Abstract
Adrenal chromaffin cells release multiple transmitters in response to sympathetic stimulation. Modest cell firing, matching sympathetic tone, releases small freely-soluble catecholamines. Elevated electrical firing rates matching input under sympathetic stress results in release of catecholamines as well as semi-soluble vaso- and neuro-active peptides packaged within the dense core of the secretory granule. This activity-dependent differential transmitter release has been shown to rely on a mechanistic shift in the mode of exocytosis through the regulated dilation of the secretory fusion pore between granule and cell surface membranes. However, biochemical description of the mechanism regulating fusion pore dilation remains elusive. In the experimental setting, electrical stimulation designed to mimic sympathetic input, is achieved through single-cell voltage-clamp. While precise, this approach is incompatible with biochemical and proteomic analysis, both of which require large sample sizes. We address this limitation in the current study. We describe a bulk chemical stimulation paradigm calibrated to match defined electrical activity. We utilize calcium and single-cell amperometric measurements to match extracellular potassium concentrations to physiological electrical stimulation under sympathetic tone as well as acute stress conditions. This approach provides larger samples of uniformly-stimulated cells for determining molecular players in activity-dependent differential transmitter release from adrenal chromaffin cells.
Keywords: action potential, amperometry, potassium, patch clamp, adrenal medulla, sympathetic, exocytosis, chromaffin
Introduction
Chromaffin cells of the adrenal medulla are a primary output of the sympathetic nervous system. Upon splanchnic stimulation chromaffin cells fire action potentials that lead to Ca2+ entry through voltage operated calcium channels. The elevated intracellular calcium causes fusion of large dense-core secretory granules with the cell surface and exocytosis of their content into the circulation. The large dense-core granules contain many transmitters, including small highly soluble molecules such as ATP, Ca2+ and catecholamines (epinephrine or norepinephrine). Chromaffin granules also contain a large semi-soluble proteinacious core that contains peptide transmitters. Release of these peptide transmitters forms an essential physiological response to acute stress. Examples of the peptide transmitters include enkephalin, an endogenous opioid analgesic; neuropeptide Y, which regulates vasodilatation and other stress responses; chromogranins, precursor molecules for the neuroactive catestatins; and atrial natriuretic factor, another vasoactive peptide (Aunis, 1998; Winkler, 1993).
Previous work has demonstrated that chromaffin secretory granules fuse with the cell surface to release their content. The mode of fusion differs in an activity-dependent manner (Elhamdani et al., 2001). Physiological electrical stimulation results in a biphasic exocytic response from chromaffin cells, depending on stimulus frequency. Under basal activity set to mimic stimulation under sympathetic tone, chromaffin cells release mainly catecholamines through a fusion process termed ‘kiss and run’ exocytosis (Fulop et al., 2005; Perrais et al., 2004). Kiss and run exocytosis is characterized by transient fusion of the granule with the cell surface during which the granule maintains its basic morphology (Fulop and Smith, 2006). Endocytosis and recycling of the granule membrane occurs through a clathrin-independent pinching off of the vesicle from the cell surface (Artalejo et al., 1995; Chan and Smith, 2003), effectively retrieving the granule intact. Previous studies have shown that under kiss and run exocytosis only the small freely soluble transmitters are released while the proteinacious granule core is retained in the granule lumen (Fulop and Smith, 2006).
Elevated sympathetic activity, as experienced under the acute stress response, drives chromaffin cells to fire at an approximately 30-fold higher rate (Kidokoro and Ritchie, 1980). The elevated excitation evokes A different mode of granule fusion from chromaffin cells. Under the stress response granule fusion proceeds past the ‘kiss and run’ configuration and fully collapses into the membrane, expelling its entire content; catecholamine and peptide transmitters. This dilation of the fusion pore has been shown to be mediated by elevated cytosolic Ca2+ driving a PKC-mediated phosphorylation event (Fulop and Smith, 2006). In this case, endocytic retrieval of excess surface membrane is achieved by bulk retrieval of membrane through a clathrin-mediated mechanism (Artalejo et al., 2002; Artalejo et al., 1995; Chan et al., 2003). Thus, driven at rates mimicking basal sympathetic tone or at rates that match the acute stress response, chromaffin cells employ two separate mechanisms of exocytosis and endocytosis and effect the preferential release of catecholamine alone versus the release of catecholamine and peptide transmitter molecules. Regulation of the fusion pore dilation represents a basic mechanism for the shift in sympathetic status from ‘breed and feed to ‘fight or flight’.
Despite the physiological importance and the biophysical characterization of this shift in exocytic mode, the molecular mechanism responsible for the transition remains virtually unknown. Only limited biochemical description of both kiss and run or full collapse exocytosis has been performed (Artalejo et al., 2002; Graham et al., 2002; Ryan, 2003). This is mainly due to the fact that the shift in exocytic mode has been achieved through precise single cell voltage stimulation provided by voltage clamp techniques. However, the electrophysiological approach does not supply adequate biological material for biochemical or proteomic analysis. Likewise, most studies utilizing chemical stimulation overwhelm the cells with high concentrations of secretagogue, making the study of differential activity-mediated shift in exocytic mode difficult. It is the purpose of this study to quantitatively match extracellular bath potassium stimulation to the specific levels of electrical activity in chromaffin cells under either sympathetic tone or under the acute stress response. This will allow for studies in which bulk bath stimulation of cells will provide adequate sample size for biochemical analysis (i.e. differential display analysis of phospho-proteins with the goal of identifying the PKC-mediated shift in fusion pore dilation). We employ FURA-based calcium measurements and carbon fiber amperometric measurements of the catecholamine release process to quantitatively match concentrations of depolarizing extracellular potassium to 0.5 Hz and 15 Hz electrical stimulation to mimic sympathetic tone and acute stress, respectively. We report that an extracellular concentration of 10 mM K+ results in identical cytosolic Ca2+ levels and catecholamine release kinetics to 0.5 Hz action potential stimulation while 30 mM external K+ matches 15 Hz stimulation in either parameter.
