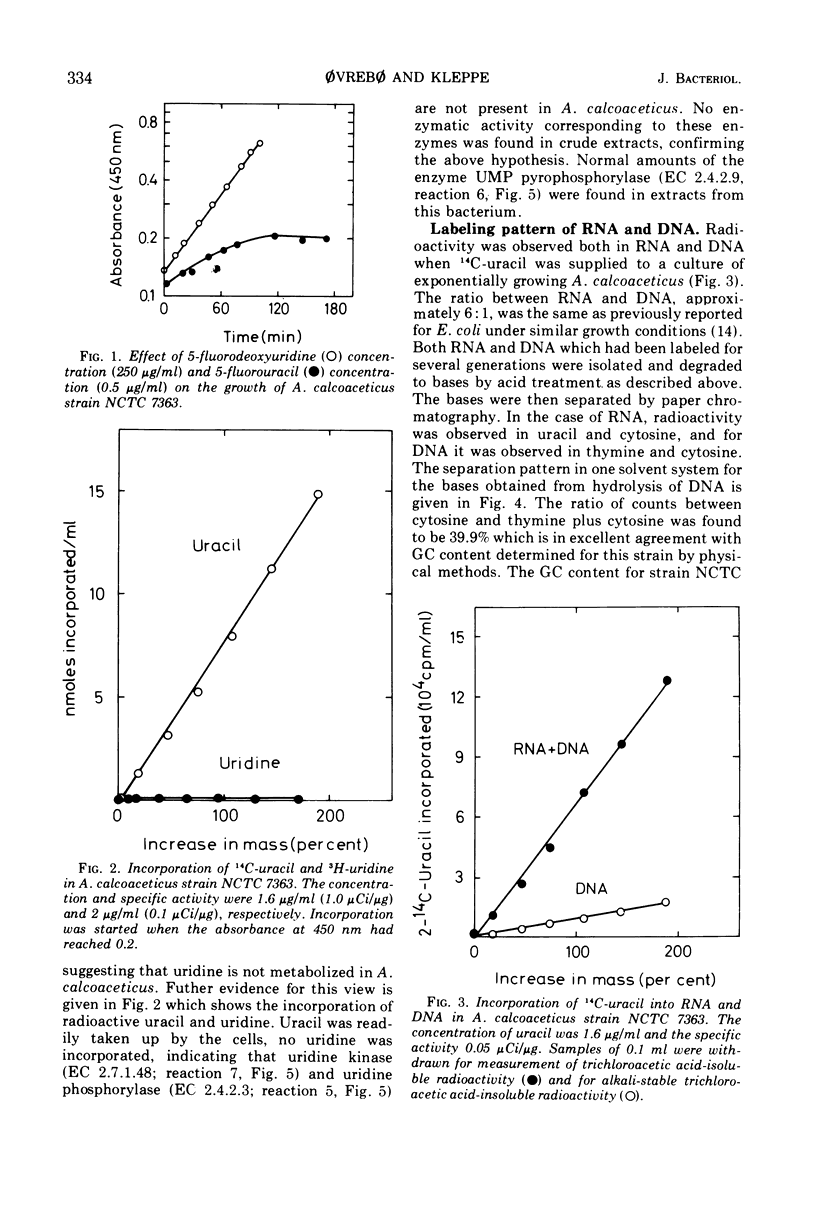
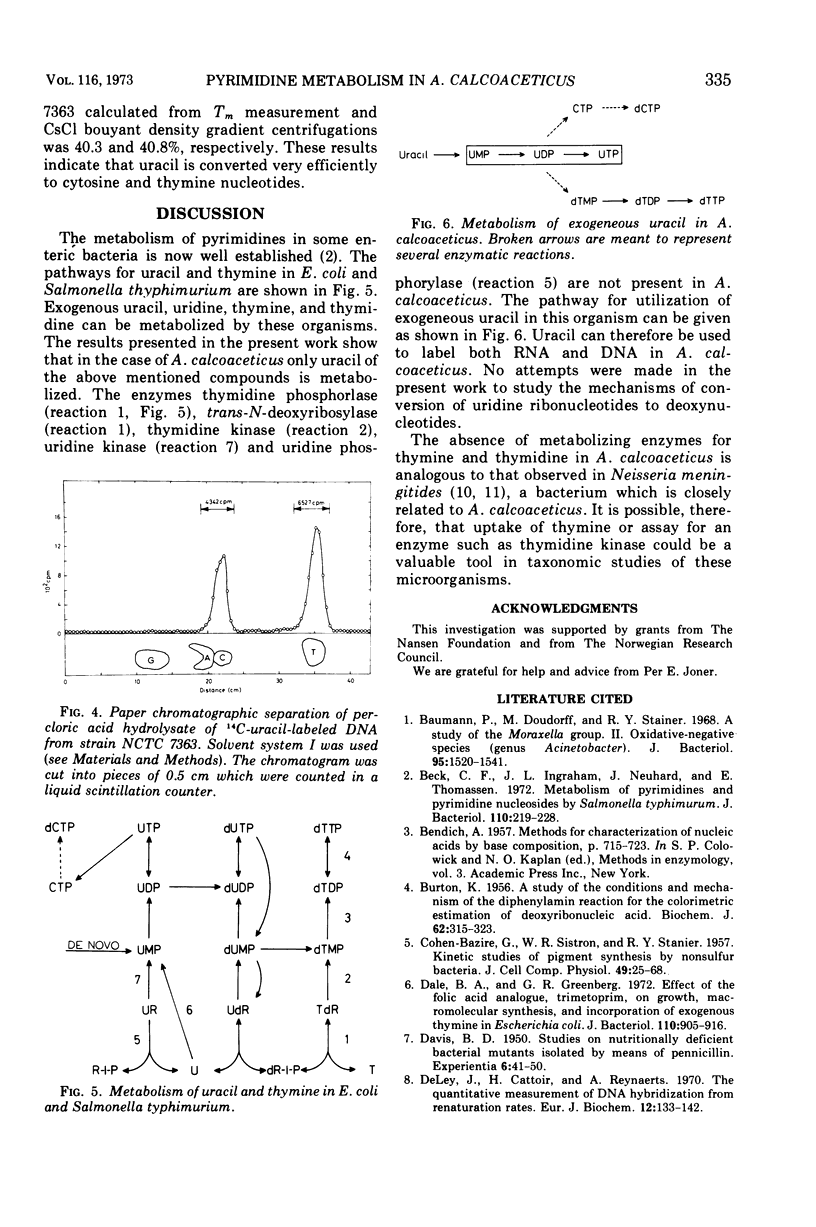
Abstract
The metabolism of thymine, thymidine, uracil, and uridine has been investigated in five different strains of Acinetobacter calcoaceticus. Attempts to isolate thymine and thymidine auxotrophic mutants were not successful. Consistent with this finding was the observation that uptake of radioactive thymine or thymidine could not be demonstrated. Search for enzymes capable of transforming thymine via thymidine to thymidine-5′-monophosphate in crude extracts was performed, and the following enzymes were absent judging from enzyme assays: thymidine phosphorylase (EC 2.4.2.4), trans-N-deoxyribosylase (EC 2.4.2.6), and thymidine kinase (EC 2.7.1.21). The enzymes responsible for the phosphorylation of thymidine-5′-monophosphate to thymidine-5′-triphosphate were present in crude extracts. Radioactive uracil was readily incorporated into both ribonucleic acid and deoxyribonucleic acid, the ratio being 6:1, and radioactivity was found only in pyrimidine bases. No uptake of uridine could be demonstrated. Uridine-5′-monophosphate pyrophosphorylase (EC 2.4.2.9) activity was detected in crude extracts, suggesting that uracil is converted directly to uridine-5′-monophosphate which is then phosphorylated to uridine-5′-triphosphate or transformed to other ribo- and deoxypyrimidine nucleotides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C. F., Ingraham J. L., Neuhard J., Thomassen E. Metabolism of pyrimidines and pyrimidine nucleosides by Salmonella typhimurium. J Bacteriol. 1972 Apr;110(1):219–228. doi: 10.1128/jb.110.1.219-228.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Greenberg G. R. Effect of the folic acid analogue, trimethoprim, on growth, macromolecular synthesis, and incorporation of exogenous thymine in Escherichia coli. J Bacteriol. 1972 Jun;110(3):905–916. doi: 10.1128/jb.110.3.905-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ley J., Cattoir H., Reynaerts A. The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem. 1970 Jan;12(1):133–142. doi: 10.1111/j.1432-1033.1970.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972 Nov;112(2):917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyssum S., Jyssum K. Utilization of thymine, thymidine and TMP by neisseria meningitidis. 1. Growth response and uptake of labelled material. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(6):683–691. doi: 10.1111/j.1699-0463.1970.tb04358.x. [DOI] [PubMed] [Google Scholar]
