Abstract
Background: Hypertension is associated with more rapid progression of chronic kidney disease. Several studies have shown that treating hypertension in patients with chronic kidney disease and proteinuria may attenuate the decline in glomerular filtration rate.
Study objective: The study evaluates the prevalence of hypertension and its association with chronic kidney disease progression in patients without and with diabetic nephropathy.
Methods: Patients with CKD stage 2-4 were followed up by a nephrologist for 12-52 months. A total of 137 patients were included in the study, 70 with non-diabetic CKD and 67 with type 2 diabetes and diabetic nephropathy. Demographic and clinical parameters were recorded at initiation and during follow-up. Glomerular filtration rate was estimated by the Cockroft-Gault formula and progression of CKD by the slope of the estimated GFR decline .
Results: Out of 70 patients in the non-diabetic group, 34 were males, (mean age 50.37±12.2 years). Out of 67 diabetic patients, 30 were (males, mean age 57.8±8.4 years). 77% in the non-diabetic group had SBP above 140 mmHg. The higher SBP was associated with older age, (53.16±10.8 vs 40.9±12.2 years, p<0.0001). Diastolic blood pressure above 90 was present in 73%. Pulse pressure above 80 had 5.7% and was associated with older age (p<0.02). Progression of chronic kidney disease correlated inversely with age, and positively with diastolic blood pressure and proteinuria (p=0.005, p=0.019 and p=0.02 respectively). Multiple regression analysis showed that only younger age and higher proteinuria were predictive for chronic kidney disease progression (p=0.00002). 6% of pts in the diabetic group had SBP below 140, 19% between 140 and 160, and 75% above 160 mmHg. Diastolic blood pressure below 80 had only 6% of patients, between 80 and 90 had 37% and above 90 mmHg had 57%. Pulse pressure below 80 mmHg had 55% and it was correlated positively with age, p=0.009. Progression of chronic kidney disease in the diabetic group correlated positively with mean arterial pressure, systolic blood pressure and proteinuria, (p=0.017, 0.036 and 0.000000 respectively) and inversely with age (p=0.0003). Multiple regression analysis showed that proteinuria, age and SBP were the only predictors for chronic kidney disease progression in diabetics.
Conclusion: Isolated systolic hypertension predominates the older age groups, proteinuria and age significantly correlate with GFR decline in both groups, and SBP is associated with more rapid progression of CKD in the diabetic patients.
Keywords: hypertension, chronic kidney disease progression, age, proteinuria
Progression of chronic kidney disease (CKD) to end-stage renaldisease (ESRD) is a world-wide major public health problem. The prevalence of CKD is high, but the accelerated incidence ofESRD far exceeds the rate of increase in the prevalence of CKD1. CKD has high costs and poor outcomes. The major outcomes of CKD are loss of kidney function and development of cardiovascular diseases. Hypertension is common in chronic kidney disease (CKD), and is a risk factor for progression of kidney disease and development and worsening of cardiovascular diseases (CVD)2. It is a cause and complication of CKD and more than 50-75% of patients with CKD have blood pressure more than 140/90 mmHg. Because CKD and hypertension are often present together and both are generally asymptomatic, evaluations of patients with either condition should be considered. The underlying diagnosis of renal disease, the level of GFR, and level of proteinuria should be evaluated, as well as the complications of decreased glomerular filtration rate (GFR), the risk for progression of kidney disease, the presence of clinical CVD and CVD risk factors and comorbid conditions2. Hypertension is associated with more rapid progression of CKD3, 4. Several studies have shown that treating hypertension in patientswith CKD and proteinuria may attenuate the decline in glomerularfiltration rate (GFR)5–7. Guidelines identify CKD as a high-risk group warranting intensivehypertension treatment2, 8. They recommend pharmacologicaltherapy and lifestyle modification to achieve a blood pressuregoal of <130/80 mmHg for patients with CKD, and equal control of systolic (SBP) and diastolic (DBP) bloodpressure.2 but, there are many studies reporting that the prevalence of controlled hypertension at the community level is <30%9–11. Recent data in the general population alsosuggest that the prevalence of isolated systolic hypertensionis high, especially among the elderly12, 13.Wide pulse pressureis also prevalentand has been shown to be a marker of arterial stiffnessand independent risk factor for cardiovascular disease14, 15.