Materials and Methods
Cell preparation
Chromaffin cells were isolated from the adrenal medullae of adult C57/BL6 mice (Jackson Laboratories, Bar Harbor ME) and cultured as described previously (Fulop et al., 2005) and plated at a concentration of 4.4×103 mm-2 (or approximately 41,000 cells per 25 mm diameter cover slip). All anesthesia and euthanasia protocols were reviewed and approved by the institutional animal care and use committee (IACUC) of Case Western Reserve University, an accredited oversight body (federal animal welfare assurance # A3145-01). The cells were super-fused in a Ringer solution of the following composition (in mM); 150 NaCl, 10 HEPES-H, 10 Glucose, 2.8 CaCl2, 2.8 KCl and 2 MgCl2. Final pH was adjusted to 7.2 and osmolarity was adjusted to 320 mOsm with mannitol. For elevated potassium stimulation, KCl was increased to either 10 mM or 30 mM. In these Ringers NaCl was decreased to maintain osmolarity at 320 mOsm. Only one recording was made per culture dish to avoid secretory fatigue. To protect against cell preparation-based data bias, both electrical and chemical stimulations were mixed such that experiments for several data sets were run on different cell preparation. All experiments were performed at room temperature. Reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Electrophysiology and electrochemistry
Electrophysiological recordings were performed in the perforated-patch configuration as previously described (Fulop et al., 2005). Briefly, cells were held at a -80 mV potential and stimulated with action potential-equivalent waveforms (APe) at 0.5 Hz or 15 Hz. All electrical stimulation protocols were delivered through an EPC-9 amplifier (HEKA Elektronik, Lambrecht Germany) under the control of Pulse (HEKA Elektronik, v. 8.4). Internal pipette solution was of the following composition (in mM); 135 CsGlutamate, 10 HEPES-H, 9.5 NaCl, 0.5 TEA-Cl and 0.53 amphotericin B.
Amperometric recordings were performed as previously described (Fulop et al., 2005). Carbon fiber electrodes with a diameter of 5 μm were obtained commercially (ALA Scientific, Westbury NY). For the amperometric measurements the carbon fiber was held at +650 mV and placed in close proximity to the cell membrane. The fiber was re-cut before each recording was made. Amperometric currents were recorded using a dedicated amplifier (VA-10x, ALA Scientific) with a head stage modified with a 1 GΩ feedback resistor to minimize noise. The signal was filtered through a 4-pole analog Bessel filter at a cut-off frequency of 1.3 kHz and sampled at 20 kHz through an ITC-1600 (Instrutech, Port Washington, NY) into IGOR PRO (WaveMetrics Inc., Lake Oswego, OR). Individual spike analysis and data pooling was performed in IGOR Pro using a macro modified from the original ‘Spike’ package (Gomez et al., 2002). The non-parametric Mann-Whitney statistical analysis was performed using Minitab (v 15; Minitab, Inc. State College, PA).
Calcium measurements
Cytosolic calcium measurements were performed in cells pre-loaded with FURA 2-AM (TefLabs, Austin TX). FURA 2-AM stock was prepared fresh daily in DMSO at 1 mM. Cells were pre-loaded by incubation in normal Ringer containing 2 μM FURA2-AM for at least 15 minutes at 37 °C. Resting and activity evoked Ca2+ influx were measured by excitation at 360 and 390 nm light. Emission intensity was measured through a photomultiplier tube (ViewFinder, TillPhotonics, Pleasanton CA). A variable aperture window was set to collect light from the cell only; other cells in the visual field as well as the patch pipette were excluded. Following the experimental protocol, the perforated patch was ruptured causing the intracellular FURA-2 to dialyze out of the cell, allowing for the measurement of the auto fluorescence of the cell. The auto fluorescence values at 360 and 390 nm wavelength excitation were subtracted from values measured during the experiment. Excitation illumination as well as acquisition of emission intensity were both controlled by Pulse (HEKA) and are reported as the ratio of emission intensity at F360 / F390 over the final 50% of the stimulus train. While no absolute estimate for the amount of FURA-2 loaded into each cell was made, it was noted that after breaking into the whole-cell configuration to allow FURA to dialyze out, cell fluorescence dropped only by 50% (FURA fluorescence was only as bright as auto-fluorescence) implying that the FURA-AM loading protocol used in these experiments resulted in very low cytosolic FURA concentrations. These concentrations of FURA would be very unlikely to significantly alter the cytosolic Ca2+-buffering and the time course of post-stimulus Ca2+ clearance.
Results
Chromaffin cells shift the mode of granule exocytosis from ‘kiss and run’ to ‘full collapse’ in an activity-dependent manner. This shift in fusion mode underlies a vital, activity-dependent, differential transmitter release and forms the basis for the sympathetic acute stress response. However, biochemical analysis of this shift remains elusive due to the limited availability of cells in the conventional single-cell voltage clamp stimulus paradigm used to detect the shift in exocytic mode. It is the overall goal of this study to develop an alternate experimental paradigm to provide adequate amounts of like-stimulated cells for biochemical and proteomic analysis. Increased populations of cells uniformly exposed to graded stimulation will provide adequate sample size for the further molecular study of the shift in exocytic mode under basal sympathetic tone versus the acute sympathetic stress response.
