Abstract
Feline immunodeficiency virus (FIV) causes a natural infection of domestic cats that resembles HIV-1 in pathogenesis and disease progression. Feline AIDS is characterized by depression of the CD4+ T cell population and fatal opportunistic infections. Maternal-fetal transmission of FIV readily occurs under experimental conditions, resulting in infected viable kittens and resorbed or arrested fetal tissues. Although both FIV and HIV use the chemokine receptor CXCR4 as a co-receptor, FIV does not utilize CD4 as the primary receptor. Rather, CD134 (OX40), a T cell activation antigen and co-stimulatory molecule, is the primary receptor for FIV. We hypothesized that placental expression of CD134 and CXCR4 may render the placenta vulnerable to FIV infection, possibly facilitating efficient vertical transmission of FIV, and impact pregnancy outcome. The purpose of this project was to quantify the relative expression of CD134 and CXCR4 mRNA from term placentas of three groups of cats: uninfected queens producing viable offspring, experimentally-infected queens producing only viable offspring, and experimentally-infected queens producing viable offspring among mostly non-viable fetuses. Total RNA was extracted from term placental tissues from all groups of cats. Real-time one-step reverse transcriptase-PCR was used to measure gene expression. The FIV receptors CD134 and CXCR4 were expressed in all late term feline placental tissues. Placentas from FIV-infected queens producing litters of only viable offspring expressed more CD134 and CXCR4 mRNA than those from uninfected queens, suggesting that infection may cause upregulation of the receptors. On the other hand, placentas from FIV-infected cats with non-successful pregnancies expressed similar levels of CD134 mRNA and slightly less CXCR4 mRNA than those from uninfected queens. Thus, it appears that cells expressing these receptors may play a role in pregnancy maintenance.
Keywords: Feline immunodeficiency virus, placenta, receptor
Introduction
Globally, the estimated number of people living with HIV is 39.5 million, 2.3 million of which are children under the age of 15 (UNAIDS/WHO, 2006). In the United States pediatric AIDS cases (defined as AIDS occurring in children under the age of 13) represent 1% of the total number of AIDS cases, with maternal-fetal transmission of the virus accounting for more than 93% of pediatric infections (CDC, 2005). Children who are infected in utero have a more rapid progression to AIDS and generally become symptomatic during the first year of life. In addition, HIV infection of pregnant women often results in poor outcome, including low birth weight babies, pre-term delivery, and an increased incidence of spontaneous abortions (D'Ubaldo et al., 1998; Kumar et al., 1995; Langston et al., 1995). Specific placental cell populations are permissive to productive HIV infection, probably contributing to transplacental transfer of virus or virus-infected cells across the placenta leading to fetal infection (David et al., 1992; McGann et al., 1994; Al-Harthi et al., 2002; Arias et al., 2003) . Although HIV infection clearly has the potential to negatively impact fetal and neonatal health, the mechanism and timing of HIV vertical transmission to the fetus is not yet clear.
FIV causes a natural infection of domestic cats that resembles HIV-1 infection in pathogenesis and disease progression. Feline AIDS is characterized by progressive depletion of the CD4+ T cell population and fatal opportunistic infections. Although both viruses use the same co-receptor, the chemokine receptor CXCR4, FIV does not utilize CD4 as the primary receptor (de Parseval et al., 2004a; Shimojima et al., 2004). The primary FIV receptor is CD134 (OX40), a T cell activation and co-stimulatory molecule (Shimojima et al., 2004). The virus binds these receptors via its surface glycoprotein, gp95 (de Parseval and Elder, 2001). Previous studies demonstrated that CXCR4 expression is essential to viral entry and that levels of CXCR4 on the host cell were the limiting factor in productive virus infection and controlled virus spread and cytopathogenicity (de Parseval et al., 2004b).