Study objective
To evaluate the prevalence of hypertension in diabetic and non-diabetic CKD patients followed on an outpatient basis, to determine the extent of control of systolic and diastolic blood pressure, and the association of hypertension with the progression of CKD.
Materials and methods
Two groups of patients with chronic kidney disease have been followed on an outpatient basis, at the nephrology department, separately, for at least 12 months, up to 52 months or until the need for renal replacement therapy. The non-diabetic group consisted of 70 patients with non-diabetic CKD (underlying renal diseases: 17 interstitial nephritis, 24 glomerulonephritis, 7 polycystic kidney disease, 22 hypertensive nephropathy) and 67 patients were included in the group with diabetic nephropathy as a result of type 2 diabetes mellitus (DM-2). The following variables were recorded: systolic, diastolic and mean arterial blood pressure, pulse pressure, body mass index, age, gender, hemoglobin, hematocrit, serum protein, albumin, total lipids, cholesterol, triglycerides, electrolytes, alkaline phosphatase, serum urea, creatinine, uric acid, 24h protein excretion, protein catabolic rate (PCR/bw/day). Glomerular filtration rate was estimated by using the Cockroft - Gault formula (eGFR). Progression of CKD was estimated by linear regression of eGFR estimates (expressed as monthly decrease of GFR in ml/min/month). Blood pressure was measured at least on three occasions if a patient had a follow-up of 12 months, or more if followed beyond one year. The mean value of two measurements with one minute pause between them for the systolic and diastolic blood pressure was recorded. The mean of all the recorded values of each variable was taken into the final statistical analysis. All patients were given angiotensin-converting enzyme inhibitor (ACEi), and when proteinuria was higher, a combination of ACE-i and angiotensin receptor blocker (ARB) was initiated. If blood pressure control was insufficient, diuretics, calcium channel blockers and beta blockers were added. ACEi or ARB were stopped when hyperkalemia occurred (serum potassium > 5.5 mEq/L), or when GFR started declining more rapidly (>30% from baseline levels in a period of 4 weeks), or when GFR was too low (less than 15 ml/min/1.73m 2).
Univariate statistical analysis was used to investigate the correlations between different variables (Spearman rho rank test) and associations between two independent variables (non-parametric Mann Whitney U test). In addition, these variables were investigated in a multivariate model using multiple regression analysis for the dependent variable being eGFR decline. Analyses were performed using the Statistica for Windows 6.0 statistical package. The p value of less than 0.05 was taken as being significant.
Results
Out of 70 patients in the non-diabetic group, 34 were males, and 36 females, and out of 67 in the diabetic group, 30 were males and 37 females. The mean ±SD follow-up in the diabetic group was 22.4±11.5 months and in the non-diabetic group 29.8±9.6 months. The mean age ±SD in the non-diabetic group was 50.37±12.2 and in the diabetic group 57.8±8.3 years. The mean ±SD GFR in the non-diabetic group was 32.1±15.8 ml/min and in the diabetic group 57.8±28.2. Table 1. shows demographic and clinical variables in both patient groups.
Table 1. Demographic and clinical variables in the 70 patients with non-diabetic CKD and 67 patients with type 2 diabetes and CKD.
Table 2. shows the prevalence of high systolic and diastolic blood pressure in both CKD patient groups. 23% of patients had lower SBP, (below 139 mmHg in the non-diabetic group), and only 6% in the diabetic group. Mean systolic blood pressure in the non-diabetic group was 154.8±16.7 mmHg and in the diabetic group 170.7±16.2. Mean diastolic blood pressure in the non-diabetic group was 96.1±8.5 mmHg and in the diabetic group 93.5±9.96 mmHg. The diabetics had isolated systolic hypertension to a greater extent than non-diabetics. Mean pulse pressure in the non-diabetic group was 58.3±12.8 and in the diabetic group 77.2±13.9 mmHg.
