Scholar]
- 59.Tutone VK, Mark PB, Stewart GA, et al. Hypertension, antihypertensive agents and outcomes following renal transplantation. Clin Transplantation. 2005;19:181–192. doi: 10.1111/j.1399-0012.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- 60.Baroletti SA, Gabardi S, Magee CC, Milford EL. Calcioum channel blockers as the treatment of choice for hypertension in renal transplant recipients: fact or fiction. Pharmacotherapy. 2003;23:788–801. doi: 10.1592/phco.23.6.788.32180. [DOI] [PubMed] [Google Scholar]
- 61.Pesavento TE, Jones PA, Julian BA, et al. Amlodipine increases cyclosporine levels in hypertensive renal transplant patients: results of a prospective study. J Am Soc Nephrol. 1996;7:831–835. doi: 10.1681/ASN.V76831. [DOI] [PubMed] [Google Scholar]
- 62.Guan D, Wang R, Lu, et al. Effects of nicardipine on blood cyclosporine levels in renal transplant patients. Transplant Proc. 1996;28:1311–1312. [PubMed] [Google Scholar]
- 63.Grekas D, Dioudis C, Kalevrosoglou I, et al. Renal hemodynamics in hypertensive renal allograft recipients: Effect of calcium antagonists and ACE inhibitors. Kidney Int. 1996;49:597. [PubMed] [Google Scholar]
- 64.Vanderschaaf MR, Hene RJ, Floor M, et al. Hypertension after renal transplantation: Calcium chanel blocker or converting enzyme blockade? Hypertension. 1995;25:77. doi: 10.1161/01.hyp.25.1.77. [DOI] [PubMed] [Google Scholar]
- 65.Bochicchio T, Sandoval G, Ron O, Perez-Grovas H, Bordes J, Herrera-Acosta J. Lisinopril prevents hyperfiltration and decreases proteinuria in post-transplant hypertensives. Kidney Int. 1990;38:873. doi: 10.1038/ki.1990.285. [DOI] [PubMed] [Google Scholar]
- 66.Psaty BM, Heckbert SR, Koepsell TD, et al. The risk of myocardial infarction associated with antihypertensive drug therapies. JAMA. 1995;274:620–625. [PubMed] [Google Scholar]
- 67.ALLHAT study. Major outcomes in high–risk hypertensive patients randomized to angiotensin –converting enzyme inhibitor or calcium channel blocker vs diuretic: The antihypertensive and lipid lowering treatment to prevent heart attack. JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 68.Bakris G, Copley JB, Vicknair N, et al. Calcium channel blockers versus other antihypertensive therapies on progression of NIDDM associated nephropathy. Kidney Int. 1996;50:1641–1650. doi: 10.1038/ki.1996.480. [DOI] [PubMed] [Google Scholar]
- 69.Bakris G, Weir MR, Shanifar S, et al. Effects of blood pressure level on progression of diabetic nephropathy: Results from the RENAL study. Arch Intern Med. 2003;163:1555–1565. doi: 10.1001/archinte.163.13.1555. [DOI] [PubMed] [Google Scholar]
- 70.Mourad G, Ribstein J, Mimran A. Converting-enzyme inhibitor versus calcium antagonist in cyclosporine-treated renal transplants. Kidney Int. 1993;43:419–425. doi: 10.1038/ki.1993.61. [DOI] [PubMed] [Google Scholar]
- 71.del Castillo D, Campistol JM, Guirado L, et al. Efficacy and safety of losartan in the treatment of hypertension in renal transplant recipients. Kidney Int. 1998;54(Suppl 68):S135. doi: 10.1046/j.1523-1755.1998.06827.x. [DOI] [PubMed] [Google Scholar]
