Abstract
Purpose
An increasing number of reports have implicated bisphosphonates as contributing to osteonecrosis of the jaw. The goal of this study was to evaluate mandible necrosis in beagle dogs treated for three years of daily treatment with oral alendronate (ALN).
Materials and Methods
Skeletally-mature female beagles were treated daily for three years with oral doses of vehicle or ALN (0.20 or 1.0 mg/kg/day). These doses approximate, on a mg/kg basis, those used for post-menopausal osteoporosis and Paget’s disease, respectively. At necropsy the 2nd molar region of the mandible was excised, stained en bloc with basic fuchsin, and assessed for matrix necrosis and intracortical bone turnover rate using histology. Matrix necrosis was defined as a region > 500um2 that was void of basic fuchsin stain, assessed using both brightfield and confocal microscopy.
Results
No animals developed exposed bone lesions in the oral cavity during the 3-year study. Matrix necrosis was observed in 25% of ALN0.2 animals and 33% of ALN1.0 animals while being noticeably absent from all vehicle animals (p < 0.05 pooled ALN doses vs VEH). These necrotic regions occurred predominately in the alveolar bone and were clearly void of patent canaliculi. Intracortical bone turnover rate of the alveolar mandible bone region was significantly lower (−75%, p < 0.05) in ALN-treated animals compared to VEH.
Conclusions
Three-years of daily oral bisphosphonate treatment significantly reduces bone turnover and increase the incidence of matrix necrosis within the mandible of dogs.
Keywords: osteonecrosis of the jaw, necrosis, remodeling suppression, alendronate
Introduction
Osteonecrosis (literally, “dead bone”) occurs when localized populations of osteocytes die 1–4. Localized osteonecrosis is a normal physiological process that occurs throughout the skeleton 1 and increases in prevalence with age 2, 3. The progression of localized osteonecrosis, from the death of a few osteocytes to a more wide-spread region of matrix necrosis, is a slow process 1. This progression is normally controlled through remodeling, which removes necrotic bone matrix, thereby limiting the negative consequences of necrosis 1. In some cases, such as occurs with osteonecrosis of the jaw (ONJ), the process of osteonecrosis becomes pathological 5–7.
Since the first literature reports linking ONJ to bisphosphonate treatment 6, 8, the public health ramifications have quickly consumed the scientific 5, 9–12 and public communities 13. Despite all this attention, very little is known about the relationship between bisphosphonates and ONJ 12. The overwhelming majority of documented ONJ cases have been in patients administered high doses of intravenous bisphosphonates for treatment/prevention of cancer-related malignancies 14–17. A smaller number of ONJ cases have been reported in patients receiving oral bisphosphonates for treatment of post-menopausal osteoporosis 9, 11, 17. It has been proposed that as ONJ becomes more universally defined, the incidence rate with oral bisphosphonates may increase 18. However, even with a lower incidence rate compared to cancer patients treated with intravenous bisphosphonates, the wide-spread use of bisphosphonates for osteoporosis makes ONJ a significant public health issue that warrants investigation.
Bisphosphonates reduce fracture risk and the development of skeletal metastases by suppressing bone turnover. Turnover suppression increases the mean tissue age because regions of older bone are remodeled less frequently. Since matrix necrosis is related to mean tissue age 2, 3, it follows that a reduction in turnover would result in an increase in necrotic bone matrix. The mandible, especially the alveolar bone region, has a high basal turnover rate 19, 20 and therefore may be particularly susceptible to an accumulation of necrotic bone matrix with bisphosphonate treatment.
The goal of this study was to evaluate osteonecrosis in the mandible of dogs treated for 3 years with clinically-relevant doses of oral alendronate. We hypothesized that tissue necrosis would be more prevalent in the mandible of animals treated with alendronate compared to vehicle-treated animals.
Methods
Experimental Design
All experimental procedures were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee prior to the start of the study. Thirty-six skeletally mature female beagles (1–2 years old) were purchased from LBL (Reelsville, IN). Upon arrival, lateral X-rays of all dogs were obtained to confirm skeletal maturity (closed proximal tibia and lumbar vertebral growth plates). Animals were housed two per cage in environmentally controlled rooms at Indiana University School of Medicine’s AALAC accredited facility. Following two weeks of acclimatization, animals were assigned to one of three treatment groups (n=12/treatment) by matching body weights. All dogs were treated for 3 years with oral doses of saline vehicle (VEH, 1 ml/kg/day) or alendronate (ALN 0.2 mg/kg/day or 1.0 mg/kg/day). The ALN 0.2 dose corresponds (on a mg/kg basis) to the dose used for the treatment of post-menopausal osteoporosis. The ALN 1.0 dose approximates, although is slightly higher than, the dose for treatment of Paget’s disease. Alendronate was dissolved in saline and administered to the dogs orally with a syringe each morning after an overnight fast and at least 2 hours prior to feeding. In order to measure bone formation rate, animals were administered intravenous fluorochrome label (calcein, 0.20 mL/kg) using a 2-12-2-5 labeling schedule (2 days of injection, 12 day ‘off’ period, 2 days of injections, 5 day ‘off’ period, necropsy). Following three years of treatment, animals were euthanized by intravenous administration of sodium pentobarbital (0.22 mg/kg Beuthanasia-D Special). The left half of each mandible was wrapped in saline soaked gauze and frozen at −20°C until analysis.