Table 2. Prevalence of hypertension in patients with diabetic and non-diabetic chronic kidney disease.
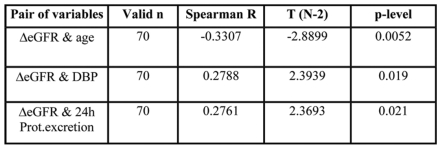
Table 3, 4 and 5 show the results of the statistical analysis in the non-diabetic group. Spearman rank correlation test showed that progression of CKD correlated inversely with age, and positively with diastolic blood pressure and proteinuria (p=0.005, p=0.019 and p=0.021 respectively) (Table 3). The initial GFR correlated inversely with SBP and pulse pressure. The higher the initial GFR, the lower SBP and pulse pressure, (p=0.009 and p=0.002 respectively) (Table 4). Higher SBP and pulse pressure above 80 mmHg were associated with older age, (p=0.00033 and p=0.013 respectively). (Table 5) When multiple regression analysis was performed, only younger age and higher proteinuria appeared to be predictive for CKD progression, (p<0.0085 and p<0.0003 respectively). Figure 1 shows the correlation between proteinuria and mean GFR decline in the non-diabetic CKD patients.
Table 3. Variables significantly correlating with eGFR decline in the non-diabetic group.
Table 4. Variables significantly correlating with initial eGFR in the non-diabetic group.
Table 5. Variables significantly associated with high systolic, diastolic, mean arterial pressure and pulse pressure in the non-diabetic group.
Figure 1. Correlation between proteinuria and mean GFR decline in the non-diabetic group of patients.
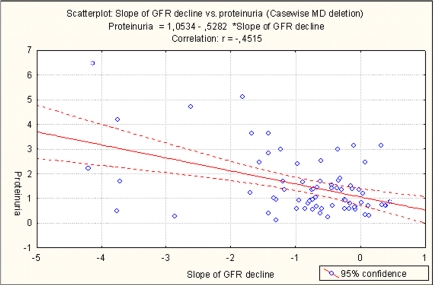
Table 6 and Table 7show the results of the statistical analysis in the diabetic group. Progression of CKD in the diabetics correlated positively with mean arterial pressure, SBP and proteinuria, p=0.017, 0.036 and 0.000000 respectively, and inversely with age, (p=0.0003) (Table 7). Pulse pressure below 80 mmHg had 55% of patients. Pulse pressure correlated positively with age, (p=0.009), and diastolic blood pressure and initial GFR correlated inversely with age, p=0.0219 and 0.0048 respectively (Table 8). When multiple regression analysis was performed, proteinuria, age and SBP were the only predictors for CKD progression (p< 0.0003, p<0.0007 and p<0.0156 respectively). Figure 2 shows the correlation between proteinuria and mean GFR decline in the diabetic CKD patients.
Table 6. Variables correlating with eGFR decline in the diabetic group.
Table 7. Variables with significant correlation to age in the diabetic group.
Figure 2. Correlation between proteinuria and mean GFR decline in the diabetic group of patients.
