Abstract
Intramolecular crosslink of hemoglobin tetramers solved the problem of urine elimination and short intravascular retention time of cell free hemoglobin infusion. It also produced a family of crosslinked hemoglobins with P50 between 18 and 30 mmHg. However, it did not solve the problem of MAP increases in infused animals. It was proven that extravasation of hemoglobin into interstitial fluid was responsible for MAP increases. Extravasation and the MAP increase was avoided using a hemoglobin polymer with average size near 25 MDa. In spite of a very high oxygen affinity, this polymer delivered oxygen to tissues, producing either vasodilation or vasoconstriction according to oxygen needs. It was also proven that cell free hemoglobins are more efficient than red cells in delivering oxygen to tissues.
Keywords: Blood substitutes, Crosslinking, Extravasation, Zero-link polymers
RESULTS AND DISCUSSION
Purified hemoglobins appear to be ideally suited for use as transfusional agents in clinical settings. They carry oxygen and their colloidal nature may act as a plasma expander. However, outside of the red cells, their oxygen affinity becomes higher than that of blood, suggesting low oxygen delivery to tissues. Also, after infusion they rapidly disappear from circulation and are profusely eliminated with the urine. Most important, they produce an increase of the mean arterial pressure (MAP) in infused animals.
Urine elimination was avoided by preventing the dissociation of hemoglobin into dimers. This was obtained by reacting oxy or deoxy hemoglobin with 3,5-bis-dibromo salicyl activated aliphatic dicarbocylic acid. These reagents react with the partner β82 lysines on the two sides of the β-cleft of hemoglobin, introducing covalent crosslinking bridges of various length according to the number of carbon atoms of the activated carboxylic acid. Notably, oxygen affinity was inversely proportional to the length of the carbon bridges. Fumaryl (four carbons length) crosslinked hemoglobin had a P50 near 17 mmHg, while the P50 of sebacoyl (10 carbons length) hemoglobin (DECA) was near 30 mmHg. The oxygen binding cooperativity of these compounds was also inversely proportional to their oxygen affinity. The value of the Hill parameter n went from near 1.0 for fumaryl Hb to higher than 2.0 for sebacoyl Hb [1].
By this means it became possible to eliminate the hemoglobinuria, prolong the intravascular retention time from 30–40 min to about 3.5 h, and modulate practically at will the oxygen affinity of the crosslinked hemoglobins. These compounds, after infusion, still produced an increased MAP, however [2].
The MAP increase is a serious problem. As shown in Fig. 1, in the cat the increase is only 10–20% above normal, however, the vasoconstriction responsible for the increase is not uniformly distributed among the various internal organs. As shown by Ulatowski et al., blood flow is severely decreased in critical organs like kidneys, intestine and pituitary systems [3].
Figure 1.
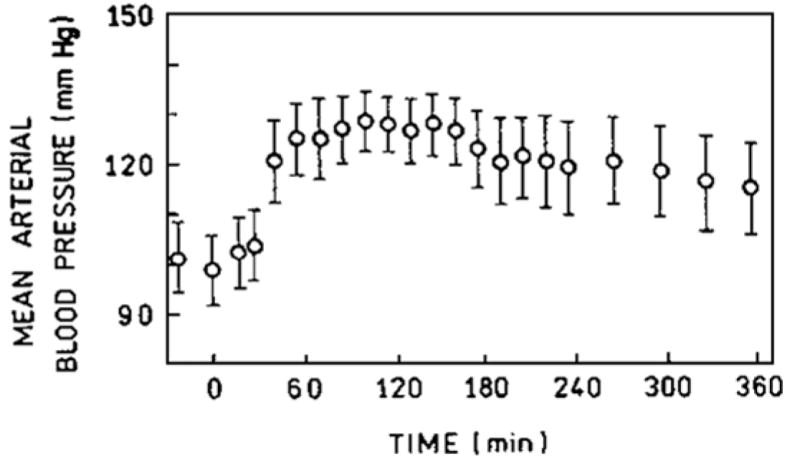
Transient of the pressure response after infusion in the cat of sebacoyl crosslinked HbA.