Fura recordings match cytosolic Ca2+ under external K+ stimulation to electrical APe stimulation
Upon activity-evoked elevations in Ca2+, only a small subset of secretory granules are competent to undergo fusion and release their transmitters. These granules are said to be docked and primed and represent the ‘readily-releasable pool’ (RRP) of granules. Under normal physiological conditions these RRP granules number in the low 100's (Smith et al., 1998) out of a total cellular complement of 10,000 – 20,000 granules. Calcium acts in a localized sub-plasmalemmal fashion to trigger the rapid fusion of these RRP granules in a cooperative manner, requiring the binding of at least three Ca2+ molecules per granule. However, under tonic stimulation, the RRP is diminished and exocytosis is sustained by recruitment of reserve granules through the RRP to fusion. The recruitment step is dependent upon global cytosolic Ca2+, exhibits a cooperativity of 1 and is slow compared to the fusion step (Smith et al., 1998). For these reasons, granule recruitment to the RRP and not fusion from the RRP represents the rate limiting step under sustained calcium elevations (Ashery et al., 2000; Smith, 1999; Voets et al., 1999; Xu et al., 1998). Thus, while the localized sub-plasmalemmal Ca2+ concentration is important for setting the rate of granule fusion under acute stimuli, the global cytosolic Ca2+ is more important for granule fusion under sustained exocytosis such as under physiological sympathetic tone or sympathetic activation.
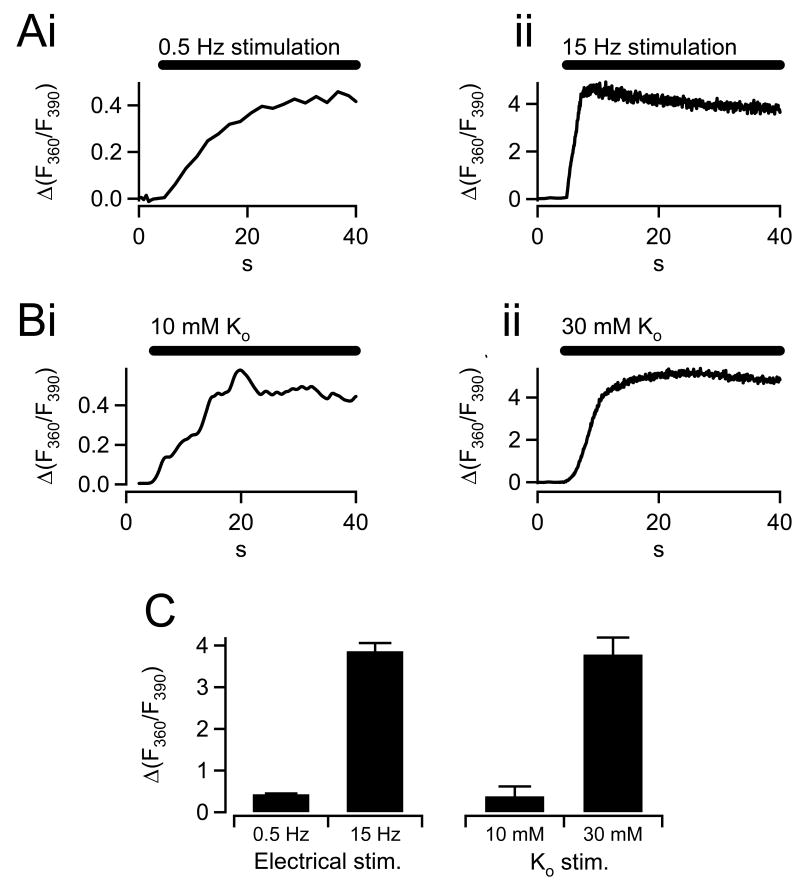
We set out to measure cytosolic Ca2+ under two electrical stimulus paradigms; action potential equivalent waveforms (APe) were delivered at 0.5 Hz to mimic sympathetic tone and 15 Hz to match the sympathetic stress response. Representative single cell fura recordings are presented in figure 1A. As expected, the low frequency stimulus (Fig. 1Ai) led to a Ca2+ accumulation that was both slower and smaller than that under high frequency stimulation (Fig. 1Aii). Next we assayed calcium accumulation under a variety of Ringers prepared to include elevated potassium. We found that stimulating cells with 10 mM external potassium concentration resulted in fura records that consistently matched those recorded under 0.5 Hz APe electrical stimulation (Fig. 1Bi). Likewise we also found that 30 mM external potassium resulted in fura records that best matched 15 Hz stimulation (Fig. 1 Bii). Pooled data from all records under 0.5 and 15 Hz APe stimulation as well as 10 and 30 mM K+ stimulation are provided in panel C and show that the 0.5 Hz, 10 mM paired data are statistically identical as are the 15 Hz and 30 mM K+ data.
Figure 1.
Graded cytosolic Ca2+ elevations elicited by action potential and potassium stimulation. Isolated chromaffin cells were loaded with the cell permeant AM ester of FURA. A) Cells were patch clamped and held in the perforated voltage-clamp configuration and stimulated with either 0.5 Hz (i) or 15 Hz (ii) trains of action potential waveforms to simulate basal sympathetic tone versus acute sympathetic stress activation. Data are plotted as the -fold increase in the fluorescence ratio over pre-stimulus values and represent activity-mediated relative increases in cytosolic Ca2+. B) Isolated cells were exposed to bath stimulation by perfusion with osmotically-balanced Ringer solutions containing elevated potassium. Representative FURA traces from single cells subjected to 10 and 30 mM external potassium are plotted in panels i and ii. The slower onset of the FURA signal in the potassium condition is likely due to temporal limitations of diffusion-delimited perfusion-based stimulation versus voltage-clamp electrical stimulation. C) Average steady state values for the ΔFURA360/FURA390 signal were measured under 0.5 Hz and 15 Hz electrical stimulation (n=10 and 12 cells, respectively) as well as 10 mM and 30 mM (n=8 and 9 cells, respectively), pooled and are co-plotted for comparison. FURA signals measured under 0.5 Hz and 10 mM potassium stimulation sets as well as under 15 Hz and 30 mM potassium stimulation sets were statistically identical (paired student's T-test, significance denoted by ‘*’ at a p < 0.01).