Discussion
Our study showed that there was a very poor control of hypertension in both, non-diabetic and diabetic patients with CKD. Only 22.8% of patients with non-diabetic CKD had SBP less than 140 mmHg and a similar proportion, 27% had DBP less than 90 mmHg. The diabetic patients had worse control of SBP, only 6% had SBP less than 140 mmHg, but better control of DBP, 43% had less than 90 mmHg. A very insignificant number of patients in both study groups achieved the target blood pressure levels of less than 130/80 or 125/75 mm Hg (in proteinuric CKD patients) according to K/DOQI guidelines2. Other studies have also showed poor control of hypertension in CKD patients in spite of guideline recommendations. In 88% of patients in the study of De Nicola, the target blood pressure was not achieved, and in 84% the blood pressure was above target at the first visit to the nephrology unit16. Another study showed that only 26.6% of patients in the general population had adequately controlled blood pressure and that poor blood pressure control was associated with age >65 yrs, diabetes and CKD17. Better control of hypertension in our study was associated with higher initial GFR, indicating that volume expansion that accompanies later stages of CKD was not adequately treated with diuretic therapy. The study of De Nicola16 also showed that among patients with uncontrolled hypertension there were only 37% of patients who were treated with diuretics and at insufficient doses in half of the cases. So, it is plausible to conclude that, in general, CKD patients are not adequately and sufficiently treated with antihypertensive therapy, and particularly with agents aimed to reduce their extracellular volume expansion.
Proteinuria adversely affected GFR decline in both, diabetic and non-diabetic patient groups in our study. Proteinuria was not correlated to either SBP or DBP, as well as mean or pulse pressure. In the diabetic group proteinuria independently of SBP affected GFR decline. The study of Ruggenenti also confirmed that proteinuria is the best independent predictor of both disease progression and end-stage renal failure in non-diabetic proteinuric nephropathies and that the risk of end-stage renal failure is independent of the mean arterial pressure18.
The MDRD (Modification of diet in renal disease) study group confirmed that there was a 0.77 hazard ratio for kidney failure if the patients were in the low target blood pressure group19.In our study, blood pressure did not appear to affect GFR decline in the non-diabetic group of patients, most probably because only an insignificant number of patients achieved a low target blood pressure. The majority of the patients had high blood pressure, and moreover, 67% of them had mean arterial pressure above 107 mmHg. In the diabetic group, systolic blood pressure was associated with faster GFR decline, independently of proteinuria. These results are in accordance to the study of Rossing et al that have shown in type 2 diabetic patients, that the SBP, together with higher baseline albuminuria, hemoglobin A1c and lower GFR and hemoglobin were significantly associated with doubling of serum creatinine or ESRD20. On the other hand, the study of Leehey et al21 showed that diabetic patients with CKD in whom mean blood pressure was controlled to less than 140/80 mmHg, hypoalbuminemia and proteinuria, but not blood pressure were independently associated with progression of kidney disease. But the univariate analysis in their study showed that the mean systolic blood pressure was associated with progression of CKD. Moreover, when SBP was <140 mmHg there was no association with GFR decline, but above that level the rate of GFR decline correlated positively with the increase of SBP.
Our study showed that older age was a protective factor for GFR decline in both patient groups. There is a lack of studies for GFR decline in elderly patients, but the study of Leehey21 also showed in the univariate analysis that younger age in type 2 diabetics with CKD was associated with faster progression. This requires further investigation in a larger population study.
Conclusions
In spite of recommendations for vigorous control of hypertension in the CKD population, our study showed that in clinical practice it is still far from optimal.
Older age was associated with isolated systolic hypertension in both patients groups, but its prevalence was more pronounced in the diabetic group where patients were significantly older than the patients in the non-diabetic group. But, nonetheless, older age was a protective factor for faster progression
Proteinuria adversely affected progression in both patient groups.
High SBP adversely affected progression only in the diabetic group.
Higher initial GFR was associated with better control of hypertension.