Matrix necrosis
Mandibles were thawed to room temperature and a portion encompassing the 2nd molar was isolated (by making two parallel buccal-lingual cuts) using a band saw with a diamond coated blade while under constant irrigation. Tissue segments were processed by bulk staining en bloc in basic fuchsin 21 with slight modifications. Basic fuchsin stains cells and empty spaces within the bone matrix (lacunae, canaliculi, Haversian canals, and microcracks) (Figure 1) but is absent in necrotic matrix due to the lack of patent canalicular networks 1, 22. Using 1% basic fuchsin dissolved in increasing concentrations of ethanol, specimens were stained according to the following schedule: 48 hours 80% (with one change to fresh 80% solution after 24 hours), 48 hours in 95% (with one change to fresh 95% solution after 24 hours), 48 hours in 100% (with one change to fresh 100% solution after 24 hours). Bones were placed under vacuum (20 in Hg) for all stages. This staining protocol differs from that of microdamage staining in the duration of stain, with microdamage specimens stained 4 hours per step 23 and these specimens stained 24 hours per step. This ‘over staining’ allows a clearer assessment of necrotic regions. Following staining, bones were embedded undecalcified as previously described 23. Two coronal plane histological sections (100–200 µm thick) were cut using a diamond wire saw (Histosaw, Delaware Diamond Knives).
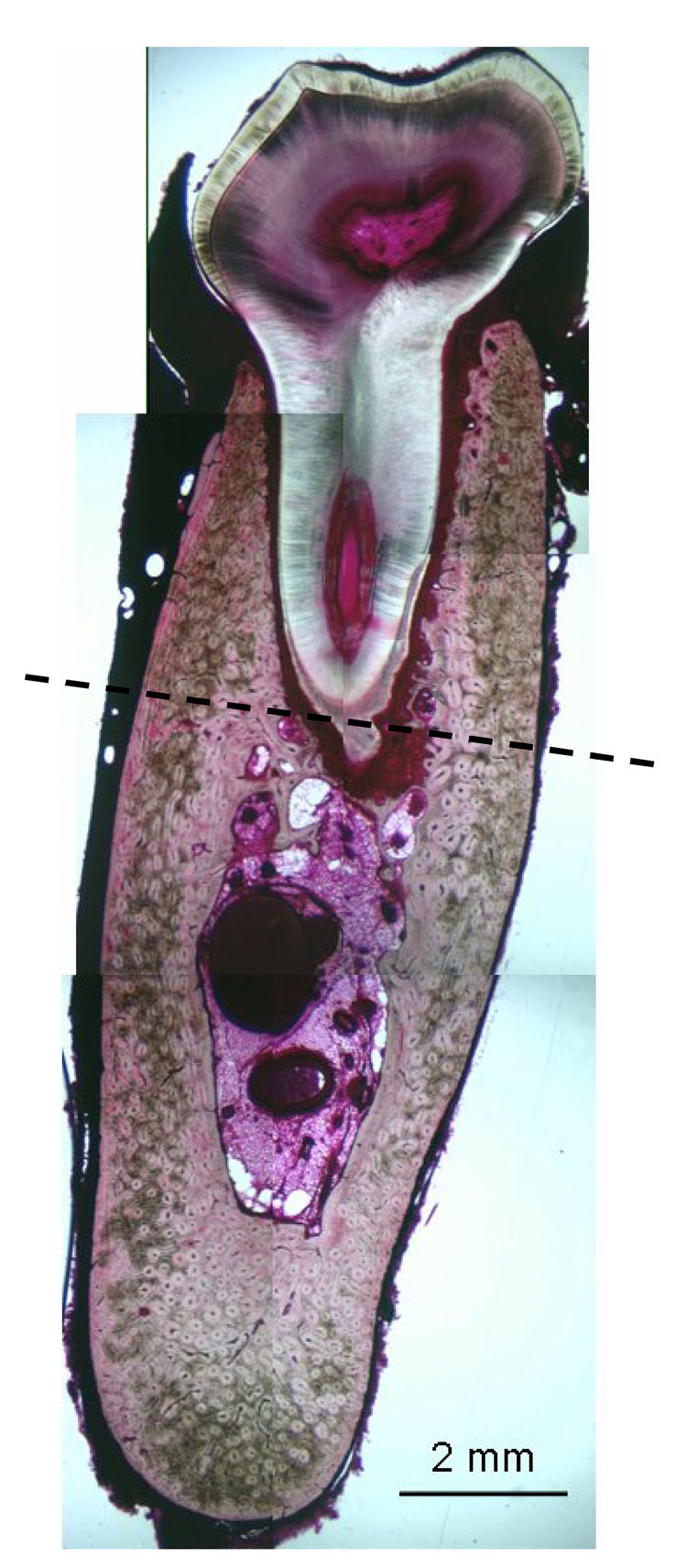
Figure 1.
Basic fuchsin stained histological section through the 2nd molar region. Dotted line at the base of the tooth root separates the alveolar (above line) and non-alveolar (below line) regions for classifying locations of necrosis and bone turnover rate.
