Abstract
Assessing airway inflammation is important for investigating the underlying mechanisms of many lung diseases, including asthma, chronic obstructive pulmonary disease (COPD), bronchiectasis, primary ciliary dyskinesia (PCD) and cystic fibrosis. A growing interest has recently directed toward non-invasive methods for the assessment of airway inflammation. Measurement of exhaled nitric oxide in exhaled air is an exciting innovative technique that gives new insights into the pathophysiology of lung disease and asthma in particular, with many potential clinical applications. Careful standardisation of measurement techniques has facilitated the use of this new measurement in paediatric respiratory medicine. Non-invasiveness and instantaneous results potentially make it a suitable instrument for use in children starting from the age of 4, with useful applications both in asthma diagnosis and monitoring.
Keywords: asthma, exhaled nitric oxide, airway inflammation, non-invasive monitoring, primary ciliary dyskinesia, cystic fibrosis
Airway inflammation is centrally important in asthma and other lung diseases. Currently, asthma is viewed as a chronic inflammatory airway disease, and international guidelines place great importance on treating inflammation in this condition1. Methods used in everyday clinical practice are not suitable for the assessment of airway inflammation. Symptoms and their perceptions vary widely between individuals and do not accurately reflect the extent of airway inflammation. Lung function measurements are used to monitor disease activity; however, it has been recognized that changes in lung function tests are not closely related to the degree of inflammation, and intensive inflammatory processes may well precede changes in lung function2, 3.
Conversely, direct assessment of airway inflammation using bronchoscopy cannot be used for monitoring due to its invasive nature. Sputum induction is another way of obtaining cells from the lower airways and it has been demonstrated that its use improves asthma control4, 5. This sampling method, however, requires inhalation of a hypertonic salt solution, which may provoke bronchoconstriction in asthmatic patients. Furthermore, processing the sputum samples requires ~2 hours work by a well-trained person to obtain reliable readings, and, at the moment, these features limit its wide clinical use.
The need to monitor inflammation in the lungs has led to the exploration of exhaled gases and condensates. Noninvasive monitoring may assist in differential diagnosis of pulmonary diseases, assessment of disease severity and response to treatment. Because these techniques are completely noninvasive, they can be used repeatedly to give information about kinetics, they can be used in patients with severe disease, which has been previously difficult to monitor, and they can be used to monitor disease in children, including infants. This article reviews whether exhaled nitric oxide (eNO) measurement is suitable for routine use in clinical practice.
Markers of airway inflammation in asthma
Invasive markers
In asthma, fiberoptic bronchial biopsies have become the “gold standard” for measuring inflammation in the airway wall, but this is an invasive procedure that is not suitable for routine clinical practice and cannot be repeated often. It is also unsuitable for use in children and patients with severe disease.
Noninvasive markers
A number of markers have been and are being considered as noninvasive markers of airway inflammation. Examples include blood eosinophil counts or serum eosinophil cationic protein (ECP), urinary eicosanoid metabolites, exhaled gasses, mediators in breath condensate or induced sputum (Table 1)6. Along these lines, the least invasive technique is the collection of exhaled breath samples. Some of them, including nitric oxide (NO) and carbon monoxide (CO), can be measured in the gas phase, while others, such as hydrogen peroxide, adenosine, interleukins and pH, can be determined in the exhaled breath condensate (EBC).
Table 1. Biomarkers in asthma.
Definition of abbreviations: EPO: eosinophil peroxidase; sIL-2R: soluble interleukin-2; receptor; LT: leukotriene; cysLT’s: cysteinyl leukotriene. (Revised from Reference 6).
Blood markers
Measurement of blood eosinophil counts and serum ECP levels if correctly performed, are reproducible and consistent 7. However, blood eosinophil counts have been shown to correlate weakly to eosinophil numbers in biopsies 8 and have poor disease specificity. The correlation of serum ECP with the number of eosinophils in biopsies is variable: although in some studies, a correlation has been reported, this has not been invariably confirmed 8, 9. Serum ECP also lacks disease specificity. Increased levels of ECP can be found in various diseases including cystic fibrosis, whereas conversely, a large degree of overlap exists between normal and asthmatic individuals with varying severity10, 11. Both eosinophil counts and ECP levels respond to factors known to influence the degree of airway inflammation such as changes in treatment or allergen exposure12, 13.
Urinary markers
Urinary eosinophil peroxidase (EPX) offers an even less invasive alternative to serum ECP12, 14, especially for children. Another line of investigation is to measure eicosanoid metabolites in urine such as leukotriene (LT) E4 or 9a, 11bPGF2 15. These measurements are reliable but require skilled expertise. How precisely they reflect ongoing inflammation in the airways needs to be further evaluated as increased urinary LTE4 levels are not limited to asthma and they do not discriminate between asthma and normal subjects16.
Exhaled breath condensate (EBC)
Breath analysis is currently a research procedure, but there is increasing evidence that it may have an important place in the diagnosis and management of lung diseases in the future17. Exhaled breath condensate is collected by cooling or freezing exhaled air. As the procedure is totally noninvasive and does not influence airway caliber, a major advantage of this technique is that it is extremely well tolerated even by patients with severe airway obstruction and children.
The most common approach is for the subject to breathe via a mouthpiece through a non-rebreathing valve block in which inspiratory and expiratory air is separated. During expiration, the breathing air flows through a condenser, which is cooled to –20° C. Exhaled condensate is usually analyzed by gas chromatography and/or extraction specrophotometry, or by immunoassays. The obtained fluid, however, is a complex diluted solution of diverse biomarkers with various chemical stabilities, including a variety of constituents 18. Due to the complexity of EBC and the fact that it is a much-diluted sample, there are still several unsolved issues surrounding this sampling method. Proposed biomarkers in EBC include adenosine, ammonia, hydrogen peroxide, ions, isoprostanes, prostaglandins, leukotrienes, nitrogen oxides, pH and cytokines. Concentrations of these mediators are influenced by airway diseases, including asthma, Chronic Obstructive respiratory Disease (COPD), Cystic Fibrosis (CF) and bronchiectasis, and are modulated by therapeutic intervention19. Differences in condensate chemistry are thought to reflect changes in the airway lining fluid caused by inflammation and oxidative stress. Condensate from asthmatic subjects contains increased levels of leukotriene B4/C4/D4/E4 in addition to several markers of oxidative stress including hydrogen peroxide, nitrotyrosine and 8-isoprostane20. Measurement of cytokines has proven less successful to date.
Although the analysis of these various molecules in breath condensate remains to be fully validated, it would seem that they could provide useful information in the disease monitoring. Significant differences in hydrogen peroxide levels were observed between controlled and non-controlled asthma21, whereas others have shown that 8-isoprostane levels are less sensitive to steroid treatment than eNO or exhaled ethane22.
Induced sputum
It has been shown that in subjects with obstructive airway disorders, an increased sputum eosinophil percentage has a higher sensitivity and specificity for the diagnosis of asthma than blood eosinophil counts or serum ECP23. The degree of sputum eosinophilia was shown to correlate with the clinical severity of the disease, in some studies 24. In addition, preliminary reports indicate that analysis of induced sputum could help in diagnosing associated conditions such as gastro-esophageal reflux by identifying lipid-laden macrophages 25 or associated left heart failure by screening for haemosiderine-laden macrophages recognized by Prussian-blue staining26. The possible role of sputum in diagnosing eosinophilic bronchitis as a cause of nonproductive cough has also been highlighted27.
An important characteristic of induced sputum is its responsiveness to interventions known to affect the degree of inflammation in asthma. As for eNO, allergen exposure increases the percent eosinophils and metachromatic cells in sputum. This was initially demonstrated following exposure to high doses of allergen given under laboratory conditions to elicit dual asthmatic reactions, which also caused an increase in circulating eosinophil counts28.
Treatment can also influence sputum eosinophil percentages. Monotherapy with short-acting inhaled b2-agonists has been shown to increase eosinophil counts. However, this effect is not observed when b2-agonists, either short- or long- acting, are given in combination with inhaled steroids20, 29. Anti-inflammatory asthma treatment decreases sputum eosinophil numbers. This has been illustrated for theophylline30, 31, antileukotrienes32, but especially for steroids33–35.
Exhaled air
Carbon monoxide
Other gases that have been measured in exhaled air include carbon monoxide (CO) and hydrocarbons such as ethane and pentane36, 37. Both are considered to be representative of the level of oxidative stress. Comparison with normal subjects indicates that exhaled CO is increased in non-steroid but not in steroid-treated asthma38, 39.
It is unclear to what extents exhaled CO and NO differ in their steroid sensitivity. The observation that children with persistent asthma, despite treatment with steroids which reduces their NO levels, have significantly higher exhaled CO compared with those with infrequent episodic asthma has led to the proposal that exhaled CO is less steroid-sensitive than eNO 37.
Hydrocarbons
The volatile hydrocarbons ethane and pentane are among the numerous end products of lipid peroxidation of peroxidised polyunsaturated fatty acids that can be measured by gas chromatography from single breath samples. Increased levels have been measured in non-steroid treated asthma. Others have described elevated pentane levels during episodes of acute asthma that returned to normal once the acute asthma subsided40. When the hydrocarbon breath test is standardized for clinical use it will likely provide a noninvasive and extremely sensitive instrument for the assessment of oxidative stress status in adults as well as in children in the future.