Amperometric analysis of catecholamine release under paired electrical and K+ stimulation
Catecholamine release from chromaffin cells can be measured with high resolution by electrochemical carbon fiber amperometry (Chow et al., 1992; Wightman et al., 1991). Briefly, this technique takes advantage of the fact that catecholamines readily oxidize in a positive electrical field, releasing 2 electrons per reaction. By placing a carbon fiber next to a cell and clamping the fiber's potential to +650 mV, catecholamines can be detected as an electrical current. The technique is capable of resolving not only quantal catecholamine release but also the kinetics of the initial opening of the fusion pore early in the secretion process (Albillos et al., 1997; Alvarez de Toledo et al., 1993; Fulop and Smith, 2006). For this reason we turned to amperometry to quantify the kinetics of catecholamine release from granules evoked to undergo fusion by either 0.5 Hz or 15 Hz APe stimulation. Results from these experiments are provided in figure 2.
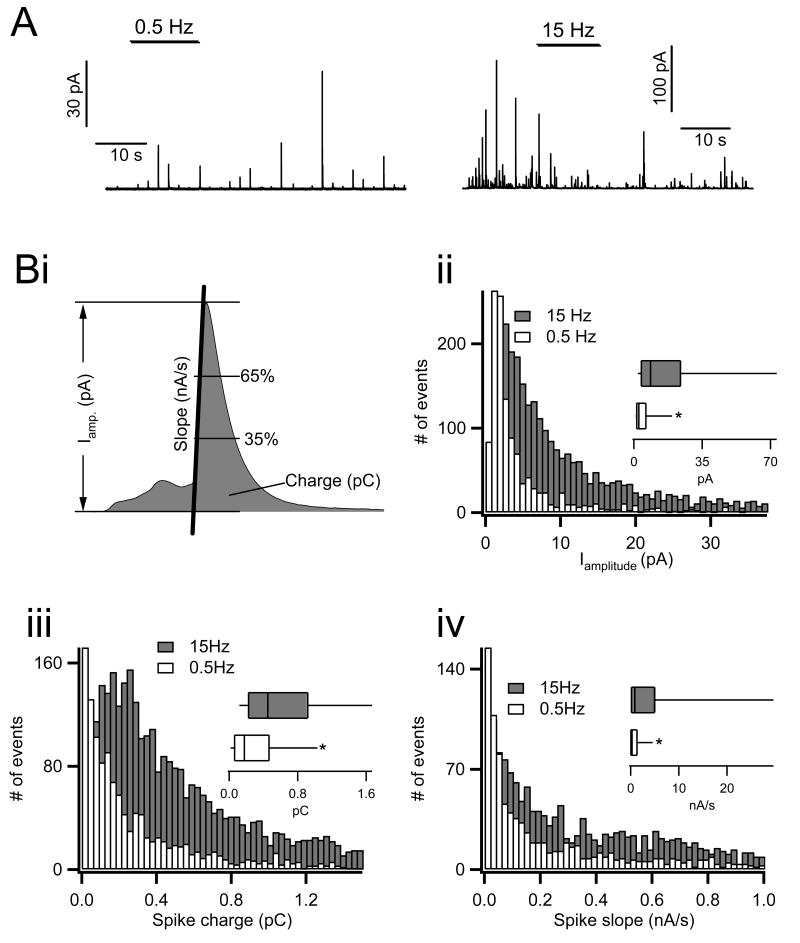
Figure 2.
Amperometric analysis of catecholamine release under 0.5 versus 15 Hz electrical stimulation. Chromaffin cells were held in the perforated patch configuration, held at -80 mV command potential and stimulated with action potential equivalent voltage waveforms. A) Catecholamine release was measured by single cell carbon fiber amperometry. Representative traces from cells stimulated under each condition are provided (note the difference in scale). B) Single amperometric spikes were identified first by an automated detection algorithm and then by eye. Three kinetic parameters were measured for each secretory event. i. These included total spike amplitude (in pA), charge (pC, an index of the total amount of catecholamine released) and initial spike slope (nA/s, the rate of catecholamine release measured between 35% and 65% of total spike height, a parameter proportional to the fusion pore diameter). ii-iv. Histograms were generated from the raw data to show population distributions of each parameter and are presented. Insets in each panel show box-and-whisker plots for each data set. Parameters for the box-and-whisker plots were as follows; smallest non-outlier observation, first quartile, median, third quartile and largest non-outlier observation. Statistical significance for each parameter was determined by a Mann-Whitney non-parametric median analysis and asterisks in each box-and-whisker plot indicate statistical significance (P < 0.001, n=14 cells for each condition).