Brightfield histological measurements were made using a semiautomatic analysis system (Bioquant OSTEO 7.20.10, Bioquant Image Analysis Co.) attached to a microscope (Nikon Optiphot 2 microscope, Nikon). Under bright-field, regions of bone void of basic fuchsin stain >500 µm2 were considered necrotic. This was chosen as it exceeds the average size of two adjacent osteons or interstitial regions 24. Limiting areas defined as necrotic to a larger area such as this also reduces inter-individual variability among animals in the analyses. For all animals, two complete cross-sections of the mandible (Figure 1) were assessed for regions of necrosis. Histological evaluation was conducted, blinded to treatment, by one investigator. All animals were classified as yes/no for matrix necrosis and the mean two-dimensional size of necrotic regions was measured. Necrotic regions were classified as being in the alveolar bone (above the most distally observed portion of the tooth roo t19), or non-alveolar (the remainder of the tissue).
Regions identified as being void of stain using brightfield microscopy were also examined using confocal microscopy (Bio-Rad MRC1024 Laser Scanning Confocal Microscope). Necrotic regions were imaged (2–4 separate fields of view for each necrotic zone) using a 568-nm wavelength excitation and a 605DF32 emission filter, and a 40x water dipping objective 25.
Bone turnover rate
Intracortical bone formation rate was measured using dynamic histomorphometry. One of the two histological sections assessed for matrix necrosis was used for these analyses; calcein labeling could be clearly observed despite heavy basic fuchsin staining. For all measures, alveolar bone was assessed separately from non-alveolar bone, with the two regions pooled together to get values for the total mandible. Using UV light, the bone area (B.Ar), number of labeled osteons (L.Os.#), the total length of osteonal labeled surface (LS), and the mean inter-label distance (Ir.L.Dis) were measured at 10x magnification. Mineral apposition rate (MAR, um/day) was calculated as Ir.L.Dis / 12, where 12 is the number of days between labels. Intracortical bone formation rate (%/year) was calculated as (MAR ×(LS/2) / B.Ar × 100) × 365. All measures and calculations were in accordance with ASBMR recommended standards 26.
Statistics
Due to the small sample size, the incidence of animals having matrix necrosis and the size of necrotic areas were compared using one-tailed Fisher’s exact test and Mann-Whitney tests, respectively. Dynamic bone formation parameters were compared among the vehicle and the two alendronate doses using one-way analysis of variance (ANOVA) with Fishers protected least significant difference post-hoc tests. As none of the variables of interest were significantly different between the two doses of alendronate, additional comparisons were performed between vehicle and pooled alendronate doses. For all analyses, significance was determined using the criteria of p < 0.05. Data are presented as mean ± standard errors.
Results
There was no evidence of exposed bone in the oral cavity of any of the dogs during the 3 year study.
Using histology, regions of matrix necrosis were observed in the mandible of three of twelve animals (25%) treated with ALN0.2, and four of twelve animals (33%) treated with ALN1.0 (Table 1). This represents a significantly higher incidence rate in alendronate-treated animals (7 of 24; 29%) compared to vehicle-treated animals (p < 0.05), in which no regions of matrix necrosis were observed in any of the twelve animals. There was no difference in the incidence rate of necrosis between the two doses of alendronate.
Table 1.
Summary of mandible matrix necrosis following three years of bisphosphonate treatment
| Vehicle 1 ml/kg/day | Alendronate 0.2 mg/kg/day | Alendronate 1.0 mg/kg/day | Pooled Alendronate doses | |
|---|---|---|---|---|
| Animals | 12 | 12 | 12 | 24 |
| Animals with necrosis, # | 0 | 3 | 4 | 7 a |
| Necrosis area, mm2 | 0 | 1.35 ± 0.42 a | 1.57 ± 0.22 a | 1.47 ± 0.21 a |
| Location of necrosis within mandible cross-section | -- | Alveolar | Alveolar and non-alveolar | Alveolar and non-alveolar |
p < 0.05 versus vehicle.
Necrotic regions were defined by the absence of basic fuchsin stain (Figure 2). In all cases, necrotic regions could be observed in both of the tissue sections from a particular animal. The majority of necrotic regions were in the alveolar regions of the mandible although two animals (both in the ALN1.0 group) had necrotic regions in the base of the mandible bone (Table 1). Two animals treated with ALN0.2, and one animal treated with ALN1.0 had two separate regions of necrosis within the same tissue section. The average cross-sectional area of necrotic tissue among all ALN-treated animals was 1.47 ± 0.21 mm2, which represents approximately 2% of the bone tissue area. This size of necrotic areas did not differ between the two doses of ALN (Table 1).