- 72.Suwelack B, Gerhardt U, Hausberg M, Rahn KH, Hohage H. Comparison of quinaril versus atenolol: effects on blood pressure and cardiac mass after renal transplantation. Am J Cardiol. 2000;86:583–585. doi: 10.1016/s0002-9149(00)01024-9. [DOI] [PubMed] [Google Scholar]
- 73.Navar GL. The intrarenal rennin – angiotensin system in hypertension. Kidney Int. 2004;65:1522–1532. doi: 10.1111/j.1523-1755.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 74.Sennesael J, Lamote J, Violet I, et al. Comparison of perindopril and amlodipine in cyclosporine – treated renal allograft recipients. Hypertension. 1995;26:436–444. doi: 10.1161/01.hyp.26.3.436. [DOI] [PubMed] [Google Scholar]
- 75.Bochicchio T, Sandoval G, Ron O, et al. Fosinopril prevents hyperfiltration and decreases proteinuria in post-transplant hypertensives. Kidney Int. 1990;38:873–879. doi: 10.1038/ki.1990.285. [DOI] [PubMed] [Google Scholar]
- 76.Meyer MM. Post-transplant hypertension. In: Bennett WM, McCarron DA, editors. Pharmacology and management of hypertension. New York: Churchill Livingstone; 1994. pp. 181–215. [Google Scholar]
- 77.Traindl O, Falger S, Reading S, et al. The effect of lisinopril on renal function in proteinuric renal transplant recipients. Transplantation. 1993;55:1309–1313. doi: 10.1097/00007890-199306000-00019. [DOI] [PubMed] [Google Scholar]
- 78.Bergman SM, Curtis JJ. Possible mediators in hypertension: Renal factors. Semin Nephrol. 1996;16:134–1390. [PubMed] [Google Scholar]
- 79.Gaston RS, Julian BA, Curtis JJ. Post-transplant erythrocytosis: an enigma revisited. Am J kidney Dis. 1994;24:1–11. doi: 10.1016/s0272-6386(12)80153-3. [DOI] [PubMed] [Google Scholar]
- 80.Ritz E, Orth SR, Strzelezyk P. Angiotensin converting enzyme inhibitors, calcium channel blockers and their combination in the treatment of glomerular disease. J Hypertens. 1997;15(Suppl):S21–S26. [PubMed] [Google Scholar]
- 81.Moser M. Angiotensin converting enzyme inhibitors, angiotensin II receptor antagonists and calcium channel blocking agents. J Am Coll Cardiol. 1997;29:1414–1421. doi: 10.1016/s0735-1097(97)00096-x. [DOI] [PubMed] [Google Scholar]
- 82.Vergoulas G, Miserlis Gr, Trellopoulos G, Antoniadis A. Evaluation of losartan in patients with renal transplantation. Hippokratia. 2000;4:67–72. [Google Scholar]
- 83.Vergoulas G, Miserlis Gr, Gakis D, Atmatzidis E, Anoniadis A. The use of valsartan in hypertensive renal transplant recipients. Hippokratia. 2001;5:61–68. [Google Scholar]
- 84.Vergoulas G, Miserlis Gr, Papanikolaou V, et al. Angiotensin II type I receptor antagonists reduce proteinuria of hypertensive renal transplant recipients. Hippokratia. 2001;5:165–171. [Google Scholar]
- 85.Ducloux D, Saint-Hillier Y, Chalopin GM. Effect of losartan on haemoglobin concentration in renal transplant recipients: a retrospective analysis. Nephrol Dial Transplant. 1997;12:2683–2686. doi: 10.1093/ndt/12.12.2683. [DOI] [PubMed] [Google Scholar]
- 86.Home S, Holzer H, Horina J. Losartan and renal transplantation. Lancet. 1998;351:285. doi: 10.1016/S0140-6736(05)78150-2. [DOI] [PubMed] [Google Scholar]
- 87.Campistol JM, Inigo P, Jimenez W, et al. Losartan decreases plasma levels of TGF-β1 in transplant patients with chronic allograft nephropathy. Kidney Int. 1999;56:714–719. doi: 10.1046/j.1523-1755.1999.00597.x. [DOI] [PubMed] [Google Scholar]