Representative raw amperometric recordings from single cells stimulated with each electrical stimulation paradigm are shown in panel A. Consistent with previous studies (Fulop et al., 2005), the current spikes recorded from cells under 0.5 Hz stimulation are significantly smaller that those measured under 15 Hz. Data recorded from multiple cells were pooled and analyzed for spike magnitude, spike charge (an index of total catecholamine content) and initial spike slope (an index of the rate of catecholamine release, a parameter dependent to fusion pore dilation) (Lindau and Alvarez de Toledo, 2003). Figure 2 (panel Bi) provides a graphical representation of each of these parameters. Spikes often exhibited a ‘foot current’, which is generally accepted to be due to the initial opening of the fusion pore to provide a limited escape route for catecholamine release. The foot is then followed by a larger spike event whose rate of rise is proportional to the size of the exocytic fusion pore (Chow et al., 1992). An initial analysis of spike amplitude, charge and initial slope showed that they did not follow a normal, but rather a skewed distribution. These distributions are provided in panel B of figure 2. In all 3 cases 0.5 Hz APe stimulation resulted in smaller and slower amperometric spikes. Insets in each sub panel show box-and-whisker plots of each distribution. Non parametric Mann-Whitney median tests indicate that all 3 parameters, spike amplitude, spike charge and initial slope, are significantly smaller under 0.5 Hz versus 15 Hz APe stimulation (asterisks in panel B indicate statistical significance with p < 0.001 in all cases).
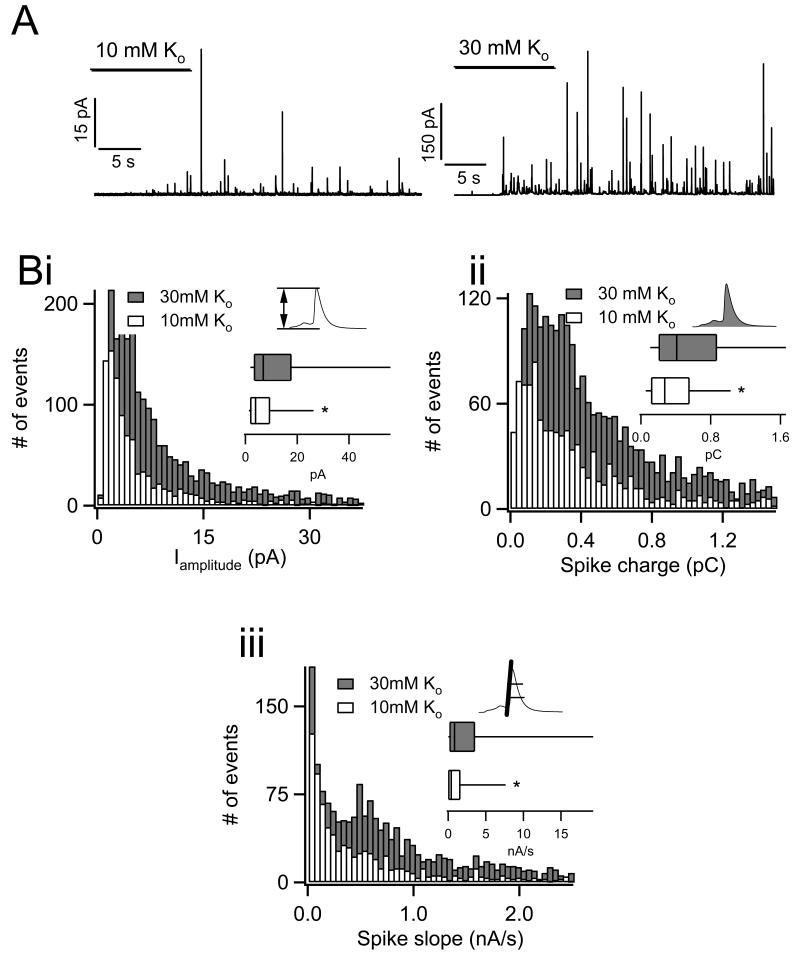
Next we repeated the same experimentation and analysis in chromaffin cells that were stimulated by bath application of Ringers containing 10 mM and 30 mM [K+]o. As in figure 2, raw amperometric records from each stimulus paradigm are provided in panel A. Again, lighter stimulation (10 mM [K+]o) resulted in smaller amplitude current spikes. Spike amplitude, charge and initial slope pooled from multiple cells again showed a skewed distribution as under APe stimulation (Fig. 3 B i, ii and iii). Likewise, box-and-whisker plots and non-parametric Mann-Whitney median tests demonstrate that all 3 parameters are significantly smaller under 10 mM than under 30 mM [K+]o stimulation (asterisks indicate significance with P < 0.001 in all cases).
Figure 3.
Amperometric analysis of catecholamine release under potassium stimulation. Isolated chromaffin cells were perfused with osmotically balanced Ringer solutions with 10 mM and 30 mM potassium. A) Catecholamine release was determined by carbon fiber amperometry. Representative raw records for each condition are provided for reference (note the difference in scale). B) Each Population histograms and box-and-whisker plots for amplitude (i), event charge (ii) and spike slope (iii) are provided (P < 0.001; n=14 cells for each condition). Icons indicate the source of each parameter in the context of spike morphology.