It was observed that the crosslinked hemoglobins, although preventing urine elimination through the small pores of the endothelium of the glomeruli, still extravasate into the interstitial fluid of internal organs through the larger pores of other capillary beds. DECA appeared in the hilar lymph of rat kidneys, Fig. 2 [4].
Figure 2.
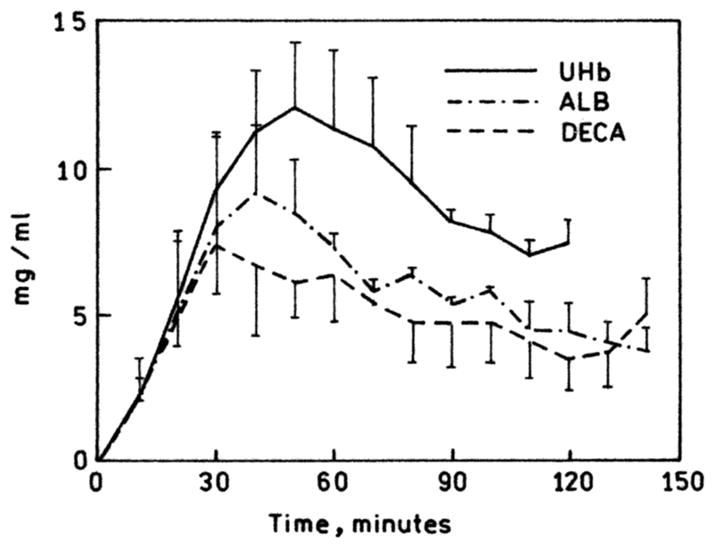
Transients of hilar lymph concentration in rats 30% exchange-transfused with unmodified hemoglobin (UMB), DECA, and marked albumin. (Adapted from Matheson et al., 2000).
Using a cranial window in the cat it was observed that only the presence of dilute DECA solutions on the abluminal side of arterioles inhibited the dilatory effect of the NO developed after addition of acetylcholine. As long as DECA remained in the luminal side of the vasculature, it did not interfere with the effect of acetylcholine [5].
This observation and the presence of DECA in the hilar lymph of rat kidneys generated the hypothesis that MAP increase after infusion of Hb solution resulted from extravasation of infused hemoglobin, because extravasation would absorb NO from the muscle layer in the abluminal side of arterioles. To prove this hypothesis it was necessary to monitor the effect of the infusion of non-extravasating hemoglobin solutions.
It was estimated that certain pores of capillary beds could be as large as 500 Å [6]. Thus it was necessary to obtain a hemoglobin polymer with comparable size. Among the various chemical treatments that are used to produce polymeric hemoglobins, the zero-link technology was preferred.
Zero-link is a procedure where the carboxyl groups on the surface of a molecule are activated with carbodiimide so as to make them able to form covalent pseudopeptide bonds with the amino groups on the surface of an adjacent molecule. It is called zero-link because no chemicals are left behind in the obtained polymers. This treatment generated extensive polymerization of bovine hemoglobin molecules (Zl-HbBv) with average size near 25 MDa. Residual small size polymers, below 1000 KDa, and non–reacted hemoglobin were eliminated by diafiltration over a 300 KDa NMW membrane. Zl-HbBv did not appear in the hilar lymph of the rat and did not produce MAP increases in the cat, as shown in Fig. 3 [7].
Figure 3.