Exhaled NO
In the last decade there has been an explosion of interest in the measurement of eNO and other volatile substances in exhaled air. Although understanding about the link between NO and airway inflammation remains incomplete, increasing evidence supports the contention that eNO may be considered as being a readout of certain aspects of airway inflammation, particularly in atopic asthma. eNO measurement has characteristics (instantaneous, noninvasive, repeatable, safe) that make it ideally suited for children. The need for developing practical, noninvasive markers to reflect asthmatic airway inflammation is universally recognized41.
Source of NO in Exhaled Air
Nitric oxide synthases
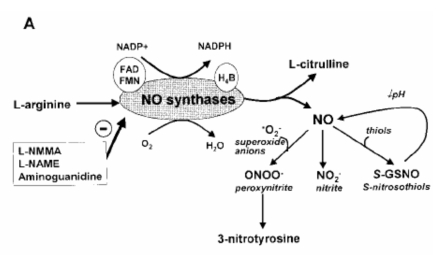
NO is derived from L-arginine by the enzyme NO synthase (NOS), of which at least three distinct isoforms exist in the human body 42. (Figure 1, panel A). Two of these enzymes are constitutively expressed and are activated by small rises in intracellular calcium concentration. Neuronal NOS (nNOS, NOS1) is predominantly expressed in neurons and endothelial NOS (eNOS, NOS3) in endothelial cells. A third enzyme that is inducible (iNOS, NOS2), may have a much greater level of activity, is independent of calcium concentration and may be induced by inflammatory cytokines.
Figure 1. Synthesis of nitric oxide (NO) and NO-related products (panel A). Sources of NO in exhaled air (panel B). (Revised from Reference 63).
Cellular sources of exhaled nitric oxide
Airway epithelial cells may express all NOS isoforms and therefore contribute to NO in the lower respiratory tract43–46. In inflammatory diseases, such as asthma, the increase in exhaled NO may reflect, at least in part, induction of NOS2. In adult asthmatic patients there is evidence of increased expression of NOS2 in airway epithelial cells47. Pro-inflammatory cytokines, which may at times be involved in asthmatic inflammation, induce the expression of NOS2 in cultured human airway epithelial cells44, 48. NOS2 may be expressed in other cell types, such as alveolar macrophages, eosinophils and other inflammatory cells49. Further evidence that the increase in exhaled NO is derived from increased NOS2 expression is the observation that corticosteroids inhibit induction of NOS2 in epithelial cells48 and in bronchial biopsies of adult asthmatic patients49, and that they also reduce exhaled NO concentrations in asthmatic patients50. (Figure 1, panel B). Epithelial cell and non-adrenergic, non-cholinergic NOS1 may also play an important role in determining both the asthmatic phenotype and the expired NO concentration.
Anatomical sites of nitric oxide formation
The levels of NO in the nose and nasopharynx are much higher than those expired from the mouth, suggesting that upper airways are a major contributor to exhaled NO, at least in normal individuals51. However, the lower respiratory tract contributes substantially to exhaled NO. Direct sampling via fibreoptic bronchoscopy in asthmatic patients shows a similar elevation of NO in trachea and main bronchi to that recorded at the mouth, thus indicating that the elevated levels in asthma are derived from the lower airways 52, 53.
Measurement of exhaled NO
Guidelines for standardized eNO measurement have been published jointly by ATS and ERS54, 55 and normal reference values with the recommended technique are available for children 4–17 years old56. Unfortunately, the application of eNO measurement to infants and young children is limited because the procedure is not well standardized for this age group.
Equipment for direct exhaled NO measurement
There are several commercially available analyzers for exhaled and nasal NO measurements: LR2000 analyzer (Logan Research Ltd, Rochester, UK), NIOX® NO analyzer (Aerocrine, Solna, Sweden), Sievers_ (Ionics Instrument, Boulder, CO, USA), and ECO Physics NO analyzer (ECOPHYSICS, Durnten, Switzerland).
The NO analyzer systems currently used in clinical investigations vary in complexity, but are based on a sensitive chemo-luminescence technique with the required accuracy. Most of the analyzers consist of a sampling system, a computerized NO analyzer with data processing, and user interface. The equipment measures the concentration of NO in sampled air online with high sensitivity in the parts per billion range (ppb), as well as pressure and flow of the sampled air. The software calculates the concentration of NO during a selected time period; displays measured and calculated data on the monitor and saves the information on disk.
The NIOX® NO analyzer has been approved for medical use in the European Union and was also cleared by the US Food and Drug administration for clinical application in patients with asthma in 200357.
Single-breath on-line measurement: eNO measurement in school - age children
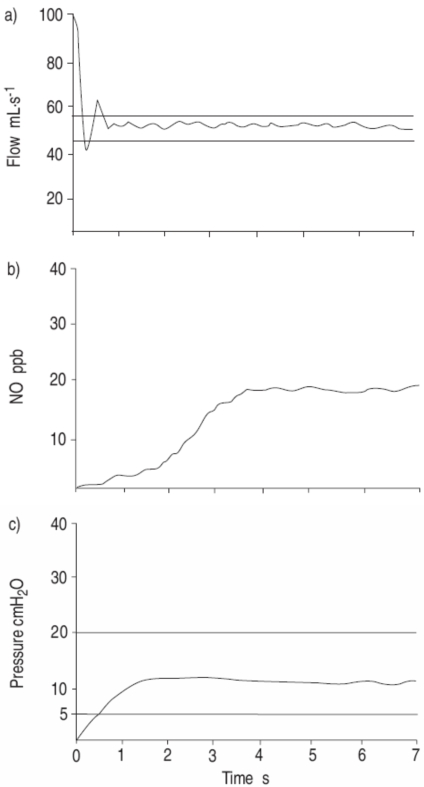
The single breath online method (SBOL) is the gold standard for eNO measurement in cooperating children (more than 4–5 years old)58. Briefly, the child should be comfortably seated and breath quietly for about 5 min to acclimatize. Inspired gas should contain low NO (v5 parts per billion (ppb)). The child inhales to near total lung capacity (TLC) and immediately exhales at a constant flow of 50 mL/sec until an NO plateau of at least 2 seconds can be identified during an exhalation of at least 4 seconds. The expiratory pressure should be maintained between 5–20 cmH2O to close the velum. Repeated exhalations (three that agree within 10% or two within 5%) should be performed with o30-s intervals and mean NO should be recorded55 (Figure 2).
Figure 2. a) Flow (airflow), b) fractional exhaled nitric oxide, and c) pressure (airway pressure) tracings for a 6-yr-old female using the single-breath on-line method at a flow rate of 50 mL/sec. The exhalation lasted 7sec, a good plateau can be identified and the expiratory pressure is maintained at 11 cmH2O during the manoeuvre. (NO: nitric oxide; ppb: parts per billion). (From Reference 55).
However, the application of single-breath on-line (SBOL) measurements in preschool children has been reported to be difficult by several investigators 55. There are several advantages in the SBOL eNO measurement that have made this the "gold standard" technique. These include the following. 1) Maintenance of constant flow. Exhaled NO is flow dependent, low flows result in higher levels and vice versa. The SBOL method keeps flow constant. 2) Exhalation from total lung capacity. SBOL exhalations are performed from (near-) TLC, the most constant and easily found lung volume. This is important as the degree of lung expansion affects eNO with values measured at the same flow rate, from the functional residual capacity (FRC), being 20% lower than those from TLC. 3) Exclusion of nasal NO. Velum closure is simple and reliable with single-breath exhalations 4) A low constant flow is easily attained. Exhaled NO can also be measured off-line, collecting exhaled air in a reservoir, again with a recommended expiratory flow of 50 ml/sec, but the standardization of this procedure is less precise than for the online method.
eNO measurement in preschool children
On-line eNO measurement during spontaneous breathing has been applied in children aged 2–5 years old 59. eNO is measured on-line during spontaneous breathing and the exhalation flow is manually adjusted to 50 ml/s by changing the exhalation resistance. The method is not well standardized, however, and still requires passive co-operation in as much as the child needs to breathe slowly and regularly through a mouthpiece.
eNO measurement in infants
There is limited experience with single-breath methods for measuring eNO in infants. eNO can be measured during tidal breathing using a facemask. Hall et al, 60 recently proposed a method for the on-line collection of tidal eNO that enables breath-to-breath monitoring in newborns and infants. A modification of the raised volume rapid thoraco-abdominal compression technique (RVRTC) has also been used to measure eNO during a single, slow forced exhalation 61. With both techniques, it is important to use a two-compartment facemask to avoid contamination by NO from the naso-pharynx. Unfortunately, the latter method requires sedation, specialized equipment and skilled operators.