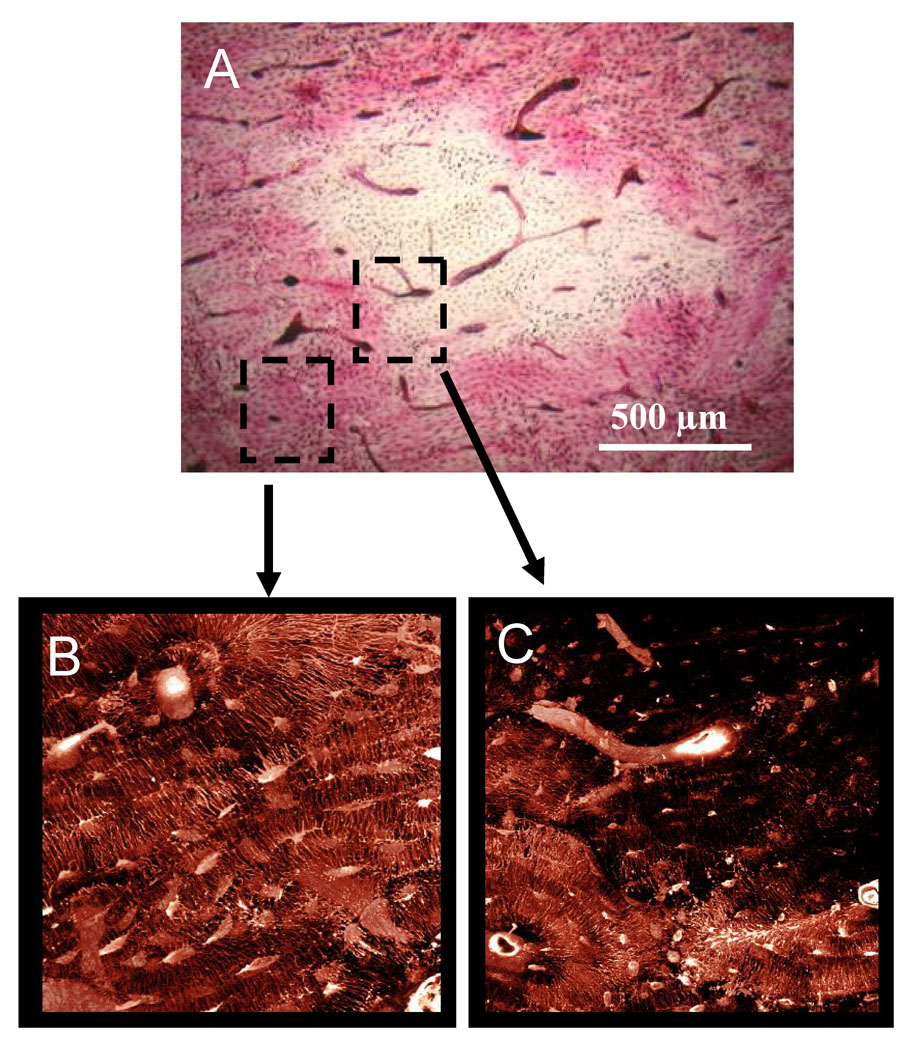
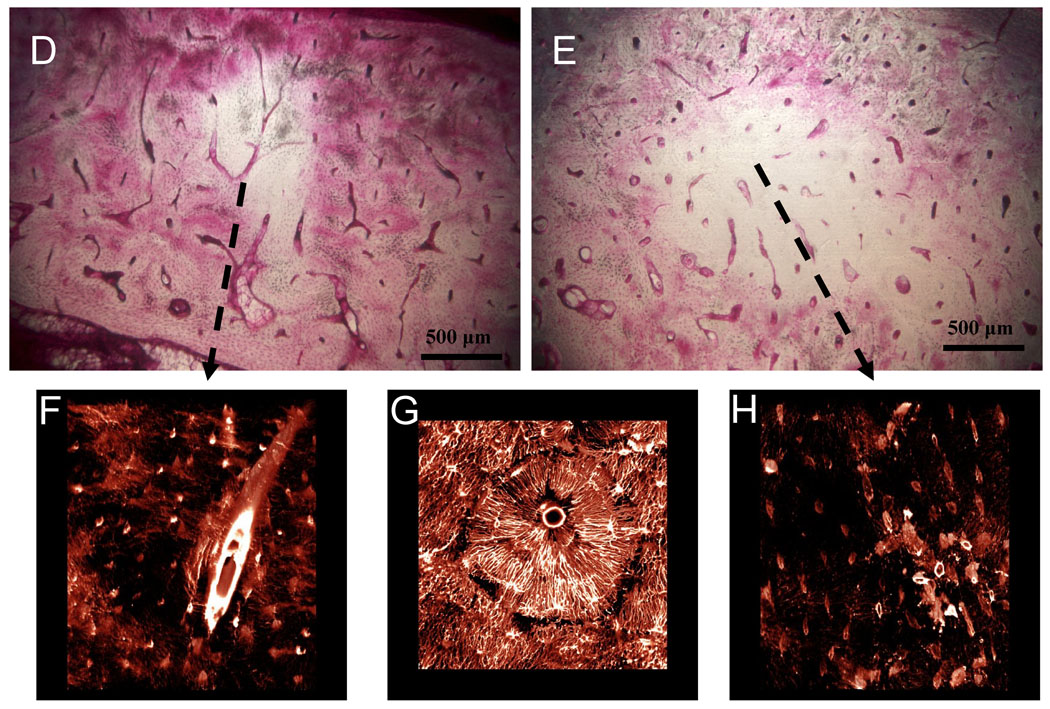
Figure 2.