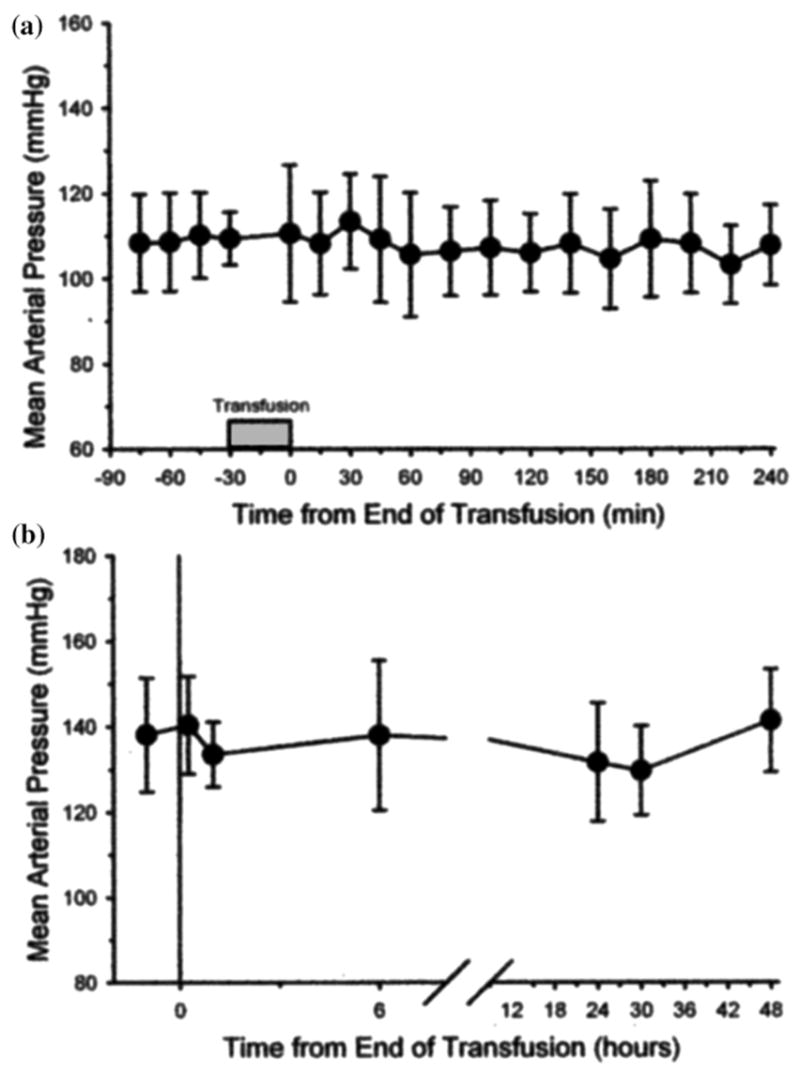
Exchange transfusion of ZL-HbBv produced no change in mean arterial pressure (±SD) over a 4-h observation period in anesthetized cats (A) or over a 48-h observation period in unanesthetized cats (B). (Adapted from Matheson et al., 2002).
The oxygen affinity of Zl-HbBv was near 4.0 mmHg, with no oxygen binding cooperativity [7]. Still it was an efficient oxygen transporter. When infused in the cat, cranial window observations indicated a 10% decrease in the diameter of pial arterioles. In the absence of MAP increases this was interpreted as a regulatory vasoconstriction, which avoided excessive oxygen delivery. In fact, when the viscosity of the blood was increased about 3 times by addition of PVP, which decreases blood flow, infusion of the polymer produced a compensatory 50% vasodilation of the arterioles, far exceeding that obtained with infusion of albumin solutions, Fig. 4 [8]. The vasocontriction was proven not to be NO dependent [9], and it was eliminated only upon addition of inhibitors of cytochrome P450 system. This is now generating the hypothesis that cytochrome P450 is a sensor of oxygen tension in tissues [10].
Figure 4.
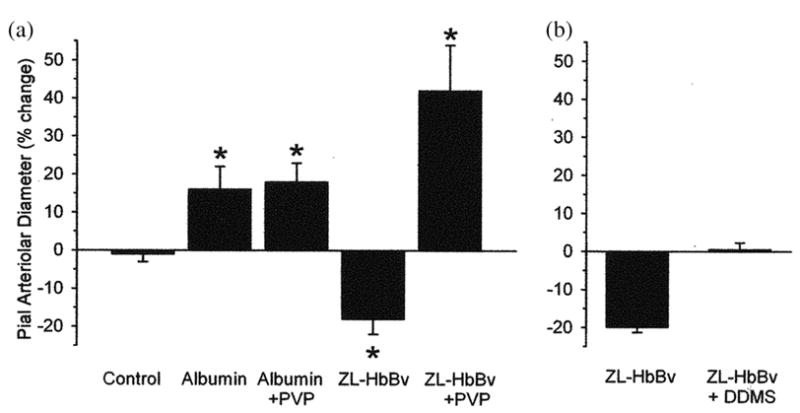
(A). Percent change (±SE) in pial arteriolar diameter measured through a closed cranial window in anesthetized cats after a 40% exchange-transfusion with 5% albumin or 6% ZL-HbBv. The constriction observed with ZL-HbBv at reduced hematocrit and blood viscosity was reversed to dilation in a group in which plasma viscosity was simultaneously increased by co-infusion of 20% polyvinylpyrrolidone (PVP). *P < .05 from time control group. (B). The constrictor response to ZL-HbBv exchange-transfusion in anesthetized rats was blocked by superfusing the cranial window with the cytochrome P450 w-hydroxylase inhibitor N-methylsulfonyl-12,12-dibromododec-11-enamide (DDMS; 50 μM). This indicates that the vasoconstriction in panel A was not NO dependent. (Adapted from Rebel et al., 2003).