- 88.Vergoulas G, Miserlis Gr, Papanikolaou V, et al. Losartan versus losartan in the treatment of hypertension of renal transplant recipients. Hippokratia. 2001;5:156–164. [Google Scholar]
- 89.Bakris G, Weir MR. Angiotensin – converting enzyme inhibitor-associated elevations in serum creatinine. Is this a cause for concern? Arch Int Med. 2000;160:685–693. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]
- 90.Elmfeldt D, Olofsson B, Meredith P. The relationship between dose and antihypertensive effect of four AT1 – receptor blockers. Differences in potency and efficacy. Blood Pressure. 2002;11:292–301. doi: 10.1080/080370502320779502. [DOI] [PubMed] [Google Scholar]
- 91.Hansson L. The relationship between dose and antihypertensive effect for different AT1 – receptor blockers. Blood Pressure. 2001;10(Suppl 30):33–39. doi: 10.1080/08037050152518348. [DOI] [PubMed] [Google Scholar]
- 92.Meredith PA. Clinical comparative trials of angiotensin II type I (AT1) – receptor blockers. Blood Pressure. 2001;10(Suppl 3):11–17. doi: 10.1080/08037050152518311. [DOI] [PubMed] [Google Scholar]
- 93.Shihab PS, Bennet WM, Tanner AM, et al. Andiotensin II blockade decreases TGF-beta and matrix proteins in cyclosporine nephropathy. Kidney Int. 1997;52:660–673. doi: 10.1038/ki.1997.380. [DOI] [PubMed] [Google Scholar]
- 94.Weir MR, Wei C. The role of angiotensin II and TGF-beta on the progression of chronic allograft nephropathy. JRAAS. 2001;2(Suppl 1):S88–S1190. doi: 10.1177/14703203010020013201. [DOI] [PubMed] [Google Scholar]
- 95.Stigant CE, Cohen J, Vivera M, et al. ACE inhibitors and angiotensin II antagonists in renal transplantation: an analysis of safety and efficacy. Am J Kidney Dis. 2000;35:58–63. doi: 10.1016/S0272-6386(00)70302-7. [DOI] [PubMed] [Google Scholar]
- 96.Urata H, Healy B, Stewart RW, Bumpus FM, Husain A. Angiotensin II-forming pathways in normal and failing human hearts. Cir Res. 1990;66:883–860. doi: 10.1161/01.res.66.4.883. [DOI] [PubMed] [Google Scholar]
- 97.Ellis ML, Patterson JH. A new class of antihypertensive therpy: angiotensin II receptor antagonists. Pharmacotherapy. 1996;16:849–860. [PubMed] [Google Scholar]
- 98.Balcells E, Meng QC, Johnson WH, Oparil S, Dell'Italia LJ. Angiotensin II formation from ACE and chymase in human and animal hearts: methods and species considerations. Am J Physiol. 1997;273:H1769–H1774. doi: 10.1152/ajpheart.1997.273.4.H1769. [DOI] [PubMed] [Google Scholar]
- 99.Hollemberg NK, Fisher ND, Price DA. Pathways for angiotensin II generation in intact human tissue: evidence from comparative pharmacological interruption of the rennin system. Hypertension. 1998;32:387–392. doi: 10.1161/01.hyp.32.3.387. [DOI] [PubMed] [Google Scholar]
- 100.Siragy HM, de Gasparo M, Carey RM. Angiotensin type 2 receptor mediates valsartan – induced hypotension in conscious rats. Hypertension. 2000;35:1074–1077. doi: 10.1161/01.hyp.35.5.1074. [DOI] [PubMed] [Google Scholar]
- 101.Benz J, Oshrain C, Henry D, Avery C, Chiang YT, Gatlin M. Valsartan a new angiotensin II receptor antagonist: a double–blind study compring the incidence of cough with lisinopril and hydrochlorothiazide. J Clin Pharmacol. 1997;37:101–107. doi: 10.1002/j.1552-4604.1997.tb04767.x. [DOI] [PubMed] [Google Scholar]