Matrix necrosis in the mandible of dogs treated for 3 years with alendronate. The second molar region was stained en bloc with basic fuchsin with regions of matrix necrosis defined as those regions void of stain uptake. Necrotic bone matrix can be observed using brightfield microscopy (A, D, E). Due to its fluorescent properties, stained regions can be visualized using confocal microscopy, revealing patent canalicular networks (B,G). Conversely, necrotic regions which are void of stain can are without patent canalicular networks (C, F, H).
When viewed using confocal microscopy, regions classified as necrotic using brightfield microscopy were absent of stained osteocyte lacunae and canalicular networks (Figure 2). The vasculature within these necrotic regions appeared sufficiently stained, suggesting the necrotic tissue is not avascular and that the absence of matrix staining was due to the inability of the stain to move from the vessel into the matrix because of disrupted canalicular networks. Bone regions showing clear stain uptake using brightfield microscopy displayed clearly stained osteocyte lacunae, canalicular networks, and blood vessels, and therefore were considered viable bone (Figure 2). No regions considered to be viable with brightfield microscopy were found to have compromised staining patters when viewed using confocal microscopy.
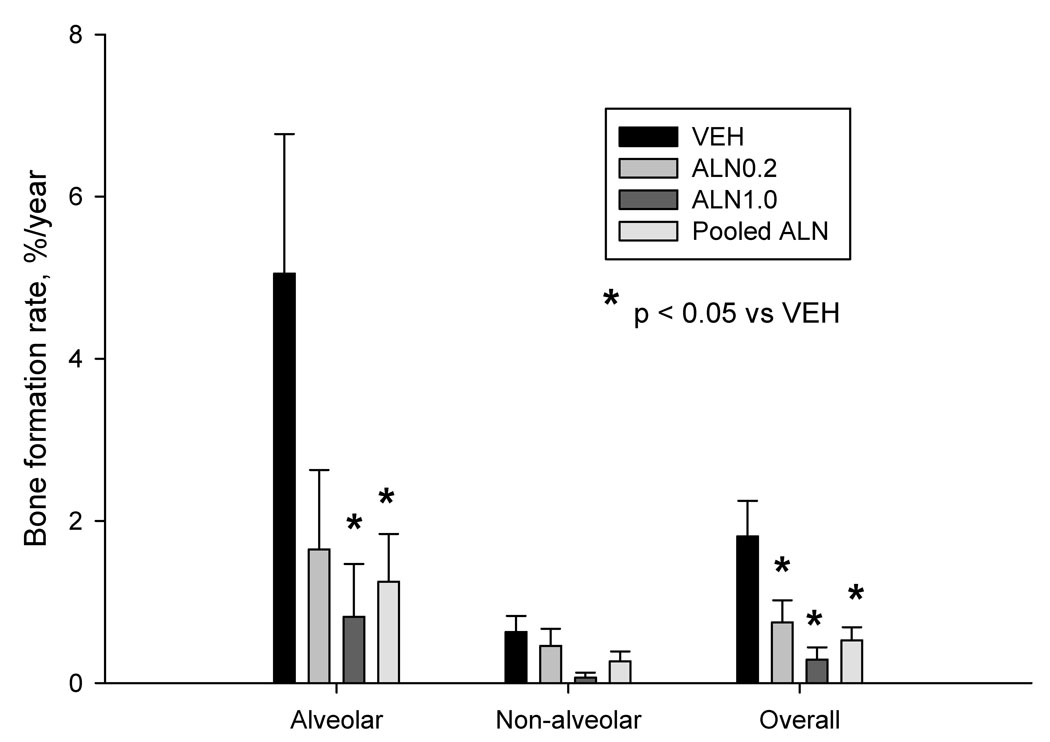
Intracortical bone formation rate of the overall mandible cross-section was significantly lower than vehicle with ALN0.2 (−58%), ALN1.0 (−84%), or when doses were pooled (−71%) (Figure 3). This was mainly due to suppression of turnover in the alveolar bone region, which among all animals was 84% greater than the turnover rate in non-alveolar bone. Alveolar bone formation was significantly lower than vehicle for ALN1.0 (−84%) and the pooled ALN doses (−75%) (Figure 3). There was no significant difference among groups in non-alveolar bone formation rate. Lower bone formation rate in ALN-treated animals was the result of both fewer labeled osteons and reduced mineral apposition rate (Table 2). There was no significant difference between the two doses of ALN for any of the dynamic histomorphometry variables.
Figure 3.
Bisphosphonates significantly reduce intracortical bone formation rate in the mandible. There was a significant reduction in the overall bone formation rate of the mandible with both doses of alendronate (ALN) or when the two doses of ALN were pooled. There was no significant difference between the two doses of ALN. The greatest suppression of turnover was noted in the alveolar portion of the mandible with no significant effect of ALN treatment on turnover suppression in the non-alveolar portion. Across all groups turnover was 84% lower in the non-alveolar region compared to the alveolar region. * p < 0.05 versus VEH.
Table 2.