How to interpret eNO concentrations
In non-smokers, exhaled NO concentration (eNO) between 5–20 ppb can be considered normal. Exhaled NO is significantly higher in asthmatic (24.3±14.8 ppb) than in healthy children (9.9 ±3.4 ppb) 62. Values >20 ppb are considered as elevated. Elevated eNO is seen in asthma, but other conditions, including viral infection of the airways, bronchiolitis obliterans syndrome and chronic obstructive pulmonary disease (COPD), may also be associated with moderately increased concentrations of exhaled NO 63. In asthmatic patients, values can increase up to 3–5-fold when compared with normal values.
Markedly increased eNO values are indicative of eosinophilic airway inflammation and predict a good response to steroid treatment. Values <5 ppb are seen in cystic fibrosis (CF) and in patients with PCD.
Factors Affecting Exhaled NO Measurements
Exhaled and nasal NO in healthy subjects is independent of age, sex, and lung function 62. There is no evidence for significant diurnal variation 64, and exhaled NO measurements are highly reproducible in normal subjects 65, 66.
There are several major factors, which may change NO levels in normal subjects (Table 2"). Some routinely used tests can transiently reduce exhaled NO; for example, repeated spirometry 67, 68, physical exercise 69, sputum induction 70. Environmental factors such as NO ozone and chlorine dioxide are known to increase exhaled NO levels 71–73. Habitual factors such as smoking 74, 75, alcohol ingestion 76, 77 reduce exhaled NO. Upper respiratory infection significantly increases exhaled NO 78, 79 and nasal NO 80.
Table 2. Factors affecting exhaled and nasal NO measurements in healthy subjects.
Definition of abbreviations: ACE: angiotensin-converting enzyme; URTI: upper respiratory tract infection. (Revised from Reference 63).
eΝΟ and lung diseases
eΝΟ and atopy
Exhaled NO is elevated in allergic/ atopic adults and children 81. It is further increased as a result of allergen exposure such as during the late phase response to allergen challenge 82, 83, during the grass pollen season 84, or during exposure to indoor allergens 85, 86. Children 81 with atopic asthma have higher levels of exhaled NO than do patients with non-atopic asthma, even without airway hyper responsiveness 87.
eΝΟ and Asthma
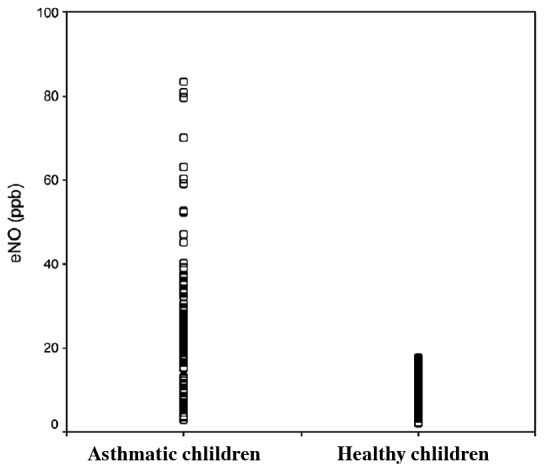
Increased levels of exhaled NO have been widely documented in patients with asthma (Figure 3) 88, 89. The increased levels of exhaled NO in asthma have a predominant lower airway origin 90 and are most likely due to activation of NOS2 in airway epithelial and inflammatory cells 91.
Figure 3. Scatter gram of exhaled NO in normal and in asthmatic children. eNO was significantly higher in asthmatic (24.3±14.8 ppb) than in healthy children (9.9 ±3.4 ppb); p<0.0001. (From Reference 62).
Asthma Diagnosis
An elevation of exhaled NO is not specific for asthma, but an increased level may be useful in differentiating asthma from other causes of chronic cough 92. The diagnostic value of exhaled NO measurements to differentiate between healthy subjects with or without respiratory symptoms and patients with confirmed asthma has been recently analyzed by Dupont and colleagues93 with 90% specificity and 95% positive predictive value when exhaled NO 15 ppb is used as a cut-off for asthma.
Exhaled and nasal NO may be used to identify subjects with atopy, because non-atopic asthmatics have normal exhaled NO94. There is a strong association between elevated exhaled and nasal NO and skin prick test scores, total IgE95, and blood eosinophilia96 in mild asthma. Elevated nasal NO is also related to the size of skin test reactivity in asymptomatic asthmatic subjects97. This may denote “sub clinical” airway inflammation.
Recent studies have demonstrated that exhaled NO is superior to conventional tests, including baseline respiratory function and bronchodilator responsiveness, in identifying patients with probable asthma, both in children and adults19.
Another potential use of exhaled NO levels in patient management is the prediction of future asthma. An elevated exhaled NO may be found in patients with “sub clinical” forms of asthma (normal lung function, negative bronchodilator tests, and elevated sputum eosinophilic cationic protein concentrations) 98, 99. Elevated levels of NO in patients with “sub clinical asthma” are not in conflict with the specificity of exhaled NO as a marker to diagnose asthma, as lack of current asthma symptoms does not exclude the diagnosis of asthma.
Differential diagnosis
In asthma, levels of eNO can be increased several-fold when compared with healthy subjects 100; although there is an important overlap between different patients 63, 101 (Figure 3). It is important to mention, however, that the typical high increase in eNO is mostly seen in atopic-asthmatic patients, and some studies have shown that normal levels of eNO can be detected in non-atopic asthmatic subjects102.
The eNO measurement is also useful in the differential diagnosis of patients with dry cough103. Patients with chronic cough that is not attributable to asthma have lower NO values, as compared with healthy volunteers and patients with asthma104, including those with cough due to gastro-oesophageal reflux105.
Relationship to other markers of asthma
The traditional means of monitoring asthma have limitations. Lung function and PC 20, measurements are not directly related to airway inflammation, have little room for improvement in mild asthma (FEV 1), and are affected by bronchodilators. Both parameters are slow to change and are not able to distinguish the effect of different doses of steroids. There are several areas in which exhaled NO measurements may be advantageous over the traditional means of asthma monitoring: screening for atopy, monitoring the impact of hazardous environmental factors, identification and monitoring of asthma exacerbations, and assessment of the adequacy of anti-inflammatory treatment.
Exhaled NO in patients with asthma is correlated with sputum eosinophils106–108 and methacholine reactivity109, 110.
Asthma monitoring
It is difficult to monitor the response of different classes of anti-inflammatory drugs in asthma, as there is no single test that can be used to quantify airway inflammation. Peripheral blood markers are unlikely to be adequate as the most important mediator and cellular responses occur locally within airways. Eosinophils in induced sputum originate from more proximal rather than small airway17. It is clear that different markers of airway inflammation should be considered together to monitor asthma110.
Exhaled NO has been used to monitor the effect of anti-inflammatory treatment in asthma111 and asthma exacerbations, both spontaneous and induced by steroid reduction112, 113. There is a lack of long-term serial studies of exhaled NO, together with other markers of airway inflammation in sputum and exhaled condensate, lung function and symptoms. Exhaled NO behaves as a “rapid response” marker, which is extremely sensitive to steroid treatment, as it may be significantly reduced even after 6 h following a single treatment with a nebulized steroid114, or within 2 to 3 d after inhaled corticosteroids111, reaching maximal effect after 2 to 4 wk of treatment112, 115.
Corticosteroids
Systemic corticosteroids have no effect on exhaled NO in normal subjects, but they decease its levels in patients with asthma116.
Inhaled corticosteroids reduce exhaled NO in asthmatic patients 111 and this effect is dose-related106. However, a plateau effect on exhaled NO measured after 6 to 12 h since the last treatment may be seen at a dose of 400 μg budesonide and higher 106. A gradual reduction in exhaled NO is seen during the first week of regular treatment with maximal effect between 3 wk (105) or 4 wk106, 115.
The reduction in exhaled NO by anti-inflammatory treatment is explained by the suppression of inflammation, resulting in reduced levels of inflammatory cytokines and, therefore, a decrease in the signal for NO overproduction by iNOS.
Inhaled β2-agonists
Neither short-acting β2-agonists nor long- acting β2-agonists (LABAs) reduce exhaled NO116. This is consistent with the fact that they do not have any anti-inflammatory effects in asthma.
Leukotriene antagonists
The leukotriene receptor antagonist pranlukast blocks the increase in exhaled NO when inhaled corticosteroids are withdrawn117, and montelukast rapidly reduces exhaled NO by 15 to 30% in children with asthma118. Antileukotrienes have a moderate effect in patients with asthma and seasonal allergic rhinitis119.
Disease control and severity
Based on the relationship between changes in clinical measures of asthma control and exhaled NO level, measurement of exhaled NO seems to be a useful marker for monitoring the efficacy of asthma control57. This suggestion is further confirmed by the findings of others, showing that eNO predicts asthma relapse in asymptomatic asthmatic patients after the withdrawal of corticosteroids120.
Treatment with inhaled corticosteroids reduces exhaled NO levels, and therefore exhaled NO cannot be directly related to asthma severity. Exhaled NO levels are almost three times higher in children with recent symptoms than in symptom-free subjects121, and are further elevated during the asthma attack in both adults122 and children123, 124.