Other experiments demostrated that fumaryl-Hb, with P50 near 18 mmHg, and DECA, with P50 near 30 mmHg, also delivered oxygen to tissues. Blood flow of infused cats was directly proportional to the amount of oxygen transported by the infused cell-free hemoglobins [11–12]. Thus, it appears that the efficacy of hemoglobin based carriers is little dependent on their oxygen affinity characteristics, being very good between P50’s of 4 to 30 mmHg.
It was noted that the vasoactive regulation of the carriers produced normoxia even when the amount of cell-free hemoglobin was less than half the amount of the red cell-hemoglobin eliminated by previous isovolumic exchange transfusions [7]. This suggested that cell-free hemoglobins are more efficient oxygen transporters than red cells.
This proposition was proven using superfusions in Ussing chambers of rabbit ileal intestinal membranes. The oxygen delivery of 3% solutions of DECA was compared to that of equivalent hemoglobin suspensions of bovine red cells. Metabolite transport from the mucosa to the serosa part on the membranes stopped when the perfusing fluid was equilibrated with 30% rather then the usual 95% oxygen. DECA at 3%, far below the hemoglobin content of blood, restored metabolite transport at 30% oxygen. The equivalent suspension of bovine red cells did not, Fig. 5 [13].
Figure 5.
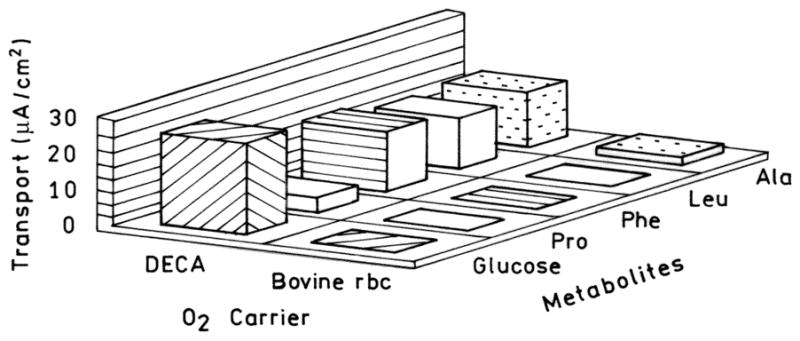
Transport of glucose and amino acids through a rabbit jejunal membrane voltage clamped in a Ussing chamber. The Ringer’s perfusate was equilibrated with 30% O2, 5% CO2 and contained either 3 g=dl DECA or an equivalent suspension of bovine red cells. After 4 h of perfusion, red cells failed to support transport. (Adapted from Bucci et al., 2003).
This phenomenon probably implies that while cell-free carriers bathe the tissues, delivering oxygen directly to them, the oxygen delivered by the red cells is retarded by the red cell membrane and distance.
In conclusion, it appears that, as long as they do not extravasate, a variety of efficient HBOC can be obtained with different oxygen affinity characteristics. This flexibility may allow production of oxygen carriers specific for different clinical setting. The basic problems affecting development of HBOC appear to be solved, in the laboratory. This does not exclude other problems emerging from expanded animal and human trials, especially when industrial production is attempted.