Vertical transmission of FIV occurs commonly in vitro (O'Neil et al., 1996; Allison and Hoover, 2003; Weaver et al., 2005). We recently reported frequent reproductive failure in FIV-B-2542-infected cats delivered late in gestation by cesarean section (Weaver et al., 2005). While preliminary evidence of immunopathology was identified in the study, the interaction between the virus and its cellular receptors was not analyzed. In the present study, we hypothesized that placental expression of CD134 and CXCR4 may facilitate efficient vertical transmission of FIV and impact pregnancy outcome. Our objective was to quantify the relative expression of CD134 and CXCR4 mRNA from term placentas of experimentally-infected and control queens. We report that the FIV receptors CD134 and CXCR4 were expressed in late term feline placental tissues and that altered placental expression of CD134 and CXCR4 was dependent upon FIV-status of queens and appeared to be correlated with pregnancy outcome.
Materials and Methods
2.1. Animals and virus
All procedures utilizing cats (Felis domesticus) were performed with approval of the Mississippi State University Institutional Animal Care and Use Committee. As previously reported (Weaver et al., 2005), cats were reproductively mature, specific-pathogen-free (SPF) animals of less than 12 months old and obtained from a commercial cattery. Ten cats were inoculated intravenously with 1 ml of a plasma pool containing approximately 1.33 × 104 copies/ml of FIV-B-2542 originally provided by Dr. Edward Hoover, (Rogers and Hoover, 1998). Ten cats were sham inoculated with 1 ml of pooled normal plasma. Infection was confirmed within 6 weeks p.i. by detection of FIV provirus by PCR and for seroconversion by ELISA, and cats were allowed to naturally breed with SPF toms. Toms used to breed uninfected females were never exposed to infected females and vice versa. Breeding was observed, dates were recorded, and pregnancy was confirmed by palpation and ultrasonography.
2.2. Cesarean delivery and tissue harvest
Kittens were delivered during week 8 gestation (approximately day 56) by cesarean section. Each kitten or fetus was removed with the associated placental membranes. All placental tissues were snap frozen in liquid nitrogen and stored at −80°C (Weaver et al., 2005).
2.3. Generation of Study Groups
The relative expression of CD134 and CXCR4 mRNA in term placental tissue from FIV-negative (Group 1) and FIV-positive cats were evaluated. The infected cats were subdivided into two groups, one of which consisted of cats who delivered only viable offspring (Group 2), the other of which consisted of cats who delivered a majority of non-viable offspring (Group 3). Due to the paucity of placental tissues associated with fetal resorption, placentas sampled from Group 3 cats 9730 and 9813 were taken from placentas associated with their viable fetuses. Pregnancy outcome study groups are listed in Table 1.
Table 1.
Pregnancy outcome of the study groupsa
| Group | Queen Number |
FIV Status | Viable kittens |
Arrested fetuses |
Fetal resorptions |
|---|---|---|---|---|---|
| 1 | 9522 | − | 3 | 0 | 0 |
| 9746 | − | 6 | 0 | 0 | |
| 9801 | − | 6 | 0 | 0 | |
| 2 | 9806 | + | 3 | 0 | 0 |
| 9809 | + | 1 | 0 | 0 | |
| 9810 | + | 1 | 0 | 0 | |
| 3 | 9730 | + | 2 | 1 | 2 |
| 9813 | + | 1 | 0 | 2 | |
| 13226 | + | 0 | 5 | 0 | |
Placental tissues were taken from each associated queen according to the following groupings: Group 1: FIV(−) queens producing viable offspring; Group 2: FIV(+) queens producing litters of only viable offspring; Group 3: FIV(+) queens producing viable offspring among majority non-viable litters.
2.4. Detection of FIV provirus in placental tissues
FIV provirus was detected in placental DNA samples using PCR targeting the FIV gag gene, followed by Southern hybridization as described (Weaver et al., 2005).