How to interpret eNO data
In asthma, the use of exhaled NO has been proposed to diagnose asthma, to monitor the response to anti-inflammatory therapy, to evaluate compliance and to predict upcoming asthma exacerbations. Furthermore, it is also proposed that treatment, which is guided by the monitoring of non-invasive markers, such as sputum eosinophils and exhaled NO, could improve overall asthma control.
Elevated eNO values seen in untreated patients confirm the diagnosis of asthma suggestive of ongoing eosinophilic airway inflammation. Successful anti-inflammatory treatment causes a decrease in eNO, which remains low if the patient is stable. If eNO is not decreased, this may be caused by non-compliance or by insufficient dose of corticosteroids. Low eNO values seen in patients stable on anti-inflammatory treatment increase when airway inflammation worsens, for example, due to allergen exposure.
eΝΟ and cystic fibrosis
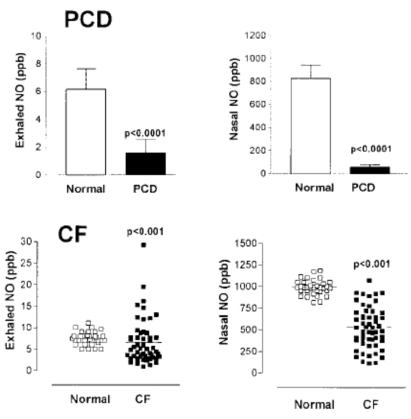
Exhaled and nasal NO levels are significantly lower in patients with cystic fibrosis (CF) than in normal subjects, despite the intense neutrophilic inflammation in the airways (Figure 4)125 leading to the release of superoxide anions, which convert NO to nitrate and may result in the formation of peroxynitrite126. Increased oxidative stress in CF is likely to be a consequence of this neutrophilic inflammation, malnutrition, and IL-10 deficiency127, 128. Although there is a trend toward both exhaled and nasal NO being higher in patients who were not homozygous for the ΔF508 CF transmembrane regulator mutation129, 130, there is no strong association between exhaled NO and disease severity in CF131 or infection with Pseudomonas.
Figure 4. Exhaled and nasal NO in primary ciliary dyskinesia (PCD) and cystic fibrosis (CF). (From Reference 130 and 135).
There are several possible reasons for the low levels of NO in patients with CF. First, there is a deficiency of NOS2 in patients with CF131. Constitutive expression of NOS2, which has been demonstrated in normal human airway epithelium, and of non-CF mouse is essentially absent in the epithelium of CF airways132. Neutrophils enhance expression of NOS2 in normal human bronchial epithelial cells but not in CF epithelial cells133. The low expression of NOS2 would account for the low levels of NO in nasal as well as exhaled air.
eNO and primary ciliary diskinesia
Primary ciliary dyskinesia (PCD) presents to general practitioners with symptoms pertinent to a variety of specialists because of the involvement of ciliated epithelium in the upper/lower respiratory tract, ears, eyes and genital tract. There is no easy, reliable screening test for PCD, and thus, the majority of patients remain undiagnosed.
It has been decisively shown that measurement of nasal NO can be used in clinical practice in various specialities to screen suspected patients for PCD, both adults134 and children135 (Figure 4). Such low values of exhaled and nasal NO are not seen in any other condition and are therefore of diagnostic value. Measurement of exhaled NO might be used as a screening procedure to detect PCD amongst patients with recurrent chest infections or male infertility due to immotile spermatozoa, and the diagnosis of PCD is then confirmed by the saccharine test, nasal nitric oxide, ciliary beat frequency and electron microscopy136.
NO plays an important role in bactericidal activity in the lungs, sodium and chloride transport in nasal epithelium, and ciliary beating, so that a lack of endogenous NO production might contribute to the characteristic recurrent chest infections in patients with PCD. Despite the lower levels of exhaled NO in children with PCD, no differences were found in the mean levels of NO metabolites in exhaled breath condensate137, suggesting that detection of NO in exhaled and nasal breath, but not in the EBC, may be the method of choice in the diagnosis of PCD.
Conclusions
Exhaled nitric oxide has been established as a marker of eosinophilic airway inflammation in asthma and recent studies have included eNO measurement in treatment algorithms. This standardized technique is simple, reproducible and is ready for routine monitoring.
Advances in technology have recently resulted in smaller devices that are easier to use and cheaper. It may be possible to introduce such analyzers in the very near future in family practice and even into patients' homes, so that patients themselves will be able to monitor their own markers and adjust their treatment accordingly.
Exhaled NO may be useful as a diagnostic tool in patients with asthma and in wheezing infants. eNO is better than lung function and bronchodilator responsiveness in identifying preschool children with asthma138. Exhaled NO levels can differentiate asthmatic young children from non-asthmatic cases with chronic cough139. In children with recurrent wheezing, raised eNO values suggest the presence of eosinophilic airway inflammation and may be useful in identifying patients most likely to respond to inhaled corticosteroid (ICS) therapy and furthermore, high eNO levels have been used to predict the benefits of ICS therapy140. Exhaled NO measurement is also useful in asthma follow-up; may predict asthma relapse after discontinuing steroids141 and can predict the failure of attempts to reduce ICS dosages in children with good symptom control142.
References
- 1.American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis. 1987;136:225–244. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 2.Rytila P, Metso T, Heikkinen K, Saarelainen P, Helenius IJ, Haahtela T. Airway inflammation in patients with symptoms suggestive of asthma but with normal lung function. Eur Respir J. 2000;16:824–830. doi: 10.1183/09031936.00.16582400. [DOI] [PubMed] [Google Scholar]
- 3.Toorn LM, Prins J, Overbeek SE, et al. Airway inflammation is present during clinical remission of atopic asthma. Am J Respir Crit Care Med. 2001;164:2107–2113. doi: 10.1164/ajrccm.164.11.2006165. [DOI] [PubMed] [Google Scholar]
- 4.Green RH, Brightling CE, McKenna S, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–1721. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 5.Vignola AM, Rennar SI, Hargreave FE, et al. Standardised methodology of sputum induction and processing. Future directions. Eur Respir J. 2002;20(Suppl. 37):51s–55s. [PubMed] [Google Scholar]
- 6.Kips JC, Kharitonov SA, Barnes PJ. Noninvasive assessment of airway inflammation in asthma. Eur Respir Mon. 2003;23:164–179. [Google Scholar]
- 7.Kips JC, Pauwels RA. Serum eosinophil cationic protein in asthma: what does it mean? Clin Exp Allergy. 1998;28:1–3. doi: 10.1046/j.1365-2222.1998.00194.x. [DOI] [PubMed] [Google Scholar]
- 8.Niimi A, Amitani R, Suzuki K, Tanaka E, Murayama T, Kuze F. Serum eosinophil cationic protein as a marker of eosinophilic inflammation in asthma. Clin Exp Allergy. 1998;28:233–240. doi: 10.1046/j.1365-2222.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- 9.Hoshino M, Nakamura Y. Relationship between activated eosinophils of the bronchial mucosa and serum eosinophil cationic protein in atopic asthma. Int Arch Allergy Immunol. 1997;112:59–64. doi: 10.1159/000237432. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson AC, Vaughan R, Brown H, Curtis C. Evaluation of serum eosinophilic cationic protein as a marker of disease activity in chronic asthma. J Allergy Clin Immunol. 1995;95:23–28. doi: 10.1016/s0091-6749(95)70148-6. [DOI] [PubMed] [Google Scholar]
- 11.Fujisawa T, Terada A, Atsuta J, Iguchi K, Kamiya H, Sakurai M. Clinical utility of serum levels of eosinophil cationic protein (ECP) for monitoring and predicting clinical course in childhood asthma. Clin Exp Allergy. 1998;28:19–25. doi: 10.1046/j.1365-2222.1998.00148.x. [DOI] [PubMed] [Google Scholar]