Intracortical bone turnover of the mandible
| Vehicle 1 ml/kg/day | Alendronate 0.2 mg/kg/day | Alendronate 1.0 mg/kg/day | Pooled Alendronate doses | |
|---|---|---|---|---|
| N | 12 | 12 | 12 | 24 |
| Alveolar bone | ||||
| Bone area, mm2 | 15.6 ± 1.7 | 13.3 ± 1.1 | 17.4 ± 2.3 | 15.2 ± 1.3 |
| Labeled osteons, #/mm2 | 0.52 ± 0.18 | 0.25 ± 0.12 | 0.13 ± 0.10 | 0.20 ± 0.08 |
| MAR, um/day | 1.37 ± 0.25 | 0.56 ± 0.25 a | 0.39 ± 0.28 a | 0.37 ± 0.15 a |
| Non-alveolar bone | ||||
| Bone area, mm2 | 43.1 ± 2.7 | 48.0 ± 3.1 | 50.1 ± 3.3 | 49.0 ± 2.2 |
| Labeled osteons, #/mm2 | 0.062 ± 0.02 | 0.038 ± 0.01 | 0.004 ± 0.002 a | 0.02 ± 0.01 a |
| MAR, um/day | 0.80 ± 0.25 | 0.72 ± 0.23 | 0.21 ± 0.14 | 0.50 ± 0.15 |
| Overall mandible | ||||
| Bone area, mm2 | 58.6 ± 3.5 | 61.3 ± 3.2 | 67.4 ± 2.9 | 64.2 ± 2.2 |
| Labeled osteons, #/mm2 | 0.18 ± 0.04 | 0.09 ± 0.03 a | 0.03 ± 0.02 a | 0.06 ± 0.02 a |
Data (mean ± SE) represent one tissue section per animal.
p < 0.05 versus vehicle.
Discussion
The existence of focal necrotic regions within the bone matrix is a normal component of the skeleton’s lifecycle, occurring when the bone matrix outlives its’ resident osteocytes 1–4. There is minimal consequence of focal internal matrix necrosis and assuming adequate levels of bone turnover it is likely removed in an efficient manner. Reducing the rate of turnover, as with bisphosphonate treatment, would be expected to increase the prevalence of matrix necrosis, as those regions that normally develop would exist for a longer period of time and may become larger in size. Our results are consistent with these theoretical expectations, showing that animals treated with clinically-relevant doses of alendronate for 3 years have a significant accumulation of necrotic bone within the mandible. These necrotic regions are void of patent canaliculi but had retained their vasculature, the latter observation suggesting this is not an ‘avascular necrosis’.
While this study shows clear evidence of increased mandible matrix necrosis in animals treated for three years with clinically relevant doses of alendronate, it does not address the mechanism responsible for this result. One hypothesis is that matrix necrosis is a result of a reduction in bone turnover. Assuming under normal conditions there is equilibrium between the rate of matrix necrosis formation and its removal 1–3, the suppression of removal due to bisphosphonate-induced turnover suppression would result in an increased level of necrosis. Given the high basal turnover rate of the mandible compared to other skeletal sites 19, 20, it would be expected that relatively little matrix necrosis would normally exist at this site and that any change in turnover rate would result in an accumulation of necrosis. Our data are consistent with this hypothesis as the majority of necrotic regions were localized to the alveolar bone of the mandible; a location which we show has over 6x higher turnover compared to the remainder of the mandible. However, two necrotic areas were observed in the mandible base, a site with relatively low turnover suggesting there may be a component of necrosis accumulation that is independent of turnover suppression.
An alternative hypothesis is that bisphosphonates directly affect osteocyte viability, effectively decreasing their lifespan and increasing the rate of necrotic matrix formation. Evidence from osteocyte culture studies argue against this hypothesis, as concentrations of bisphosphonates estimated to exist in vivo were found to reduce osteocyte apoptosis both in vitro 27 and in vivo 28. At higher concentrations, bisphosphonates have been shown to have direct cytotoxic effects on other cells, including fibroblasts 29 and endothelial cells 30. As bisphosphonates accumulate in the skeleton in a dose-dependent manner 31, 32 it is possible that with prolonged exposure the local concentrations of drug reach very high levels, perhaps exceeding those studied by Plotkin et al 27. Such concentrations could have a cytotoxic effect on osteocytes. This hypothesis, one of skeletal accumulation independent of turnover suppression, would be consistent with our finding regions of necrosis in the base of the mandible (a site with low turnover) only in those dogs treated with the higher dose of alendronate.
The connection between mandible matrix necrosis, as observed in the current study, and osteonecrosis of the jaw (ONJ), defined as exposed oral lesions, remains unclear. We hypothesize that increased levels of matrix necrosis represent an early stage of ONJ but that additional perturbations of the oral cavity are necessary to manifest exposed oral lesions. Both tooth extraction and periodontal disease have been implicated as having an interaction with bisphosphonates in the manifestation of ONJ 33, 34. The most plausible link between bisphosphonates and both dental extraction and periodontal disease is their contrasting effects on bone turnover. In response to extraction the turnover rate is accentuated, most notably in the alveolar bone surrounding the extraction site (up to 400%/year) 35. Increased turnover also occurs with periodontal disease or other instances in which infection is present in the oral region (osteomyelitis). Therefore in the presence of bisphosphonates, the normal enhancement of turnover in each of these instances is prevented, perhaps leading to an accumulation of non-viable osteocytes which progresses to necrotic bone matrix, and eventually the clinical manifestation of ONJ.