2.5. Detection of antibody to FIV by ELISA
FIV was detected in feline plasma from both groups of FIV-infected queens using the SNAP Combo FeLV Ag/FIV Antibody Test Kit (Idexx Laboratories, Westbrook, ME) according to the manufacturer's instructions. Positive results were scored when the sample produced a blue spot in the appropriate position on the membrane. A sample from a seronegative cat was included as a negative control in a parallel reaction.
2.6. RNA extraction
RNA was isolated from 10 randomly sectioned placental tissue samples from 9 cats using TRIzol Reagent (Invitrogen, Corp. Carlsbad, CA) according to the manufacturer's instructions. Briefly, cells were lysed in 1 ml TRIzol Reagent. Homogenates were allowed to sit for 5 min at ambient temperature (20—25°C) and supplemented with 0.2 ml chloroform. Samples were covered and vigorously shaken for 15 s. The resulting mixture was allowed to sit at ambient temperature (20—25°C) for 15 min. Samples were centrifuged at 12,000 × g for 15 min at 4°C. Following centrifugation, the colorless upper aqueous phase containing RNA was transferred to a fresh tube. RNA was precipitated from the aqueous phase with the addition of 0.5 ml isopropanol and incubated at ambient temperature for 10 min. The RNA precipitate was pelleted by centrifugation at 12,000 × g for 8 min at 4°C, and the supernatant was removed. The RNA pellet was washed by vortexing with 1 ml of 75% ethanol and centrifuged at 12,000 x g (5 min, 4°C). The ethanol wash was removed, and the RNA pellet was air-dried for 5 min. After drying, the RNA pellet was dissolved in 100 μl of RNase-free water and incubated for 15 min in a 55—60°C water bath. The resulting RNA was stored at −80°C until use.
2.7. Real-time reverse transcriptase-PCR
Cytokine mRNA sequences for the cat and other comparative mammals (human, dog, cow, and pig) were obtained from the National Center for Biotechnology Information (NCBI) and aligned using the European Molecular Biology Laboratory-European Bioinformatics Institute (EMBL-EBI) ClustalW alignment tool. The human mRNA was blast-searched against the human genome to locate the exon/intron boundaries. These boundaries were used to find the homologous boundaries in the feline sequence. We used Beacon Designer (PREMIER Biosoft) to design primer/probe sets (Table 2) targeting the feline receptors CD134 and CXCR4, and the internal control gene (β-actin). All PCR amplicons spanned an intron-exon junction. The target probes were 5'labeled with the reporter dye FAM (6-carboxyfluorescein) and 3' labeled with the quencher dye TAMRA (6-carboxytetramethylrhodamine). The probe for the housekeeping gene was 5'labeled with the reporter dye HEX (hexachloro-6-carboxyfluorescein) and 3' labeled with the quencher dye TAMRA. Primers and probes were obtained commercially (MWG-BIOTECH, Inc., High Point, NC). Primers and probes used to quantify FIV targeted the FIV gag gene (Weaver et al., 2005).
Table 2.
Sequences of PCR primers and TaqMan probes specific for FIV receptors and the internal control mRNA.