- 12.Kristjansson S, Strannegard IL, Strannegard O, Peterson C, Enander I, Wennergren G. Urinary eosinophil protein X in children with atopic asthma: a useful marker of anti-inflammatory treatment. J Allergy Clin Immunol. 1996;97:1179–1187. doi: 10.1016/s0091-6749(96)70182-3. [DOI] [PubMed] [Google Scholar]
- 13.van Velzen E, van den Bos JW, Benckhuijsen JA, van Essel T, de Bruijn R, Aalbers R. Effect of allergen avoidance at high altitude on direct and indirect bronchial hyperresponsiveness and markers of inflammation in children with allergic asthma. Thorax. 1996;51:582–584. doi: 10.1136/thx.51.6.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmerman B, Lanner A, Enander I, Zimmerman RS, Peterson CG, Ahlstedt S. Total blood eosinophils, serum eosinophil cationic protein and eosinophil protein X in childhood asthma: relation to disease status and therapy. Clin Exp Allergy. 1993;23:564–570. doi: 10.1111/j.1365-2222.1993.tb00895.x. [DOI] [PubMed] [Google Scholar]
- 15.Kumlin M, Stensvad F, Larsson L, Dahlen B, Dahlen SE. Validation and application of a new simple strategy for measurements of urinary leukotriene E4 in humans. Clin Exp Allergy. 1995;25:467–479. doi: 10.1111/j.1365-2222.1995.tb01079.x. [DOI] [PubMed] [Google Scholar]
- 16.Dahlen SE, Kumlin M. Can asthma be studied in the urine? Clin Exp Allergy. 1998;28:129–133. doi: 10.1046/j.1365-2222.1998.00186.x. [DOI] [PubMed] [Google Scholar]
- 17.Kharitonov SA, Barnes PJ. Clinical aspects of exhaled nitric oxide. Eur Respir J. 2000;16:781–792. doi: 10.1183/09031936.00.16478100. [DOI] [PubMed] [Google Scholar]
- 18.Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001;163:1693–1722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- 19.Horvath I. Airway inflammation: exhaled NO measurement in clinical practice. Breathe. 2005;1(3):229–235. [Google Scholar]
- 20.Hanazawa T, Kharitonov SA, Barnes PJ. Increased nitrotyrosine in exhaled breath condensate of patients with asthma. Am J Respir Crit Care Med. 2000;162:1273–1276. doi: 10.1164/ajrccm.162.4.9912064. [DOI] [PubMed] [Google Scholar]
- 21.Dohlman AW, Black HR, Royall JA. Expired breath hydrogen peroxide is a marker of acute airway inflammation in pediatric patients with asthma. Am Rev Respir Dis. 1993;148:955–960. doi: 10.1164/ajrccm/148.4_Pt_1.955. [DOI] [PubMed] [Google Scholar]
- 22.Montuschi P, Corradi M, Ciabattoni G, Nightingale J, Kharitonov SA, Barnes PJ. Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. Am J Respir Crit Care Med. 1999;160:216–220. doi: 10.1164/ajrccm.160.1.9809140. [DOI] [PubMed] [Google Scholar]
- 23.Pizzichini E, Pizzichini MM, Efthimiadis A, Dolovich J, Hargreave FE. Measuring airway inflammation in asthma: eosinophils and eosinophilic cationic protein in induced sputum compared with peripheral blood. J Allergy Clin Immunol. 1997;99:539–544. doi: 10.1016/s0091-6749(97)70082-4. [DOI] [PubMed] [Google Scholar]
- 24.Louis R, Lau LC, Bron AO, Roldaan AC, Radermecker M, Djukanovic R. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med. 2000;161:9–16. doi: 10.1164/ajrccm.161.1.9802048. [DOI] [PubMed] [Google Scholar]
- 25.Parameswaran K, Anvari M, Efthimiadis A, Kamada D, Hargreave FE, Allen CJ. Lipid-laden macrophages in induced sputum are a marker of oropharyngeal reflux and possible gastric aspiration. Eur Respir J. 2000;16:1119–1122. doi: 10.1034/j.1399-3003.2000.16f17.x. [DOI] [PubMed] [Google Scholar]
- 26.Leigh R, Sharon RF, Efthimiadis A, Hargreave FE, Kitching AD. Diagnosis of left-ventricular dysfunction from induced sputum examination (letter) Lancet. 1999;354:833–834. doi: 10.1016/S0140-6736(99)80018-X. [DOI] [PubMed] [Google Scholar]
- 27.Brightling CE, Pavord ID. Eosinophilic bronchitis - what is it and why is it important? Clin Exp Allergy. 2000;30:4–6. doi: 10.1046/j.1365-2222.2000.00740.x. (editorial; comment) [DOI] [PubMed] [Google Scholar]
- 28.Gauvreau GM, Doctor J, Watson RM, Jordana M, O’Byrne PM. Effects of inhaled budesonide on allergen-induced airway responses and airway inflammation. Am J Respir Crit Care Med. 1996;154:1267–1271. doi: 10.1164/ajrccm.154.5.8912734. [DOI] [PubMed] [Google Scholar]
- 29.Kips JC, O’Connor BJ, Inman MD, Svensson K, Pauwels RA, O’Byrne PM. A long-term study of the antiinflammatory effect of low-dose budesonide plus formoterol versus high-dose budesonide in asthma. Am J Respir Crit Care Med. 2000;161:996–1001. doi: 10.1164/ajrccm.161.3.9812056. [DOI] [PubMed] [Google Scholar]
- 30.Aizawa H, Iwanaga T, Inoue H, Takata S, Matsumoto K, Takahashi N. Once-daily theophylline reduces serum eosinophil cationic protein and eosinophil levels in induced sputum of asthmatics. Int Arch Allergy Immunol. 2000;121:123–128. doi: 10.1159/000024307. [DOI] [PubMed] [Google Scholar]
- 31.Tohda Y, Muraki M, Iwanaga T, Kubo H, Fukuoka M, Nakajima S. The effect of theophylline on blood and sputum eosinophils and ECP in patients with bronchial asthma. Int J Immunopharmacol. 1998;20:173–181. doi: 10.1016/s0192-0561(98)00026-5. [DOI] [PubMed] [Google Scholar]
- 32.Pizzichini E, Leff JA, Reiss TF, Hendeles L, Boulet LP, Wei LX. Montelukast reduces airway eosinophilic inflammation in asthma: a randomized, controlled trial. Eur Respir J. 1999;14:12–18. doi: 10.1034/j.1399-3003.1999.14a04.x. [DOI] [PubMed] [Google Scholar]
- 33.Gershman NH, Wong HH, Liu JT, Fahy JV. Low- and high-dose fluticasone propionate in asthma; effects during and after treatment. Eur Respir J. 2000;15:11–18. doi: 10.1183/09031936.00.15101100. [DOI] [PubMed] [Google Scholar]
- 34.Fahy JV, Boushey HA. Effect of low-dose beclomethasone dipropionate on asthma control and airway inflammation. Eur Respir J. 1998;11:1240–1247. doi: 10.1183/09031936.98.11061240. [DOI] [PubMed] [Google Scholar]
- 35.Veen JC, Smits HH, Hiemstra PS, Zwinderman AE, Sterk PJ, Bel EH. Lung function and sputum characteristics of patients with severe asthma during an induced exacerbation by double blind steroid withdrawal. Am J Respir Crit Care Med. 1999;160:93–99. doi: 10.1164/ajrccm.160.1.9809104. [DOI] [PubMed] [Google Scholar]
- 36.Paredi P, Kharitonov SA, Barnes PJ. Elevation of exhaled ethane concentration in asthma. Am J Respir Crit Care Med. 2000;162:1450–1454. doi: 10.1164/ajrccm.162.4.2003064. [DOI] [PubMed] [Google Scholar]
- 37.Uasuf CG, Jatakanon A, James A, Kharitonov SA, Wilson NM, Barnes PJ. Exhaled carbon monoxide in childhood asthma. J Pediatr. 1999;135:569–574. doi: 10.1016/s0022-3476(99)70054-5. [DOI] [PubMed] [Google Scholar]
- 38.Horvath I, Donnelly LE, Kiss A, Paredi P, Kharitonov SA, Barnes PJ. Raised levels of exhaled carbon monoxide are associated with an increased expression of heme oxygenase-1 in airway macrophages in asthma: a new marker of oxidative stress. Thorax. 1998;53:668–672. doi: 10.1136/thx.53.8.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zayasu K, Sekizawa K, Okinaga S, Yamaya M, Ohrui T, Sasaki H. Increased carbon monoxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med. 1997;156:1140–1143. doi: 10.1164/ajrccm.156.4.96-08056. [DOI] [PubMed] [Google Scholar]
- 40.Olopade CO, Christon JA, Zakkar M, Hua C, Swedler WI, Scheff PA. Exhaled pentane and nitric oxide levels in patients with obstructive sleep apnea. Chest. 1997;111:1500–1504. doi: 10.1378/chest.111.6.1500. [DOI] [PubMed] [Google Scholar]
- 41.National Heart, Lung and Blood Institute. Global strategy for asthma management and prevention. NHLBI Workshop Report. NIH Publication. 1995;95 95. [Google Scholar]
- 42.Nathan C, Xie Q-W. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994;269:13725–13728. [PubMed] [Google Scholar]