References
- 1.Hsu CY, Vittinghoff E, Lin F, Shlipak MG. The incidence of end-stage renal disease is increasing faster than the prevalence of chronic renal insufficiency. Ann Intern Med. 2004;141:95–101. doi: 10.7326/0003-4819-141-2-200407200-00007. [DOI] [PubMed] [Google Scholar]
- 2.Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5) suppl 1:S1–S290. [PubMed] [Google Scholar]
- 3.Shulman NB, Ford CE, Hall WD, et al. Prognostic value of serum creatinine and effect of treatment of hypertension on renal function Results from the hypertension detection and follow-up program. The Hypertension Detection and Follow-Up Program Cooperative Group. Hypertension. 1989;13(suppl5):I80–I93. doi: 10.1161/01.hyp.13.5_suppl.i80. [DOI] [PubMed] [Google Scholar]
- 4.Walker WG, Neaton JD, Cutler JA, Neuwirth R, Cohen JD. Renal function change in hypertensive members of the Multiple Risk Factor Intervention Trial. Racial and treatment effects. The MRFIT Research Group. J Am Med Assoc. 1992;268:3085–3091. [PubMed] [Google Scholar]
- 5.Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123:754–762. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]
- 6.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. J Am Med Assoc. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 7.Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 8.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 9.Mulrow PJ. Detection and control of hypertension in the population: the United States experience. Am J Hypertens. 1998;11:744–746. doi: 10.1016/s0895-7061(98)00066-1. [DOI] [PubMed] [Google Scholar]
- 10.Berlowitz DR, Ash AS, Hickey EC, et al. Inadequate management of blood pressure in a hypertensive population. N Engl J Med. 1998;339:1957–1963. doi: 10.1056/NEJM199812313392701. [DOI] [PubMed] [Google Scholar]
- 11.Julius S. Worldwide trends and shortcomings in the treatment of hypertension. Am J Hypertens. 2000;13(suppl):S57–S61. doi: 10.1016/s0895-7061(00)00273-9. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhry SI, Krumholz HM, Foody JM. Systolic hypertension in older persons. J Am Med Assoc. 2004;292:1074–1080. doi: 10.1001/jama.292.9.1074. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd-Jones DM, Evans JC, Larson MG, O’Donnell CJ, Roccella EJ, Levy D. Differential control of systolic and diastolic blood pressure: factors associated with lack of blood pressure control in the community. Hypertension. 2000;36:594–599. doi: 10.1161/01.hyp.36.4.594. [DOI] [PubMed] [Google Scholar]
- 14.Bielak LF, Turner ST, Franklin SS, Sheedy PFII, Peyser PA. Age-dependent associations between blood pressure and coronary artery calcification in asymptomatic adults. J Hypertens. 2004;22:719–725. doi: 10.1097/00004872-200404000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Haider AW, Larson MG, Franklin SS, Levy D. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138:10–16. doi: 10.7326/0003-4819-138-1-200301070-00006. [DOI] [PubMed] [Google Scholar]
- 16.De Nicola L, Minutolo R, Gallo C, et al. Management of hypertension in chronic kidney disease: The Italian multicentric study. JN. 2005;18:397–404. [PubMed] [Google Scholar]
- 17.Triolo L, Cattaruzza MS, Sicoli R, et al. Blood pressure control and comorbidity in a nephrology clinic. JN. 2004;17:808–812. [PubMed] [Google Scholar]
- 18.Ruggenenti P, Perna A, Mosconi L, Pisoni R, Remuzzi G. Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. Kidney Int. 1998;53:1209–1216. doi: 10.1046/j.1523-1755.1998.00874.x. [DOI] [PubMed] [Google Scholar]
- 19.Sarnak MJ, Greene T, Wang X, et al. Ann Intern Med. 2005;142:342–351. doi: 10.7326/0003-4819-142-5-200503010-00009. [DOI] [PubMed] [Google Scholar]
- 20.Rossing K, Christensen PK, Hovind P, Tarnow L, Rossing P, Parving HH. Progression of nephropathy in type 2 diabetic patients. Kidney Int. 2004;66:1596–605. doi: 10.1111/j.1523-1755.2004.00925.x. [DOI] [PubMed] [Google Scholar]
- 21.Leehey DJ, Kramer HJ, Daoud TM, Chatha MP, Isreb MA. Progression of kidney disease in type 2 diabetes – beyond blood pressure control: an observational study. BMC Nephrology. 2005;6:8. doi: 10.1186/1471-2369-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]