| Receptor | Primer | Sequence (5'-3') | Length | Accession | Probe sequence (5'-3') |
|---|---|---|---|---|---|
| CD134 | Sense | CAGGTTATGGGATGGAGAGTCG | 22 | AY738589 | TGACCAGGACACCAAGTGCCTCCAGTG |
| Anti- sense |
TGCAAGGCTCGTAGTTCACG | 20 | |||
| CXCR4 | Sense | AAGGCAGTCCATGTCATCTACAC | 23 | AJ009816 | ACCTCTACAGCAGTGTCCTCATCCTGGC |
| Anti- sense |
AGACCACCTTTTCAGCCAACAG | 22 | |||
| β-actin | Sense | GACTACCTCATGAAGATCCTCACG | 24 | AB051104 | ACAGTTTCACCACCACCGCCGAGC |
| Anti- sense |
CCTTGATGTCACGCACAATTTCC | 23 |
The real-time reverse transcriptase-PCR (rtRT-PCR) used an iCycler (BioRad Laboratories, Valencia, CA): 50°C, 30 min; 95°C, 5 min; 45 × (95°C, 15 s; 60°C, 1 min). Each reaction contained 12.5 μl of the commercial reaction mix, 0.5 μl of Thermoscript™ Plus/Platinum® Taq Mix, 1 μl of forward and reverse β-actin primers (7.5 pmol/μl), 1μl of forward and reverse target primers (10 pmol/μl), 1 μl of the respective probe (100 fmol/μl), and ∼0.06—0.27 μg RNA. For every placental RNA sample, parallel reactions were performed in replicated triplicate reactions on separate plates for each receptor. Real-time RT-PCR to quantify FIV gag was carried out as described previously (Weaver et al., 2005). Serially diluted, pooled RNA from uninfected cats was used to generate a standard curve for both duplex-partner amplicons. The standard curves were used to normalize for differences in PCR efficiency between duplex partners and between sample plate runs. Differences in the amount of template RNA in each reaction were corrected by the cycle threshold (Ct) value for β-actin. Normalized samples were divided by the calibration generating the relative expression levels.
2.8. Statistical Analysis
Statistical evaluation of litter sizes and fetal viability were done using single-factor ANOVA (Microsoft Excel-XP, Redmond, WA). To perform statistical analysis of receptor expression, the mean normalized values for the control samples and each set of treated samples (FIV-infected placentas) was done by using Monte Carlo resampling simulation with 500 iterations (Simon, 1997). Confidence intervals were calculated (α = 0.05) based on fold differences for each of the control versus treated comparisons.
Results
3.1. Pregnancy outcome
The pregnancy outcome data (Table 1) for FIV-infected and control queens was previously reported (Weaver et al., 2005), and these data were used to generate three study groups containing three randomly selected placental tissues from FIV-infected and control queens. Control cats produced a total of 15 viable kittens, a mean litter size of 5 kittens/litter. Infected queens with litters of only viable offspring produced 5 viable kittens, a mean litter size of 1.6 kittens/litter. Infected queens producing viable offspring from majority non-viable litters had a total of 13 kittens, three of which were viable (23%) and ten of which were non-viable (77%). The mean litter size was 4.3 kittens/litter. Non-viable offspring were either arrested during early development (n = 6) or fetal resorptions (n = 4). Differences in litter sizes between the three groups were significant (p = 0.05).
3.2. Detection of FIV infection
FIV provirus was detected in all but one of the placental specimens from FIV-infected cats, as reported (Weaver et al., 2005). We failed to amplify FIV provirus from the placenta of animal 9809, although infection of the corresponding kitten was confirmed by amplification of FIV provirus from kitten bone marrow. FIV infection of the inoculated queens was confirmed serologically.
3.3. Expression of CD134, CXCR4, and FIV in placental tissue
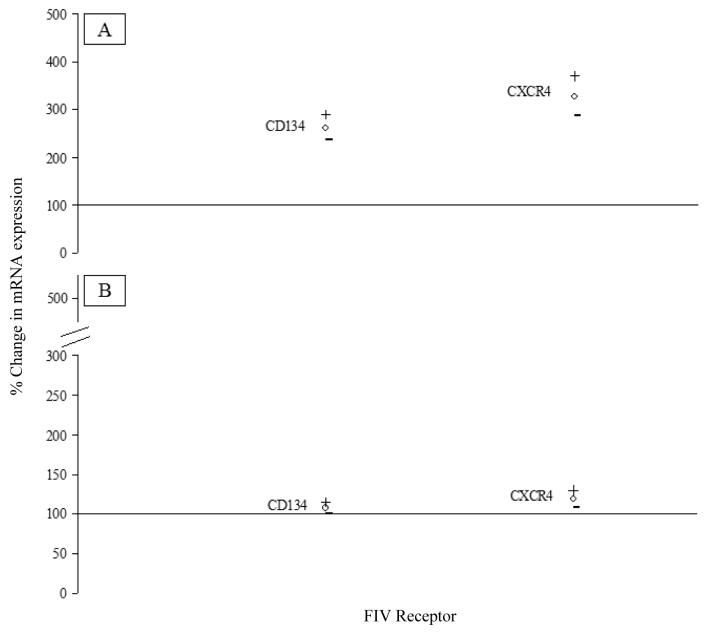
The relative expression of CD134 and CXCR4 mRNA in near term placental tissue was quantified, and data for the three groups of queens were compared as follows: control queens producing viable offspring (Group 1) versus FIV positive queens producing litters of only viable offspring (Group 2) and FIV positive queens producing viable offspring among majority non-viable litters (Group 3) versus Group 1. Confidence intervals were calculated (α = 0.05) for each of the control versus treated comparisons (Fig. 1). If the mRNA expression between two sample sets was the same, the relative expression was 100%.