- 43.Shaul PW, North AJ, Wu LC, et al. Endothelial nitric oxide synthase is expressed in cultured bronchiolar epithelium. J Clin Invest. 1994;94:2231–2236. doi: 10.1172/JCI117585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asano K, Chee CBE, Gaston B, et al. Constitutive and inducible nitric oxide synthase gene expression, regulation and activity in human lung epithelial cells. Proc Natl Acad Sci USA. 1994;91:10089–10093. doi: 10.1073/pnas.91.21.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo FH, de Raeve HR, Rice TW, Stuehr DJ, Thunnissen FBJM, Erzurum SC. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci USA. 1995;92:7809–7813. doi: 10.1073/pnas.92.17.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobzik L, Bredt DS, Lowenstein CJ, et al. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol. 1993;9:371–377. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- 47.Hamid Q, Springall DR, Riveros-Moreno V, et al. Induction of nitric oxide synthase in asthma. Lancet. 1993;342:1510–1513. doi: 10.1016/s0140-6736(05)80083-2. [DOI] [PubMed] [Google Scholar]
- 48.Robbins RA, Barnes PJ, Springall DR, et al. Expression of inducible nitric oxide synthase in human bronchial epithelial cells. Biochem Biophys Res Commun. 1994;203:209–218. doi: 10.1006/bbrc.1994.2169. [DOI] [PubMed] [Google Scholar]
- 49.Saleh D, Ernst P, Lim S, Barnes PJ, Giaid A. Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of nitric oxide synthase: effect of inhaled glucocorticoid. FASEB J. 1998;12:929–937. [PubMed] [Google Scholar]
- 50.Kharitonov SA, Yates DH, Barnes PJ. Regular inhaled budesonide decreases nitric oxide concentration in the exhaled air of asthmatic patients. Am J Respir Crit Care Med. 1996;153:454–457. doi: 10.1164/ajrccm.153.1.8542158. [DOI] [PubMed] [Google Scholar]
- 51.Lundberg JON, Weitzberg E, Nordvall SL, Kuylenstierna R, Lundberg JM, Alving K. Primarily nasal origin of exhaled nitric oxide and absence in Kartagener’s syndrome. Eur Respir J. 1994;8:1501–1504. doi: 10.1183/09031936.94.07081501. [DOI] [PubMed] [Google Scholar]
- 52.Kharitonov S, Chung KF, Evans DJ, O’Connor BJ, Barnes PJ. Increased exhaled nitric oxide in asthma is derived from the lower respiratory tract. Am J Respir Crit Care Med. 1996;153:1773–1780. doi: 10.1164/ajrccm.153.6.8665033. [DOI] [PubMed] [Google Scholar]
- 53.Massaro AF, Mehta S, Lilly CM, Kobzik L, Reilly JJ, Drazen JM. Elevated nitric oxide concentrations in isolated lower airway gas of asthmatic subjects. Am J Respir Crit Care Med. 1996;153:1510–1514. doi: 10.1164/ajrccm.153.5.8630594. [DOI] [PubMed] [Google Scholar]
- 54.American Thoracic Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 55.Baraldi E, de Jongste JC. Measurement of exhaled nitric oxide in children, 2001. Eur Respir J. 2002;20:223–237. doi: 10.1183/09031936.02.00293102. [DOI] [PubMed] [Google Scholar]
- 56.Buchvald F, Baraldi E, Carraro S, et al. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol. 2005;115:1130–1136. doi: 10.1016/j.jaci.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 57.Kharitonov SA. Exhaled markers of inflammatory lung diseases: ready for routine monitoring? Swiss Med WKLY. 2004;134:175–192. doi: 10.4414/smw.2004.10411. [DOI] [PubMed] [Google Scholar]
- 58.Baraldi EandCarraroS. Exhaled NO and breath condensate. Paediatric Respiratory Reviews. 2006;7S:S20–S22. doi: 10.1016/j.prrv.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 59.Buchvald F, Bisgaard H. FeNO measured at fixed exhalation flow rate during controlled tidal breathing in children from the age of 2 yr. Am J Respir Crit Care Med. 2001;163:699–704. doi: 10.1164/ajrccm.163.3.2004233. [DOI] [PubMed] [Google Scholar]
- 60.Hall G, Reinmann B, Wildhaber J, Frey U. Tidal exhaled nitric oxide in healthy, unsedated newborn infants with prenatal tobacco exposure. J Appl Physiol. 2002;92:59–66. doi: 10.1152/jappl.2002.92.1.59. [DOI] [PubMed] [Google Scholar]
- 61.Wildhaber JH, Hall GL, Stick SM. Measurements of exhaled nitric oxide with the single-breath technique and positive expiratory pressure in infants. Am J Respir Crit Care Med. 1999;159:74–78. doi: 10.1164/ajrccm.159.1.9805021. [DOI] [PubMed] [Google Scholar]
- 62.Hatziagorou E, Sarafidou S, Kirvassilis F, Emboriadou M, Aivazis V, Tsanakas J. Measurements of exhaled nitric oxide in healthy subjects age 5 to 14 years. Eur Respir J. 2006;28:259S. [Google Scholar]
- 63.Kharitonov SA, Barnes PJ. Exhaled Markers of Pulmonary Disease. Am J Respir Crit Care Med. 2001;163:1693–1722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- 64.ten Hasken NHT, van der Vaart H, van der Mark TW, Kolter GH, Postma DS. Exhaled nitric oxide is higher both at day and night in subjects with nocturnal asthma. Am J Respir Crit Care Med. 1998;158:902–907. doi: 10.1164/ajrccm.158.3.9712021. [DOI] [PubMed] [Google Scholar]
- 65.Purokivi M, Randell J, Hirvonen MR, Tukiainen H. Reproducibility of measurements of exhaled NO, and cell count and cytokine concentrations in induced sputum. Eur Respir J. 2000;16:242–246. doi: 10.1034/j.1399-3003.2000.16b10.x. [DOI] [PubMed] [Google Scholar]
- 66.Bartley J, Fergusson W, Moody A, Wells AU, Kolbe J. Normal adult values, diurnal variation, and repeatability of nasal nitric oxide measurement. Am J Rhinol. 1999;13:401–405. doi: 10.2500/105065899781367528. [DOI] [PubMed] [Google Scholar]
- 67.Deykin A, Massaro AF, Coulston E, Drazen JM, Israel E. Exhaled NO following repeated spirometry or repeated plethysmography in healthy individuals. Am J Respir Crit Care Med. 2000;161:1237–1240. doi: 10.1164/ajrccm.161.4.9904086. [DOI] [PubMed] [Google Scholar]
- 68.Silkoff PE, Wakita S, Chatkin J, et al. Exhaled nitric oxide after beta2-agonist inhalation and spirometry in asthma. Am J Respir Crit Care Med. 1999;159:940–944. doi: 10.1164/ajrccm.159.3.9805044. [DOI] [PubMed] [Google Scholar]
- 69.Phillips CR, Giraud GD, Holden WE. Exhaled nitric oxide during exercise: site of release and modulation by ventilation and blood flow. J Appl Physiol. 1996;80:1865–1871. doi: 10.1152/jappl.1996.80.6.1865. [DOI] [PubMed] [Google Scholar]
- 70.Piacentini GL, Bodini A, Costella S, Vicentini L, Suzuki Y, Boner AL. Exhaled nitric oxide is reduced after sputum induction in asthmatic children. Pediatr Pulmonol. 2000;29:430–433. doi: 10.1002/(sici)1099-0496(200006)29:6<430::aid-ppul3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 71.Nightingale JA, Rogers DF, Barnes PJ. Effect of inhaled ozone on exhaled nitric oxide, pulmonary function, and induced sputum in normal and asthmatic subjects. Thorax. 1999;54:1061–1069. doi: 10.1136/thx.54.12.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olin AC, Ljungkvist G, Bake B, Hagberg S, Henriksson L, Toren K. Exhaled nitric oxide among pulpmill workers reporting gassing incidents involving ozone and chlorine dioxide. Eur Respir J. 1999;14:828–831. doi: 10.1034/j.1399-3003.1999.14d18.x. [DOI] [PubMed] [Google Scholar]
- 73.van Amsterdam JG, Verlaan BP, van Loveren H, Elzakker BG, Vos SG, Opperhuizen A, Steerenberg PA. Air pollution is associated with increased level of exhaled nitric oxide in nonsmoking healthy subjects. Arch Environ Health. 1999;54:331–335. doi: 10.1080/00039899909602496. [DOI] [PubMed] [Google Scholar]
- 74.Kharitonov SA, Robbins RA, Yates DH, Keatings V, Barnes PJ. Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am J Respir Crit Care Med. 1995;152:609–612. doi: 10.1164/ajrccm.152.2.7543345. [DOI] [PubMed] [Google Scholar]
- 75.Robbins RA, Floreani AA, Von Essen SG, et al. Measurement of exhaled nitric oxide by three different techniques. Am J Respir Crit Care Med. 1996;153:1631–1635. doi: 10.1164/ajrccm.153.5.8630613. [DOI] [PubMed] [Google Scholar]
- 76.Yates DH, Kharitonov SA, Robbins RA, Thomas PS, Barnes PJ. The effect of alcohol ingestion on exhaled nitric oxide. Eur Respir J. 1996;9:1130–1133. doi: 10.1183/09031936.96.09061130. [DOI] [PubMed] [Google Scholar]
- 77.Persson MG, Gustafsson LE. Ethanol can inhibit nitric oxide production. Eur Respir J. 1992;224:99–100. doi: 10.1016/0014-2999(92)94826-h. [DOI] [PubMed] [Google Scholar]
- 78.Kharitonov SA, Yates DH, Barnes PJ. Increased nitric oxide in exhaled air of normal human subjects with upper respiratory infections. Eur Respir J. 1995:295–297. doi: 10.1183/09031936.95.08020295. [DOI] [PubMed] [Google Scholar]