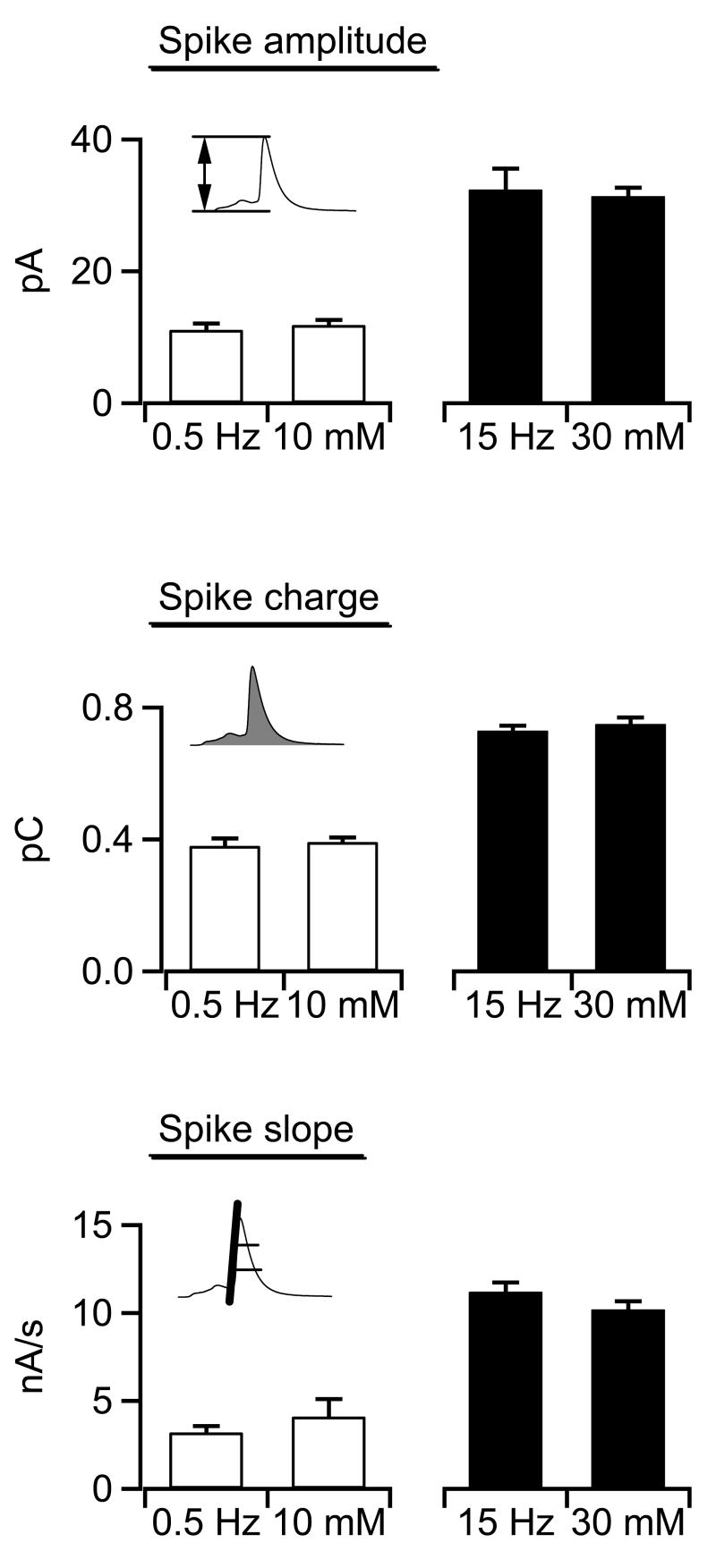
Amperometric spike parameters have been provided in many different formats in the literature. Most studies (including from our group) provide simple mean-analysis of spike parameters and base statistical significance on a standard Student's T test or ANOVA analysis. In order to frame the data presented in this study into the context of the literature, we provide the means of three spike parameters in figure 4. Again, spike amplitude, spike charge and slope are statistically identical between 0.5 Hz APe and 10 mM [K+]o stimulation as well as between 15 Hz APe and 30 mM [K+]o stimulation. Thus, by either median or more conventional mean-based statistical analysis, catecholamine secretion kinetics evoked by 10 mM K+ stimulation quantitatively match those evoked by 0.5 Hz electrical APe stimulation and those under 30 mM K+ match the 15 Hz APe condition.
Figure 4.
Summary of amperometric data analysis. For comparison, data collected under each stimulation paradigm (0.5 Hz and 15 Hz electrical stimulation and 10 and 30 mM external potassium) were pooled and are presented. Data are paired according to stimulation strength and icons indicate the parameter measured with respect to spike morphology. Mean spike amplitude (top plot), mean spike charge (middle plot) and mean spike slope (bottom plot) for events evoked under 0.5 Hz action potential stimulation and those evoked by 10 mM potassium stimulation were statistically identical (Student's paired T-test; p<0.01). Likewise, spike parameters measured under 15 Hz and 30 mM potassium stimulation were identical (n values for each condition are as in previous figures).
Discussion
Chromaffin cells represent an important output of the sympathetic nervous system. They contribute serum catecholamine under basal stimulation experience under sympathetic tone that acts to balance parasympathetic input and set the overall metabolic balance of the organism into the energy storing ‘breed and feed’ status. Systemic characteristics of the ‘breed and feed’ status that fall under the control of the balance between sympathetic catecholamine release and parasympathetic acetylcholine levels include shunting of blood from the peripheral skeletal muscle to the viscera and increased insulin release. Under elevated sympathetic input during acute stress, chromaffin cells release catecholamines at a higher rate and also release a host of neuro- and vaso-active peptides into the circulation that act in concert to shift the organism from a status of energy storage to that of energy expenditure under the ‘fight or flight’ response. Consequences of the increased transmitter release include diversion of blood flow from the internal organs to the peripheral skeletal muscle and increased glucagon release. Proper regulation of the exocytic properties of chromaffin cells represents an important control point for setting the overall energy status of the organism. Improper control of this process is linked to multiple patho-physiological states including depression, diabetes and hypertension. Thus, a fundamental understanding of the exocytic process in chromaffin cells represents a fundamental point for the control of these disease states.
Studies to this point have shown that chromaffin cells regulate the release of catecholamine and neuro- and vaso-active peptides by a simple mechanism. Under sympathetic tone, modest catecholamine release is achieved through a restricted exocytic fusion pore between secretory granule and cell exterior, allowing for the selective and partial release of the small highly-soluble catecholamines while retaining the less soluble peptide transmitters within the granule's dense proteinacious core. Likewise, global release of catecholamines as well as peptide transmitters is achieved under elevated cell activity by a Ca2+mediated PKC-dependent dilation of the fusion pore to allow a more rapid and complete expulsion of all granule contents (Fulop et al., 2005). Understanding the regulation of the activity-dependent dilation of the granule fusion pore is central to an understanding of the shift between ‘breed and feed’ and ‘fight or flight’ metabolic states.