Limitations exist for studying ONJ in humans, emphasizing the need to develop an animal model to help understand the pathophysiology of ONJ and to eventually test treatment or prevention 12. The canine model is routinely utilized in skeletal biology and offers numerous advantages for studying ONJ. First, the turnover rates in beagles are similar to humans with a slightly shorter remodeling period (3 months in dog versus 4–6 months in humans) 36, 37. This allows studies where changes in turnover may have an important physiological role to be conduced using a condensed time-frame. Secondly, the alveolar bone of the dog mandible has high levels of intra-cortical remodeling, similar to humans 19, 20, 35. One perceived limitation of the canine model is that it is not a model of osteoporosis (or osteopenia) as dogs do not lose bone when ovariectomized. However, as ONJ is not a condition that appears linked to either estrogen deficiency or low bone mass, this is not a significant limitation to the model.
The current study has various limitations. As we did not examine the entire mandible it is not possible to determine the true extent of necrosis in bisphosphonate-treated animals nor eliminate the possibility vehicle-treated animals have necrotic regions. However, three additional 1 mm tissue segments were examined in all dogs (2 sections per region). These additional sections showed no evidence of necrosis in any of the vehicle-treated animals nor did they show necrosis in any additional alendronate-treated dogs. Regions of necrosis were evident in other segments of two dogs that had necrosis in the 2nd molar region. We therefore consider the results in the 2nd molar region to be representative of the matrix necrosis in these animals. For this study, necrotic regions were defined as those > 500 µm2, which exceeds the average size of two adjacent osteons or interstitial regions 24. This explains the absence of necrosis in vehicle-treated animals. Indeed, smaller regions of the bone matrix that were void of stain could be observed in both alendronate- and vehicle-treated animals, using brightfield microscopy. Limiting areas defined as necrotic to a larger area such as > 500 µm2 also reduces inter-individual variability among animals in the analyses. Finally, this study provides no conclusive evidence that these necrotic lesions are related to the clinical condition of ONJ.
In conclusion, we have shown that following three years of treatment with clinically-relevant doses of oral alendronate, regions of bone matrix necrosis existed within the mandible of beagle dogs. We propose the matrix necrosis observed in these animals represents an early stage of a process that may eventually result in the clinical manifestation of osteonecrosis of the jaw.
Acknowledgements
The authors thank Keith Condon and Diana Jacobs for their assistance with preparation of histological samples. Confocal microscopy was conducted within The Indiana Center for Biological Microscopy. This work was supported by NIH Grants AR047838 and an animal facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR10601-01 from the National Center for Research Resources, National Institutes of Health. Alendronate was kindly provided by Merck.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Enlow DH. Functions of the Haversian system. Am J Anat. 1962;110:269–305. doi: 10.1002/aja.1001100305. [DOI] [PubMed] [Google Scholar]
- 2.Enlow DH. Osteocyte necrosis in normal bone. J Dent Res. 1966;45:213. doi: 10.1177/00220345660450011901. [DOI] [PubMed] [Google Scholar]
- 3.Frost HM. In vivo osteocyte death. J Bone Joint Surg Am. 1960;42-A:138–143. [PubMed] [Google Scholar]
- 4.Dunstan CR, Somers NM, Evans RA. Osteocyte death and hip fracture. Calcif Tissue Int. 1993;53 Suppl 1:S113–S116. doi: 10.1007/BF01673417. discussion S116-117. [DOI] [PubMed] [Google Scholar]
- 5.Migliorati CA, Casiglia J, Epstein J, et al. Managing the care of patients with bisphosphonate-associated osteonecrosis: an American Academy of Oral Medicine position paper. J Am Dent Assoc. 2005;136:1658–1668. doi: 10.14219/jada.archive.2005.0108. [DOI] [PubMed] [Google Scholar]
- 6.Ruggiero SL, Mehrotra B, Rosenberg TJ, et al. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Bilezikian JP. Osteonecrosis of the jaw--do bisphosphonates pose a risk? N Engl J Med. 2006;355:2278–2281. doi: 10.1056/NEJMp068157. [DOI] [PubMed] [Google Scholar]
- 8.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 9.Shane E, Goldring S, Christakos S, et al. Osteonecrosis of the Jaw: More Research Needed. Journal of Bone and Mineral Research. 2006;21:1503–1505. doi: 10.1359/jbmr.060712. [DOI] [PubMed] [Google Scholar]
- 10.2006 Website: http://www.nof.org/patientinfo/osteonecrosis.htm.