Fig. 1.
Percentage change in FIV receptor mRNA expression in control versus treated comparisons. (A) Percentage change in CD134 and CXCR4 mRNA in placentas from infected queens producing litters of viable offspring (Group 2) compared to control cats (Group 1); (B) Percentage change in CD134 and CXCR4 mRNA in placentas from Group 1 cats compared to infected queens producing viable offspring among majority non-viable litters (Group 3). Results are shown as 95% upper (+) and lower (−) confidence intervals around the mean (○). Relative expression was 100% if the mRNA expression between the two sample sets was the same.
The mRNA for FIV receptors CD134 and CXCR4 was expressed in late term placental tissues from all groups (Fig. 1 a and b). Group 2 queens expressed more CD134 (mean 261%; lower and upper confidence intervals 235% and 290%, respectively) and CXCR4 (mean 327%; lower and upper confidence intervals 287% and 371%, respectively) mRNA compared to placentas from Group 1 (Fig. 1a). Groups 1 and 3 queens expressed similar levels of CD134 (mean 107%; lower and upper confidence intervals 100% and 114%, respectively) mRNA, while Group 1 expression of CXCR4 (mean 118%; lower and upper confidence intervals 107% and 130%, respectively) mRNA was slightly higher than that of Group 3 queens (Fig. 1b).
3.4. Relative expression of FIV RNA in placentas
The relative expression of FIV between the two groups of infected cats was quantified using rtRT-PCR targeting the FIV gag gene. There were no differences in the levels of FIV expression between the two groups (p > 0.05).
Discussion
Transmission of FIV in utero often results in reproductive failure (Rogers and Hoover, 1998; Weaver et al., 2005). O'Neil et al. (1996), reported no significant difference in the litter sizes between chronically and acutely infected queens (51% and 47%, respectively), however, higher incidences of fetal non-viability were associated with acutely infected versus chronically infected queens (53% and 30%, respectively) (O'Neil et al., 1995). FIV-B-2542-infected queens transmitted the virus to more than 50% of their offspring (O'Neil et al., 1996; Weaver et al., 2005) and the rate of in utero infection increased with gestational age (Rogers and Hoover, 1998). Despite the evidence supporting efficient in utero transmission, the mechanisms by which the virus traverses the placenta and enters the fetal circulation have not been described.
The interaction between the virus and its binding receptor(s) establishes cell tropism and is the first event in the virus replicative cycle, yet little is known about the expression of FIV receptors at the feline maternal-fetal interface. HIV and FIV utilize CD4 (Dalgleish et al., 1984; Klatzmann et al., 1984) and CD134 (de Parseval et al., 2004a; Shimojima et al., 2004), as their primary receptors, respectively. HIV-1 selectively targets CD4-bearing cells, resulting in their depletion. However, FIV has a cell tropism that broadens with disease progression, consistent with the expression of CD134 (Shimojima et al., 2004). HIV and FIV share usage of a common co-receptor, the chemokine receptor CXCR4. Progression to disease in HIV-1 infections is correlated with the ability to bind CXCR4 (Connor et al., 1997; Lu et al., 1997). Similarly, susceptibility to infection with FIV is directly related to cellular expression of CXCR4, levels of which have been shown to be the limiting factor in productive infection of feline cells (Willett et al., 2002; de Parseval et al., 2004b). Despite the use of different primary receptors, the shared usage of CXCR4 as a cellular co-receptor by HIV and FIV establishes similarities in cell tropism between the two lentiviruses, thus reiterating the usefulness of the FIV-infected cat as a model for lentivirus-induced immunopathology at the maternal-fetal interface.