- 79.Murphy AW, Platt-Mills TA, Lobo M, Hayden F. Respiratory nitric oxide levels in experimental human influenza. Chest. 1999;114:452–456. doi: 10.1378/chest.114.2.452. [DOI] [PubMed] [Google Scholar]
- 80.Ferguson EA, Eccles R. Changes in nasal nitric oxide concentration associated with symptoms of common cold and treatment with a topical nasal decongestant. Acta Otolaryngol. 1997;117:614–617. doi: 10.3109/00016489709113447. [DOI] [PubMed] [Google Scholar]
- 81.Frank TL, Adisesh A, Pickering AC, et al. Relationship between exhaled nitric oxide and childhood asthma. Am J Respir Crit Care Med. 1998;158:1032–1036. doi: 10.1164/ajrccm.158.4.9707143. [DOI] [PubMed] [Google Scholar]
- 82.Kharitonov SA, O’Connor BJ, Evans DJ, Barnes PJ. Allergen-induced late asthmatic reactions are associated with elevation of exhaled nitric oxide. Am J Respir Crit Care Med. 1995;151:1894–1899. doi: 10.1164/ajrccm.151.6.7767537. [DOI] [PubMed] [Google Scholar]
- 83.Paredi P, Leckie MJ, Horvath I, Allegra L, Kharitonov SA, Barnes PJ. Exhaled carbon monoxide is elevated following allergen challenge in patients with asthma. Eur Respir J. 1999;13:48–52. doi: 10.1183/09031936.99.13104899. [DOI] [PubMed] [Google Scholar]
- 84.Baraldi E, Carra S, Dario C, et al. Effect of natural grass pollen exposure on exhaled nitric oxide in asthmatic children. Am J Respir Crit Care Med. 1999;159:262–266. doi: 10.1164/ajrccm.159.1.9804063. [DOI] [PubMed] [Google Scholar]
- 85.Piacentini GL, Bodini A, Costella S, et al. Exhaled nitric oxide in asthmatic children exposed to relevant allergens: effect of flunisolide. Eur Respir J. 2000;15:730–734. doi: 10.1034/j.1399-3003.2000.15d17.x. [DOI] [PubMed] [Google Scholar]
- 86.Simpson A, Custovic A, Pipis S, Adisesh A, Faragher B, Woodcock A. Exhaled nitric oxide, sensitization, and exposure to allergens in patients with asthma who are not taking inhaled steroids. Am J Respir Crit Care Med. 1999;160:45–49. doi: 10.1164/ajrccm.160.1.9809091. [DOI] [PubMed] [Google Scholar]
- 87.Salome CM, Roberts AM, Brown NJ, Dermand J, Marks GB, Woolcock AJ. Exhaled nitric oxide measurements in a population sample of young adults. Am J Respir Crit Care Med. 1999;159:911–916. doi: 10.1164/ajrccm.159.3.9802108. [DOI] [PubMed] [Google Scholar]
- 88.Saleh D, Ernst P, Lim S, Barnes PJ, Giaid A. Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of nitric oxide synthase: effect of inhaled glucocorticoid. FASEB J. 1998;12:929–937. [PubMed] [Google Scholar]
- 89.Kharitonov SA, Yates DH, Robbins RA, Logan-Sinclair R, Shinebourne EA, Barnes PJ. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994;343:133–135. doi: 10.1016/s0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- 90.Kharitonov SA, Chung FK, Evans DJ, O’Connor BJ, Barnes PJ. The elevated level of exhaled nitric oxide in asthmatic patients is mainly derived from the lower respiratory tract. Am J Respir Crit Care Med. 1996;153:1773–1780. doi: 10.1164/ajrccm.153.6.8665033. [DOI] [PubMed] [Google Scholar]
- 91.Persson MG, Zetterstrom O, Agrenius V, Ihre E, Gustafsson LE. Single- breath nitric oxide measurements in asthmatic patients and smokers. Lancet. 1994;343:146–147. doi: 10.1016/s0140-6736(94)90935-0. [DOI] [PubMed] [Google Scholar]
- 92.Chatkin JM, Ansarin K, Silkoff PE, et al. Exhaled nitric oxide as a noninvasive assessment of chronic cough. Am J Respir Crit Care Med. 1999;159:1810–1813. doi: 10.1164/ajrccm.159.6.9809047. [DOI] [PubMed] [Google Scholar]
- 93.Dupont LJ, Demedts MG, Verleden GM. Prospective evaluation of the accuracy of exhaled nitric oxide for the diagnosis of asthma [abstract] Am J Respir Crit Care Med. 1999;159:A861. [Google Scholar]
- 94.Ludviksdottir D, Janson C, Hogman M, Hedenstrom H, Bjornsson E, Boman G. Exhaled nitric oxide and its relationship to airway responsiveness and atopy in asthma: BHR-Study Group. Respir Med. 1999;93:552–556. doi: 10.1016/s0954-6111(99)90154-3. [DOI] [PubMed] [Google Scholar]
- 95.Ho LP, Wood FT, Robson A, Innes JA, Greening AP. Atopy influences exhaled nitric oxide levels in adult asthmatics. Chest. 2000;118:1327–1331. doi: 10.1378/chest.118.5.1327. [DOI] [PubMed] [Google Scholar]
- 96.Silvestri M, Spallarossa D, Yourukova VF, Battistini E, Fregonese B, Rossi GA. Orally exhaled nitric oxide levels are related to the degree of blood eosinophilia in atopic children with mild-intermitten asthma. Eur Respir J. 1999;13:321–326. doi: 10.1034/j.1399-3003.1999.13b17.x. [DOI] [PubMed] [Google Scholar]
- 97.Moody A, Fergusson W, Wells A, Bartley J, Kolbe J. Increased nitric oxide production in the respiratory tract in asymptomatic Pacific Islanders: an association with skin prick reactivity to house dust mite. J Allergy Clin Immunol. 2000;105:895–899. doi: 10.1067/mai.2000.105318. [DOI] [PubMed] [Google Scholar]
- 98.Sovijarvi ARA, Saarinen A, Helin T, et al. Increased nitric oxide in exhaled air in patients with asthmatic symptoms not fulfilling the functional criteria of asthma. Eur Respir J. 1998;12:431S. [Google Scholar]
- 99.Withers NJ, Bale KL, Laszlo G. Levels of exhaled nitric oxide as a screening tool for undiagnosed asthma: results of a pilot study. Eur Respir J. 1998;12:393S. [Google Scholar]
- 100.Persson MG, Zetterstrom O, Argenius V, Ihre E, Gustaffson LE. Single breath nitric oxide measurements in asthmatic patients and smokers. Lancet. 1994;343:146–147. doi: 10.1016/s0140-6736(94)90935-0. [DOI] [PubMed] [Google Scholar]
- 101.Horvath I, Loukides S, Wodehouse T, et al. Comparison of exhaled and nasal nitric oxide and exhaled carbon monoxide levels in bronchiectatic patients with and without primary ciliary dyskinesia. Thorax. 2003;58:68–72. doi: 10.1136/thorax.58.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gratziou C, Lignos M, Dassiou M, Roussos C. The influence of atopy on exhaled nitric oxide in patients with asthma and/or rhinitis. Eur Respir J. 1999;14:897–901. doi: 10.1034/j.1399-3003.1999.14d28.x. [DOI] [PubMed] [Google Scholar]
- 103.Nogami H, Shoji S, Nishima S. Exhaled nitric oxide as a simple assessment of airway hyperresponsiveness in bronchial asthma and chronic cough patients. J Asthma. 2003;40:653–659. doi: 10.1081/jas-120019036. [DOI] [PubMed] [Google Scholar]
- 104.Dupont LJ, Rochette F, Demedts MG, Verleden GM. Exhaled nitric oxide correlates with airway hyperresponsiveness in steroid-naive patients with mild asthma. Am J Respir Crit Care Med. 1998;157:894–8. doi: 10.1164/ajrccm.157.3.9709064. [DOI] [PubMed] [Google Scholar]
- 105.Parameswaran K, Kamada D, Borm A, et al. Sputum cell counts and exhaled nitric oxide in patients with non-asthmatic cough and gastro-esophageal reflux. Eur Respir J. 1998;12:248S. [Google Scholar]
- 106.Jatakanon A, Kharitonov SA, Lim S, Barnes PJ. Effect of differing doses of inhaled budesonide on markers of airway inflammation in patients with mild asthma. Thorax. 1999;54:108–114. doi: 10.1136/thx.54.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gibson PG, Henry RL, Thomas P. Noninvasive assessment of airway inflammation in children: induced sputum, exhaled nitric oxide, and breath condensate. Eur Respir J. 2000;16:1008–1015. [PubMed] [Google Scholar]
- 108.Mattes J, von Storm G, Reining U, et al. NO in exhaled air is correlated with markers of eosinophilic airway inflammation in corticosteroid-dependent childhood asthma. Eur Respir J. 1999;13:1391–1395. [PubMed] [Google Scholar]
- 109.Jatakanon A, Lim S, Kharitonov SA, Chung KF, Barnes PJ. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 1998;53:91–95. doi: 10.1136/thx.53.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dupont LJ, Rochette F, Demedts MG, Verleden GM. Exhaled nitric oxide correlates with airway hyperresponsiveness in steroid-naοve patients with mild asthma. Am J Respir Crit Care Med. 1998;157:894–898. doi: 10.1164/ajrccm.157.3.9709064. [DOI] [PubMed] [Google Scholar]