- Jyssum S. Utilization of thymine, thymidine and TMP by Neisseria meningitidis. 2. Lack of enzymes for specific incorporation of exogenous thymine, thymidine and TMP into DNA. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(6):778–788. doi: 10.1111/j.1699-0463.1971.tb00111.x. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- MARSHAK A., VOGEL H. J. Microdetermination of purines and pyrimidines in biological materials. J Biol Chem. 1951 Apr;189(2):597–605. [PubMed] [Google Scholar]
- Munch-Petersen A. Thymidine breakdown and thymine uptake in different mutants of Escherichia coli. Biochim Biophys Acta. 1967 Jun 20;142(1):228–237. doi: 10.1016/0005-2787(67)90530-8. [DOI] [PubMed] [Google Scholar]
- OKAZAKI R., KORNBERG A. DEOXYTHYMIDINE KINASE OF ESCHERICHIA COLI. I. PURIFICATION AND SOME PROPERTIES OF THE ENZYME. J Biol Chem. 1964 Jan;239:269–274. [PubMed] [Google Scholar]
- Pintér M., Bende I. Computer analysis of Acinetobacter iwoffi (Moraxella iwoffii) and Acinetobacter anitratus (Moraxella glucidolytica) strains. J Gen Microbiol. 1967 Feb;46(2):267–272. doi: 10.1099/00221287-46-2-267. [DOI] [PubMed] [Google Scholar]
- Smith J. T. Production of thymineless mutants in gram-negative bacteria (Aerobacter, Proteus). J Gen Microbiol. 1967 Apr;47(1):131–137. doi: 10.1099/00221287-47-1-131. [DOI] [PubMed] [Google Scholar]
- Thornley M. J. A taxonomic study of Acinetobacter and related genera. J Gen Microbiol. 1967 Nov;49(2):211–257. doi: 10.1099/00221287-49-2-211. [DOI] [PubMed] [Google Scholar]
- Twarog R. Enzymes of the isoleucine-valine pathway in Acinetobacter. J Bacteriol. 1972 Jul;111(1):37–46. doi: 10.1128/jb.111.1.37-46.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twarog R., Liggins G. L. Enzymes of the tryptophan pathway in Acinetobacter calco-aceticus. J Bacteriol. 1970 Oct;104(1):254–263. doi: 10.1128/jb.104.1.254-263.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