The expression of CXCR4 at both the protein and mRNA levels in the human placenta was described by Kumar et al. (2004), who reported that CXCR4 was expressed to two-fold higher levels in early versus late human placentas. In addition, the expression of CXCR4 was shown in all layers of the human placenta including the trophoblasts, stroma, and endothelium (Bustamante et al., 2005). In a previous study, abundant CXCR4 transcripts were detected in late term feline placentas, but analysis of placental CXCR4 expression was limited to a comparison of levels in FIV-infected versus control queens using single-factor ANOVA. The levels of CXCR4 expression between the two groups were virtually identical (p = 0.98), indicating that FIV did not affect expression of this chemokine receptor late in gestation (Weaver et al., 2005).
In the present study, we used real-time RT-PCR to quantify the relative expression of CD134 and CXCR4 mRNA in placental samples from three study groups: FIV negative (control) queens producing viable offspring (Group 1), FIV positive queens producing litters of only viable offspring (Group 2), and FIV positive queens producing viable offspring among majority non-viable litters (Group 3). Statistical analysis of the mean normalized Ct values for the control versus treated comparisons was done by using Monte Carlo resampling simulation (Simon, 1997) because the number of placental samples obtained from queens was limited due to pregnancy failure in FIV-infected queens. Monte Carlo random resampling analysis results in accurate estimates with fewer replications and is especially useful in the estimation of unpaired data sets where sample numbers are small and parametric statistics are more prone to error. Furthermore, confidence intervals for small sample sizes with a limited range of values can be calculated using random sampling (Chu and Ette, 2005).
We report that CD134 and CXCR4 mRNA was expressed in all placental samples and that CXCR4 was the more abundantly expressed receptor. To our knowledge, this is the first report describing the expression of CD134 at the feline maternal-fetal interface. Placentas from Group 2 cats expressed higher percentages of CD134 and CXCR4 mRNA than those of the controls (Group 1) (Fig. 1a). Other investigators reported that upregulation of CD134 and CXCR4 on CD4+ T cells occurs as a consequence of T cell activation, enhancing the effector function of the T cells (de Parseval et al., 2004a ; Joshi et al., 2005), and that increased surface expression of CXCR4 on CD4+ T cell populations results in increased susceptibility of these cells to FIV infection (Joshi et al., 2005). Although we have not evaluated placental T cells directly, upregulation of CD134 and CXCR4 in the placental tissues may provide evidence of FIV-induced T cell activation in these tissues.
Unlike the Group 2 queens, the FIV-infected queens producing non-viable fetuses (Group 3) expressed placental CD134 mRNA at a similar level to that of Group 1 (controls), while control queens expressed slightly more CXCR4 mRNA than Group 3 queens. Collectively these results may implicate a role for cells bearing these receptors in feline pregnancy maintenance.
We reported previously (Weaver et al., 2005) that FIV was detectable in 93% of placentas examined. A comparison of relative viral RNA expression between the two groups of infected cats evaluated in the present study revealed no significant difference in viral expression between the two. The expression late in gestation of both the primary receptor (CD134) and co-receptor (CXCR4) utilized by FIV may explain the frequent placental infection. Further investigation is needed to clarify the role of cells bearing these receptors in vertical transmission of the virus and to determine whether poor pregnancy outcome is a direct result of viral infection of the fetus or an indirect result of virus-induced immunopathology.