- 102.Conlin PR, Gerh WC, Fof J, Roehm JB, Boccuzzi SJ. Four year persistence patterns among patients initiating therapy with angiotensin II receptor antagonist losartan versus other antihypertensive drug classes. Clin Theraput. 2001;23:1999–2009. doi: 10.1016/s0149-2918(01)80152-1. [DOI] [PubMed] [Google Scholar]
- 103.Lorenz M, Billensteiner E, Bodingbauer M, et al. The effect of ACE inhibitor and angiotensin II blocker therapy on early posttransplant kidney graft function. Am J Kidney Dis. 2004;43:1065–1070. doi: 10.1053/j.ajkd.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 104.Klingbeil AU, Schneider M, Martus P, et al. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med. 2003;115:41–46. doi: 10.1016/s0002-9343(03)00158-x. [DOI] [PubMed] [Google Scholar]
- 105.Suwelack B, Gerhard U, hausberg M, et al. Comparison of quinapril versus atenolol.Effects on blood pressure and cardiac mass after renal transplantation. Am J Cardiol. 2000;86:583–585. doi: 10.1016/s0002-9149(00)01024-9. [DOI] [PubMed] [Google Scholar]
- 106.Isobe N, Taniguchi K, Oshima S, et al. Candesartan cilexetil improves left ventricular function, left ventricular hypertrophy and endothelial function in patients with hypertensive heart disease. Circ J. 2002;66:993–999. doi: 10.1253/circj.66.993. [DOI] [PubMed] [Google Scholar]
- 107.Huysman FT, van Heusden FH, Wetxels JF, et al. Antihypertensive effect of beta blockade in renal transplant recipients with or without host kidneys. Transplantation. 1988;46:234–237. doi: 10.1097/00007890-198808000-00009. [DOI] [PubMed] [Google Scholar]
- 108.Hausberg M, Barenbrock M, Hohage H, et al. ACE inhibitor versus beta-blocker for the treatment of hypertension in renal allograft recipients. Hypertension. 1999;33:862–868. doi: 10.1161/01.hyp.33.3.862. [DOI] [PubMed] [Google Scholar]
- 109.Chobanian AV, Bakris G, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 110.Bell DS. Advantages of a third – generation beta blocker in patients with diabetes mellitus. Am J Cardiol. 2004;93:49–52. doi: 10.1016/j.amjcard.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 111.Poole-Wilson PA, Cleland JG, Di Lenarda A, et al. Rational and design of the carvedilol or metoprolo European trial in patients with chronic heart failure: COMET. Eur J Heart Fail. 2002;4:321–329. doi: 10.1016/s1388-9842(02)00025-9. [DOI] [PubMed] [Google Scholar]
- 112.Zezina L, Vessby B, Larsson E, Backman U, Fellstrom B. Carvedilol treatment of kidney graft recipients with chronic rejection. Clin Transplantation. 1999;13:484–490. doi: 10.1034/j.1399-0012.1999.130608.x. [DOI] [PubMed] [Google Scholar]
- 113.Suter PM, Vetter W. Metabolic effects of antihypertensive drugs. J Hypertens. 1995;13:S11–S17. doi: 10.1097/00004872-199512002-00003. [DOI] [PubMed] [Google Scholar]
- 114.Gress TW, Nieto FJ, Shahar WR, Wofford MR, Brancati FL. Hypertension and antihypertensive therapy as risk factor for type 2 diabetes melltus. N Engl J Med. 2000;342:905–912. doi: 10.1056/NEJM200003303421301. [DOI] [PubMed] [Google Scholar]