Despite the obvious importance of this molecular reaction, progress in its description has been largely blocked by the lack of access to biochemical and proteomic analysis. This is mainly due to the fact that the precise stimulation of cells required to differentially activate one mode of exocytosis over the other has been accomplished by single cell patch clamp techniques. This limitation defines the overall goal of this study: to develop an equally precise method for stimulating many cells at once to differentially evoke one mode of exocytosis over the other and to do so at a scale sufficient to provide adequate biological material for standard biochemical and proteomic techniques. Bulk stimulation of a dish of cells will provide approximately 40,000 like-stimulated cells as compared to a single cell stimulated via a patch pipette. To this achieve this scale, we considered several possible approaches. We first considered bath electrical field stimulation. However, the spherical morphology and subsequent isopotential membrane of the cells makes action potential stimulation very difficult and complex (Hassan et al., 2002, 2003). Next we considered chemical stimulation with the native secretagogue, nicotine. However, while nicotine is efficient at stimulating cells, it is not likely to elicit a tightly controlled stimulation of all cells. Rather, it will depend on the density of each cells nicotinic acetylcholine receptors. Due to these considerations, we decided to take a much more direct approach: utilizing elevated extracellular potassium to depolarize the membrane potential of the cell, thereby opening voltage-gated calcium channels. This approach is not dependent upon expression of receptors, but acts directly on the fundamental electrical properties of the cell. Moreover, by varying the concentration of potassium, we are able to provide a graded stimulation of the cell. Small elevations in potassium raise the membrane potential and increase to mean open probability of calcium channels a small amount, while greater potassium concentrations open more calcium channels.
We present relative measures of cytosolic calcium under all stimulation paradigms as one of the parameters to calibrate external potassium concentrations to frequencies of APe stimulation. On first pass it might seem that the relevant Ca2+ concentration would be the transient, sub-plasmalemmal calcium elevations, since that is where the binding reaction that causes granule fusion with the cell membrane occurs. This is indeed true if only acute and short term secretion from the readily-releasable pool is being considered. However, the stimulus paradigms we employ are meant to reflect longer term, native input from the splanchnic nerve. Indeed the splanchnic nerve paces chromaffin cells at a constant rate set by the sympathetic tone (Brandt et al., 1976; Kidokoro and Ritchie, 1980; Wallace et al., 2002). Under these conditions, the rate limiting step for secretion is not the Ca2+ binding at the final fusion step, rather it is the Ca2+-mediated recruitment and priming of the granules to the releasable state (Ashery et al., 2000; Smith, 1999; Voets et al., 1999; Xu et al., 1998).
Our primary assay for matching single cell patch clamp APe stimulation to global bath stimulation with elevated potassium relies on the spike kinetics of individual amperometric currents. This technique has been used for over a decade to study the fusion of single, as well as multiple granules, from chromaffin cells with exquisite resolution (Albillos et al., 1997; Chow et al., 1994; Chow et al., 1992; Segura et al., 2000; Wightman et al., 1995; Wightman et al., 1991). Here we utilize spike amplitude, charge and slope parameters as metrics to compare stimulation intensities. Each parameter is measured in response to both chemical and electrical stimulation to calibrate potassium exposure to specific electrical stimuli designed to mimic native electrical stimulation. We report these parameters as population histograms and show that each data set exhibits a skewed distribution. Thus we determined the significance of each value by a median, rather than mean, analysis. We find that 10 and 30 mM external potassium stimulation elicits catecholamine release that quantitatively matches release evoked by electrical stimulation designed to mimic sympathetic input under ‘breed and feed’ and ‘fight or flight’ conditions, respectively. It is anticipated that these data may lead to the future proteomic and biochemical analysis of the molecular mechanisms responsible for differential fusion pore dilation and thus differential transmitter release from adrenal chromaffin cells.