Acknowledgements
We thank Dr. Edward A. Hoover, Colorado State University, for generously providing the infectious plasma pool containing FIV-B-2542. We are grateful to the veterinary staff at the College of Veterinary Medicine, Mississippi State University, for assistance with animal care and surgery. We thank Crystal E. Boudreaux for performing ELISAs. This project was supported by the National Institutes of Health (2R15AI048419-02A1) and (3R15AI048419-02A1S1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None Declared.
References
- Al-Harthi L, Guilbert LJ, Hoxie JA, Landay A. Trophoblasts are productively infected by CD4-independent isolate of HIV type 1. AIDS Res. Hum. Retrovir. 2002;18:13–17. doi: 10.1089/088922202753394673. [DOI] [PubMed] [Google Scholar]
- Allison RW, Hoover EA. Covert vertical transmission of feline immunodeficiency virus. AIDS Res. Hum. Retrovir. 2003;19:421–434. doi: 10.1089/088922203765551764. [DOI] [PubMed] [Google Scholar]
- Arias RA, Munoz LD, Munoz-Fernandez MA. Transmission of HIV-1 infection between trophoblast placental cells and T-cells take place via an LFA-1-mediated cell to cell contact. Virology. 2003;307:266–277. doi: 10.1016/s0042-6822(02)00040-5. [DOI] [PubMed] [Google Scholar]
- Bustamante S, Garcia Y, Garrido H, Bethencourt S, Tovar R, Ponce L, Corado J, Mora-Orta S. CXCR-4 and CCR-5 expression in normal term human placenta. Investig. Clin. 2005;46:25–35. [PubMed] [Google Scholar]
- CDC HIV AIDS Surveillance Report Cases of HIV infection and AIDS in the United States and Dependent Areas. 2005 [Google Scholar]
- Chu HM, Ette EI. A random sampling approach for robust estimation of tissue-to-plasma ratio from extremely sparse data. AAPS J. 2005;7:E249–258. doi: 10.1208/aapsj070124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ubaldo C, Pezzotti P, Rezza G, Branca M, Ippolito G. Association between HIV-1 infection and miscarriage: a retrospective study. DIANAIDS Collaborative Study Group. Diagnosi Iniziale Anomalie Neoplastiche AIDS. AIDS. 1998;12:1087–1093. doi: 10.1097/00002030-199809000-00016. [DOI] [PubMed] [Google Scholar]
- Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- David FJ, Autran B, Tran HC, Menu E, Raphael M, Debre P, Hsi BL, Wegman TG, Barre-Sinoussi F, Chaouat G. Human trophoblast cells express CD4 and are permissive for productive infection with HIV-1. Clin. Exp. Immunol. 1992;88:10–16. doi: 10.1111/j.1365-2249.1992.tb03031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Parseval A, Chatterji U, Sun P, Elder JH. Feline immunodeficiency virus targets activated CD4+ T cells by using CD134 as a binding receptor. Proc. Natl. Acad. Sci. U.S.A. 2004a;101:13044–13049. doi: 10.1073/pnas.0404006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Parseval A, Elder JH. Binding of recombinant feline immunodeficiency virus surface glycoprotein to feline cells: role of CXCR4, cell-surface heparans, and an unidentified non-CXCR4 receptor. J. Virol. 2001;75:4528–4539. doi: 10.1128/JVI.75.10.4528-4539.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Parseval A, Ngo S, Sun P, Elder JH. Factors that increase the effective concentration of CXCR4 dictate feline immunodeficiency virus tropism and kinetics of replication. J. Virol. 2004b;78:9132–9143. doi: 10.1128/JVI.78.17.9132-9143.