Footnotes
References
- 1.Bucci E, Razynska A, Kwansa H, Matheson-Urbaitis B, O’Hearne M, Ulatowski JA, Koehler RC. Production and characteristics of an infusible oxygen-carrying fluid based on hemoglobin intramolecularly cross-linked with sebacic acid. J Lab Clin Med. 1996;128:146–153. doi: 10.1016/s0022-2143(96)90006-2. [DOI] [PubMed] [Google Scholar]
- 2.Urbaitis BK, Razynska A, Corteza Q, Fronticelli C, Bucci E. Intravascular retention and renal handling of purified natural and intramolecularly crosslinked hemoglobins. J Lab Clin Med. 1991;117:115–121. [PubMed] [Google Scholar]
- 3.Ulatowski JA, Nishikawa T, Matheson-Urbaitis B, Bucci E, Traystman RJ, Koehler RC. Regional blood flow alterations after bovine fumaryl beta beta-crosslinked hemoglobin transfusion and nitric oxide synthase inhibition. Crit Care Med. 1996;24:558–565. doi: 10.1097/00003246-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Matheson B, Razynska A, Kwansa HE, Bucci E. Appearance of dissociable and crosslinked hemoglobins in renal hilar lymph. J Lab Clin Inv. 2000;135:459–464. doi: 10.1067/mlc.2000.106458. [DOI] [PubMed] [Google Scholar]
- 5.Asano Y, Koehler RC, Ulatowski JA, Traystman RJ, Bucci E. Effect of cross-linked hemoglobin transfusion on endothelial-dependent dilation in cat pial arterioles. Am J Physiol Heart Circ Physiol. 1998;275:1313–21. doi: 10.1152/ajpheart.1998.275.4.H1313. [DOI] [PubMed] [Google Scholar]
- 6.Rippe B, Haraldsson B. Transport of macromolecules across microvascular walls the two-pore theory. Physiol Rev. 1994;74:163–219. doi: 10.1152/physrev.1994.74.1.163. [DOI] [PubMed] [Google Scholar]
- 7.Matheson B, Kwansa HE, Rebel A, Mito T, Koehler RC, Bucci E. Vascular response to infusions of a non extravasating hemoglobin polymer. J Appl Physiol. 2002;93:1479–1486. doi: 10.1152/japplphysiol.00191.2002. [DOI] [PubMed] [Google Scholar]
- 8.Rebel A, Ulatowski JA, Kwansa H, Bucci E, Koehler RC. Cerebrovascular response to decreased hematocrit, effect of cell-free hemoglobin, plasma viscosity and CO2. Am J Physiol Heart Circ Physiol. 2003;285:H1600–1608. doi: 10.1152/ajpheart.00077.2003. [DOI] [PubMed] [Google Scholar]
- 9.Sampei K, Ulatowski JA, Asano Y, Kwansa H, Bucci E, Koehler RC. Role of nitric oxide scavenging in vascular response to cell-free hemoglobin transfusion. Am J Physiol Heart Circ Physiol. 2005;289:H1191–1201. doi: 10.1152/ajpheart.00251.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin X, Kwansa HE, Bucci E, Roman RJ, Koehler RC. Role of 20-HETE in the pial arteriolar constrictor response to decreased hematocrit after exchange transfusion of cell-free polymeric hemoglobin. J Appl Physiol. 2006;100:336–342. doi: 10.1152/japplphysiol.00890.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulatowski JA, Bucci E, Nishikawa T, Razynska A, Williams A, Takeshima R, Traystman RJ, Koehler RC. Cerebral O2 transport with hematocrit reduced by cross-linked hemoglobin transfusion. Am J Physiol Heart Circ Physiol. 1996;39(270):466–475. doi: 10.1152/ajpheart.1996.270.2.H466. [DOI] [PubMed] [Google Scholar]
- 12.Ulatowski JA, Bucci E, Razynska A, Traystman RJ, Koehler RC. Cerebral blood flow during hypoxic hypoxia with plasma-based hemoglobin at reduced hematocrit. Am J Physiol. 1998;274:H1933–1942. doi: 10.1152/ajpheart.1998.274.6.H1933. [DOI] [PubMed] [Google Scholar]
- 13.Bucci E, Watts TL, Kwansa HE, Fasano A, Matheson B, Rebel A, Koehler RC. Cell-free hemoglobin, oxygen off-load and vasoconstriction. Adv Exp Med Biol. 2003;S10:89–92. doi: 10.1055/s-2001-18180. [DOI] [PubMed] [Google Scholar]