Acknowledgments
We would like to thank Dr. Shyue-An Chan and Bryan Doreian for helpful comments during the preparation of this manuscript. This work was supported by grants from the NSF (IBN-0344768) and NIH (1R01NS052123) to CS and (T32 HL 07653) for support of TF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–12. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- Alvarez de Toledo G, Fernández-Chacón R, Fernández JM. Release of secretory products during transient vesicle fusion. Nature. 1993;363:554–8. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- Artalejo CR, Elhamdani A, Palfrey HC. Sustained stimulation shifts the mechanism of endocytosis from dynamin-1-dependent rapid endocytosis to clathrin- and dynamin-2-mediated slow endocytosis in chromaffin cells. Proc Natl Acad Sci U S A. 2002;99(9):6358–63. doi: 10.1073/pnas.082658499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo CR, Henley JR, McNiven MA, Palfrey HC. Rapid endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP, and dynamin but not clathrin. Proc Natl Acad Sci USA. 1995;92:8328–32. doi: 10.1073/pnas.92.18.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashery U, Varoqueaux F, Voets T, Betz A, Thakur P, Koch H, Neher E, Brose N, Rettig J. Munc13-1 acts as a priming factor for large dense-core vesicles in bovine chromaffin cells. Embo J. 2000;19(14):3586–96. doi: 10.1093/emboj/19.14.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aunis D. Exocytosis in chromaffin cells of the adrenal medulla. International Review of Cytology. 1998;181:213–320. doi: 10.1016/s0074-7696(08)60419-2. [DOI] [PubMed] [Google Scholar]
- Brandt B, Hagiwara S, Kidokoro Y, Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. J Physiol. 1976;263:417–39. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SA, Chow R, Smith C. Calcium dependence of action potential-induced endocytosis in chromaffin cells. Pflugers Arch. 2003;445(5):540–6. doi: 10.1007/s00424-002-0966-y. [DOI] [PubMed] [Google Scholar]
- Chan SA, Smith C. Low frequency stimulation of mouse adrenal slices reveals a clathrin-independent, protein kinase C-mediated endocytic mechanism. J Physiology (London) 2003;553(3):707–17. doi: 10.1113/jphysiol.2003.053918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RH, Klingauf J, Neher E. Time course of Ca2+ concentration triggering exocytosis in neuroendocrine cells. Proc Natl Acad Sci USA. 1994;91:12765–9. doi: 10.1073/pnas.91.26.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RH, von Ruden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356(6364):60–3. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Elhamdani A, Palfrey HC, Artalejo CR. Quantal size is dependent on stimulation frequency and calcium entry in calf chromaffin cells. Neuron. 2001;31(5):819–30. doi: 10.1016/s0896-6273(01)00418-4. [DOI] [PubMed] [Google Scholar]
- Fulop T, Radabaugh S, Smith C. Activity-dependent differential transmitter release in mouse adrenal chromaffin cells. J Neurosci. 2005;25(32):7324–32. doi: 10.1523/JNEUROSCI.2042-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Smith C. Physiological stimulation regulates the exocytic mode through calcium activation of protein kinase C in mouse chromaffin cells. Biochem J. 2006;399(1):111–9. doi: 10.1042/BJ20060654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JF, Brioso MA, Machado JD, Sanchez JL, Borges R. New approaches for analysis of amperometrical recordings. Ann N Y Acad Sci. 2002;971:647–54. doi: 10.1111/j.1749-6632.2002.tb04544.x. [DOI] [PubMed] [Google Scholar]
- Graham ME, O'Callaghan DW, McMahon HT, Burgoyne RD. Dynamin-dependent and dynamin-independent processes contribute to the regulation of single vesicle release kinetics and quantal size. Proc Natl Acad Sci U S A. 2002;99(10):7124–9. doi: 10.1073/pnas.102645099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan N, Chatterjee I, Publicover NG, Craviso GL. Mapping membrane-potential perturbations of chromaffin cells exposed to electric fields. IEEE Transactions on Plasma Sceince. 2002;30(4):1516–24. [Google Scholar]
- Hassan N, Chatterjee I, Publicover NG, Craviso GL. Numerical study of induced current perturbations in the vicinity of excitable cells exposed to extremely low frequency magnetic fields. Phys Med Biol. 2003;48(20):3277–93. doi: 10.1088/0031-9155/48/20/002. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y, Ritchie AK. Chromaffin cell action potentials and their possible role in adrenaline secretion from rat adrenal medulla. J Physiol. 1980;307:199–216. doi: 10.1113/jphysiol.1980.sp013431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M, Alvarez de Toledo G. The fusion pore. Biochim Biophys Acta. 2003;1641(23):167–73. doi: 10.1016/s0167-4889(03)00085-5. [DOI] [PubMed] [Google Scholar]
- Perrais D, Kleppe I, Taraska J, Almers W. Recapture after exocytosis causes differential retention of protein in granules of bovine chromaffin cells. J Physiol. 2004 doi: 10.1113/jphysiol.2004.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TA. Kiss-and-run, fuse-pinch-and-linger, fuse-and-collapse: the life and times of a neurosecretory granule. Proc Natl Acad Sci U S A. 2003;100(5):2171–3. doi: 10.1073/pnas.0530260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura F, Brioso MA, Gomez JF, Machado JD, Borges R. Automatic analysis for amperometrical recordings of exocytosis. J Neurosci Methods. 2000;103(2):151–6. doi: 10.1016/s0165-0270(00)00309-5. [DOI] [PubMed] [Google Scholar]
- Smith C. A persistent activity-dependent facilitation in chromaffin cells is caused by Ca2+ activation of protein kinase C. J Neurosci. 1999;19(2):589–98. doi: 10.1523/JNEUROSCI.19-02-00589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Moser T, Xu T, Neher E. Cytosolic Ca2+ acts by two separate pathways to modulate the supply of release-competent vesicles in chromaffin cells. Neuron. 1998;20(6):1243–53. doi: 10.1016/s0896-6273(00)80504-8. [DOI] [PubMed] [Google Scholar]
- Voets T, Neher E, Moser T. Mechanisms underlying phasic and sustained secretion in chromaffin cells from mouse adrenal slices. Neuron. 1999;23(3):607–15. doi: 10.1016/s0896-6273(00)80812-0. [DOI] [PubMed] [Google Scholar]
- Wallace DJ, Chen C, Marley PD. Histamine promotes excitability in bovine adrenal chromaffin cells by inhibiting an M-current. J Physiol. 2002;540(Pt 3):921–39. doi: 10.1113/jphysiol.2001.013370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Finnegan JM, Pihel K. Monitoring catecholamines at single cells. Trends Analyt Chem. 1995;14:154–8. [Google Scholar]
- Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Diliberto EJ, Viveros OH. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci USA. 1991;88:10754–8. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. The adrenal chromaffin granule: a model for large dense core vesicles of endocrine and nervous tissue. J Anat. 1993;183:237–52. [PMC free article] [PubMed] [Google Scholar]
- Xu T, Binz T, Niemann H, Neher E. Multiple kinetic components of exocytosis distinguished by neurotoxin sensitivity. Nature Neuroscience. 1998;1(3):192–200. doi: 10.1038/642. [DOI] [PubMed] [Google Scholar]