- 11.American Dental Association Council on Scientific. A. Dental management of patients receiving oral bisphosphonate therapy: Expert panel recommendations. J Am Dent Assoc. 2006;137:1144–1150. doi: 10.14219/jada.archive.2006.0355. [DOI] [PubMed] [Google Scholar]
- 12.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-Associated Osteonecrosis of the Jaw: Report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007 doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 13.Kolata G. Drug for Bones Is Newly Linked to Jaw Disease. New York: The New York Times; 2006. [Google Scholar]
- 14.Woo SB, Hellstein JW, Kalmar JR. Systematic review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 15.Lacy MQ, Dispenzieri A, Gertz MA, et al. Mayo clinic consensus statement for the use of bisphosphonates in multiple myeloma. Mayo Clin Proc. 2006;81:1047–1053. doi: 10.4065/81.8.1047. [DOI] [PubMed] [Google Scholar]
- 16.Kuehn BM. Reports of adverse events from bone drugs prompt caution. Jama. 2006;295:2833–2836. doi: 10.1001/jama.295.24.2833. [DOI] [PubMed] [Google Scholar]
- 17.Mavrokokki T, Cheng A, Stein B, et al. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in australia. J Oral Maxillofac Surg. 2007;65:415–423. doi: 10.1016/j.joms.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 18.Ruggiero SL, Fantasia J, Carlson E. Bisphosphonate-related osteonecrosis of the jaw: background and guidelines for diagnosis, staging and management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:433–441. doi: 10.1016/j.tripleo.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Huja SS, Fernandez SA, Hill KJ, et al. Remodeling dynamics in the alveolar process in skeletally mature dogs. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1243–1249. doi: 10.1002/ar.a.20396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garetto LP, Tricker ND. Remodeling of bone surrounding the implant interface. In: Garetto LP, Turner CH, Duncan RL, Burr DB, editors. Bridging the Gap Between Dental & Orthopaedic Implants; 3rd Annual Indiana Conference; Indianapolis, IN. 1998. [Google Scholar]
- 21.Burr DB, Stafford T. Validity of the bulk-staining technique to separate artifactual from in vivo bone microdamage. Clin Orthop Relat Res. 1990:305–308. [PubMed] [Google Scholar]
- 22.Frost HM. Micropetrosis. J Bone Joint Surg Am. 1960;42-A:144–150. [PubMed] [Google Scholar]
- 23.Allen MR, Iwata K, Phipps R, et al. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone. 2006;39:872–879. doi: 10.1016/j.bone.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 24.Jaworski Z, Lok E. The rate of osteoclastic bone erosion in haversian remodeling sites of adult dog's rib. Calc Tiss Res. 1972;10:103–112. doi: 10.1007/BF02012540. [DOI] [PubMed] [Google Scholar]
- 25.Bentolila V, Boyce TM, Fyhrie DP, et al. Intracortical remodeling in adult rat long bones after fatigue loading. Bone. 1998;23:275–281. doi: 10.1016/s8756-3282(98)00104-5. [DOI] [PubMed] [Google Scholar]
- 26.Parfitt A, Drezner M, Glorieux F, et al. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Journal of Bone and Mineral Research. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 27.Plotkin LI, Weinstein RS, Parfitt AM, et al. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Follet H, Li J, Phipps RJ, et al. Risedronate and alendronate suppress osteocyte apoptosis following cyclic fatigue loading. Bone. 2007;40:1172–1177. doi: 10.1016/j.bone.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 29.Correia Vde F, Caldeira CL, Marques MM. Cytotoxicity evaluation of sodium alendronate on cultured human periodontal ligament fibroblasts. Dent Traumatol. 2006;22:312–317. doi: 10.1111/j.1600-9657.2005.00434.x. [DOI] [PubMed] [Google Scholar]
- 30.Moreira MS, Katayama E, Bombana AC, et al. Cytotoxicity analysis of alendronate on cultured endothelial cells and subcutaneous tissue. a pilot study. Dent Traumatol. 2005;21:329–335. doi: 10.1111/j.1600-9657.2005.00370.x. [DOI] [PubMed] [Google Scholar]
- 31.King LE, Grynpas MD, Tomlinson G, et al. Pamidronate content and turnover in sternum, vertebral body, and iliac bones of dogs. Bone. 1997;20:405–411. doi: 10.1016/s8756-3282(97)00017-3. [DOI] [PubMed] [Google Scholar]
- 32.Komatsubara S, Mori S, Mashiba T, et al. Suppressed bone turnover by long-term bisphosphonate treatment accumulates microdamage but maintains intrinsic material properties in cortical bone of dog rib. J Bone Miner Res. 2004;19:999–1005. doi: 10.1359/JBMR.040126. [DOI] [PubMed] [Google Scholar]
- 33.Zavras AI, Zhu S. Bisphosphonates are associated with increased risk for jaw surgery in medical claims data: is it osteonecrosis? J Oral Maxillofac Surg. 2006;64:917–923. doi: 10.1016/j.joms.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Migliorati CA, Schubert MM, Peterson DE, et al. Bisphosphonate-associated osteonecrosis of mandibular and maxillary bone: an emerging oral complication of supportive cancer therapy. Cancer. 2005;104:83–93. doi: 10.1002/cncr.21130. [DOI] [PubMed] [Google Scholar]
- 35.Garetto LP, Chen J, Parr JA, et al. Remodeling dynamics of bone supporting rigidly fixed titanium implants: a histomorphometric comparison in four species including humans. Implant Dent. 1995;4:235–243. doi: 10.1097/00008505-199500440-00002. [DOI] [PubMed] [Google Scholar]
- 36.Boyce RW, Paddock CL, Gleason JR, et al. The effects of risedronate on canine cancellous bone remodeling: three-dimensional kinetic reconstruction of the remodeling site. J Bone Miner Res. 1995;10:211–221. doi: 10.1002/jbmr.5650100207. [DOI] [PubMed] [Google Scholar]
- 37.Eriksen E, Axelrod D, Melsen F. Bone Histomorphometry. New York: Raven Press; 1994. [Google Scholar]