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A, Garg H, Tompkins MB, Tompkins WA. Preferential feline immunodeficiency virus (FIV) infection of CD4+ CD25+ T-regulatory cells correlates both with surface expression of CXCR4 and activation of FIV long terminal repeat binding cellular transcriptional factors. J. Virol. 2005;79:4965–4976. doi: 10.1128/JVI.79.8.4965-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman JC, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Kumar A, Kumar S, Dinda AK, Luthra K. Differential expression of CXCR4 receptor in early and term human placenta. Placenta. 2004;25:347–351. doi: 10.1016/j.placenta.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Kumar RM, Uduman SA, Khurranna AK. Impact of maternal HIV-1 infection on perinatal outcome. Int. J. Gynaecol. Obstet. 1995;49:137–143. doi: 10.1016/0020-7292(95)02356-h. [DOI] [PubMed] [Google Scholar]
- Langston C, Lewis DE, Hammill HA, Popek EJ, Kozinetz CA, Kline MW, Hanson IC, Shearer WT. Excess intrauterine fetal demise associated with maternal human immunodeficiency virus infection. J. Infect. Dis. 1995;172:1451–1460. doi: 10.1093/infdis/172.6.1451. [DOI] [PubMed] [Google Scholar]
- Lu Z, Berson JF, Chen Y, Turner JD, Zhang T, Sharron M, Jenks MH, Wang Z, Kim J, Rucker J, Hoxie JA, Peiper SC, Doms RW. Evolution of HIV-1 coreceptor usage through interactions with distinct CCR5 and CXCR4 domains. Proc. Natl. Acad. Sci. U.S.A. 1997;94:6426–6431. doi: 10.1073/pnas.94.12.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann KA, Collman R, Kolson DL, Gonzalez-Scarano F, Coukos G, Coutifaris C, Strauss JF, Nathanson N. Human immunodeficiency virus type 1 causes productive infection of macrophages in primary placental cell cultures. J. Infect. Dis. 1994;169:746–753. doi: 10.1093/infdis/169.4.746. [DOI] [PubMed] [Google Scholar]
- O'Neil LL, Burkhard MJ, Diehl LJ, Hoover EA. Vertical transmission of feline immunodeficiency virus. Semin. Vet. Med. Surg. (Small Anim) 1995;10:266–278. [PubMed] [Google Scholar]
- O'Neil LL, Burkhard MJ, Hoover EA. Frequent perinatal transmission of feline immunodeficiency virus by chronically infected cats. J. Virol. 1996;70:2894–2901. doi: 10.1128/jvi.70.5.2894-2901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers AB, Hoover EA. Maternal-fetal feline immunodeficiency virus transmission: timing and tissue tropisms. J. Infect. Dis. 1998;178:960–967. doi: 10.1086/515692. [DOI] [PubMed] [Google Scholar]
- Shimojima M, Miyazawa T, Ikeda Y, McMonagle EL, Haining H, Akashi H, Takeuchi Y, Hosie MJ, Willett BJ. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science. 2004;303:1192–1195. doi: 10.1126/science.1092124. [DOI] [PubMed] [Google Scholar]
- Simon JL. Resampling: The New Statistics. 2nd Edition Resampling Stats, Inc.; Arlington, VA: 1997. p. 420. [Google Scholar]
- UNAIDS/WHO AIDS Epidemic Update 2006. Joint United Nations Programme on HIV/AIDS. 2006 [Google Scholar]
- Weaver CC, Burgess SC, Nelson PD, Wilkinson M, Ryan PL, Nail CA, Kelly-Quagliana KA, May ML, Reeves RK, Boyle CR, Coats KS. Placental immunopathology and pregnancy failure in the FIV-infected cat. Placenta. 2005;26:138–147. doi: 10.1016/j.placenta.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Willett BJ, Cannon CA, Hosie MJ. Upregulation of surface feline CXCR4 expression following ectopic expression of CCR5: implications for studies of the cell tropism of feline immunodeficiency virus. J. Virol. 2002;76:9242–9252. doi: 10.1128/JVI.76.18.9242-9252.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]