- 111.Pizzichini E, Pizzichini MM, Kidney JC, et al. Induced sputum, bronchoalveolar lavage and blood from mild asthmatics: inflammatory cells, lymphocyte subsets and soluble markers compared. Eur Respir J. 1998;11:828–834. doi: 10.1183/09031936.98.11040828. [DOI] [PubMed] [Google Scholar]
- 112.Kharitonov SA, Yates DH, Barnes PJ. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med. 1996;153:454–457. doi: 10.1164/ajrccm.153.1.8542158. [DOI] [PubMed] [Google Scholar]
- 113.Kharitonov SA, Yates DH, Chung KF, Barnes PJ. Changes in the dose of inhaled steroid affect exhaled nitric oxide levels in asthmatic patients. Eur Respir J. 1996;9:196–201. doi: 10.1183/09031936.96.09020196. [DOI] [PubMed] [Google Scholar]
- 114.Jatakanon A, Lim S, Barnes PJ. Changes in sputum eosinophils predict loss of asthma control. Am J Respir Crit Care Med. 2000;161:64–72. doi: 10.1164/ajrccm.161.1.9809100. [DOI] [PubMed] [Google Scholar]
- 115.Kharitonov SA, Barnes PJ, O’Connor BJ. Reduction in exhaled nitric oxide after a single dose of nebulized budesonide in patients with asthma [abstract] Am J Respir Crit Care Med. 1996;153:A799. [Google Scholar]
- 116.Lim S, Jatakanon A, John M, Gilbey T, O’Connor BJ, Barnes PJ. Effect of inhaled budesonide on lung function and airway inflammation. Am J Respir Crit Care Med. 1999;159:22–30. doi: 10.1164/ajrccm.159.1.9706006. [DOI] [PubMed] [Google Scholar]
- 117.Kobayashi H, Takahashi Y, Mitsufuji H, et al. Decreased exhaled nitric oxide in mild persistent asthma patients treated with a leukotriene receptor antagonist, pranlukast. Jpn J Physiol. 1999;49:541–544. doi: 10.2170/jjphysiol.49.541. [DOI] [PubMed] [Google Scholar]
- 118.Bisgaard H, Loland L, Oj JA. NO in exhaled air of asthmatic children is reduced by the leukotriene receptor antagonist montelukast. Am J Respir Crit Care Med. 1999;160:1227–1231. doi: 10.1164/ajrccm.160.4.9903004. [DOI] [PubMed] [Google Scholar]
- 119.Wilson AM, Orr LC, Sims EJ, Dempsey OJ, Lipworth BJ. Antiasthmatic effects of mediator blockade versus topical corticosteroids in allergic rhinitis and asthma. Am J Respir Crit Care Med. 2000;162:1297–1301. doi: 10.1164/ajrccm.162.4.9912046. [DOI] [PubMed] [Google Scholar]
- 120.Stirling RG, Kharitonov SA, Campbell D, et al. Increase in exhaled nitric oxide levels in patients with difficult asthma and correlation with symptoms and disease severity despite treatment with oral and inhaled and corticosteroids. Thorax. 1998;53:1030–1034. doi: 10.1136/thx.53.12.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jones SL, Kittelson J, Cowan JO, et al. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am J Respir Crit Care Med. 2001;164:738–743. doi: 10.1164/ajrccm.164.5.2012125. [DOI] [PubMed] [Google Scholar]
- 122.Artlich A, Busch T, Lewandowski K, Jonas S, Gortner L, Falke KJ. Childhood asthma: exhaled nitric oxide in relation to clinical symptoms. Eur Respir J. 1999;13:1396–1401. doi: 10.1183/09031936.99.13614029. [DOI] [PubMed] [Google Scholar]
- 123.Massaro AF, Gaston B, Kita D, Fanta C, Stamler JS, Drazen JM. Expired nitric oxide levels during treatment of acute asthma. Am J Respir Crit Care Med. 1995;152:800–803. doi: 10.1164/ajrccm.152.2.7633745. [DOI] [PubMed] [Google Scholar]
- 124.Baraldi E, Dario C, Ongaro R, et al. Exhaled nitric oxide concentrations during treatment of wheezing exacerbation in infants and young children. Am J Respir Crit Care Med. 1999;159:1284–1288. doi: 10.1164/ajrccm.159.4.9807084. [DOI] [PubMed] [Google Scholar]
- 125.Balfour-Lynn IM, Laverty A, Dinwiddie R. Reduced upper airway nitric oxide in cystic fibrosis. Arch Dis Child. 1996;75:319–322. doi: 10.1136/adc.75.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jones KL, Bryan TW, Jinkins PA, et al. Superoxide causes a reduction in nitric oxide gas and an increase in nitrate. Am J Physiol. 1998;275:L1120–L1126. doi: 10.1152/ajplung.1998.275.6.L1120. [DOI] [PubMed] [Google Scholar]
- 127.Yu H, Nasr SZ, Deretic V. Innate lung defenses and compromised Pseudomonas aeruginosa clearance in the malnourished mouse model of respiratory infections in cystic fibrosis. Infect Immun. 2000;68:2142–2147. doi: 10.1128/iai.68.4.2142-2147.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Grasemann H, Michler E, Wallot M, Ratjen F. Decreased concentration of exhaled nitric oxide (NO) in patients with cystic fibrosis. Pediatr Pulmonol. 1997;24:173–177. doi: 10.1002/(sici)1099-0496(199709)24:3<173::aid-ppul2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 129.Thomas SR, Kharitonov SA, Scott SF, Hodson ME, Barnes PJ. Nasal and exhaled nitric oxide is reduced in adult patients with cystic fibrosis and does not correlate with cystic fibrosis genotype. Chest. 2000;117:1085–1089. doi: 10.1378/chest.117.4.1085. [DOI] [PubMed] [Google Scholar]
- 130.Antuni JD, Kharitonov SA, Hughes D, Hodson ME, Barnes PJ. Increase in exhaled carbon monoxide during exacerbations of cystic fibrosis. Thorax. 2000;55:138–142. doi: 10.1136/thorax.55.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Downey D, Elborn JS. Nitric oxide, iNOS, and inflammation in cystic fibrosis. J Pathol. 2000;190:115–116. doi: 10.1002/(SICI)1096-9896(200002)190:2<115::AID-PATH491>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 132.Kelley TJ, Drumm ML. Inducible nitric oxide synthase expression is reduced in cystic fibrosis murine and human airway epithelial cells. J Clin Invest. 1998;102:1200–1207. doi: 10.1172/JCI2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Meng QH, Polak JM, Edgar AJ, et al. Neutrophils enhance expression of inducible nitric oxide synthase in human normal but not cystic fibrosis bronchial epithelial cells. J Pathol. 2000;190:126–132. doi: 10.1002/(SICI)1096-9896(200002)190:2<126::AID-PATH500>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 134.Loukides S, Kharitonov SA, Wodehouse T, Cole PJ, Barnes PJ. Effect of L-arginine on mucociliary function in primary ciliary dyskinesia. Lancet. 1998;352:371–372. doi: 10.1016/S0140-6736(05)60471-0. [DOI] [PubMed] [Google Scholar]
- 135.Karadag B, James AJ, Gultekin E, Wilson NM, Bush A. Nasal and lower airway level of nitric oxide in children with primary ciliary dyskinesia. Eur Respir J. 1999;13:1402–1405. doi: 10.1183/09031936.99.13614069. [DOI] [PubMed] [Google Scholar]
- 136.Bush A. Primary ciliary dyskinesia. Acta Otorhinolaryngol Belg. 2000;54:317–324. [PubMed] [Google Scholar]
- 137.Csoma Z, Bush A, Wilson NM, et al. Nitric oxide metabolites are not reduced in exhaled breath condensate of patients with primary ciliary dyskinesia. Chest. 2003;124:633–638. doi: 10.1378/chest.124.2.633. [DOI] [PubMed] [Google Scholar]
- 138.Malmberg LP, Pelkonen AS, Haahtela T, Turpeinen M. Exhaled nitric oxide rather than lung function distinguishes preschool children with probable asthma. Thorax. 2003;58:494–499. doi: 10.1136/thorax.58.6.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Avital A, Uwyyed K, Berkman N, Godfrey S, Bar-Yishay E, Springer C. Exhaled nitric oxide and asthma in young children. Pediatr Pulmonol. 2001;32:308–313. doi: 10.1002/ppul.1124. [DOI] [PubMed] [Google Scholar]
- 140.Zeiger R, Szefler S, Phillips B, et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol. 2006;117:45–52. doi: 10.1016/j.jaci.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 141.Pijnenburg M, Hofhuis W, Hop W, De Jongste J. Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission. Thorax. 2005;60:215–218. doi: 10.1136/thx.2004.023374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zacharasiewicz A, Wilson N, Lex C, et al. Clinical use of noninvasive measurements of airway inflammation in steroid reduction in children. Am J Respir Crit Care Med. 2005;171:1077–1082. doi: 10.1164/rccm.200409-1242OC. [DOI] [PubMed] [Google Scholar]