Abstract
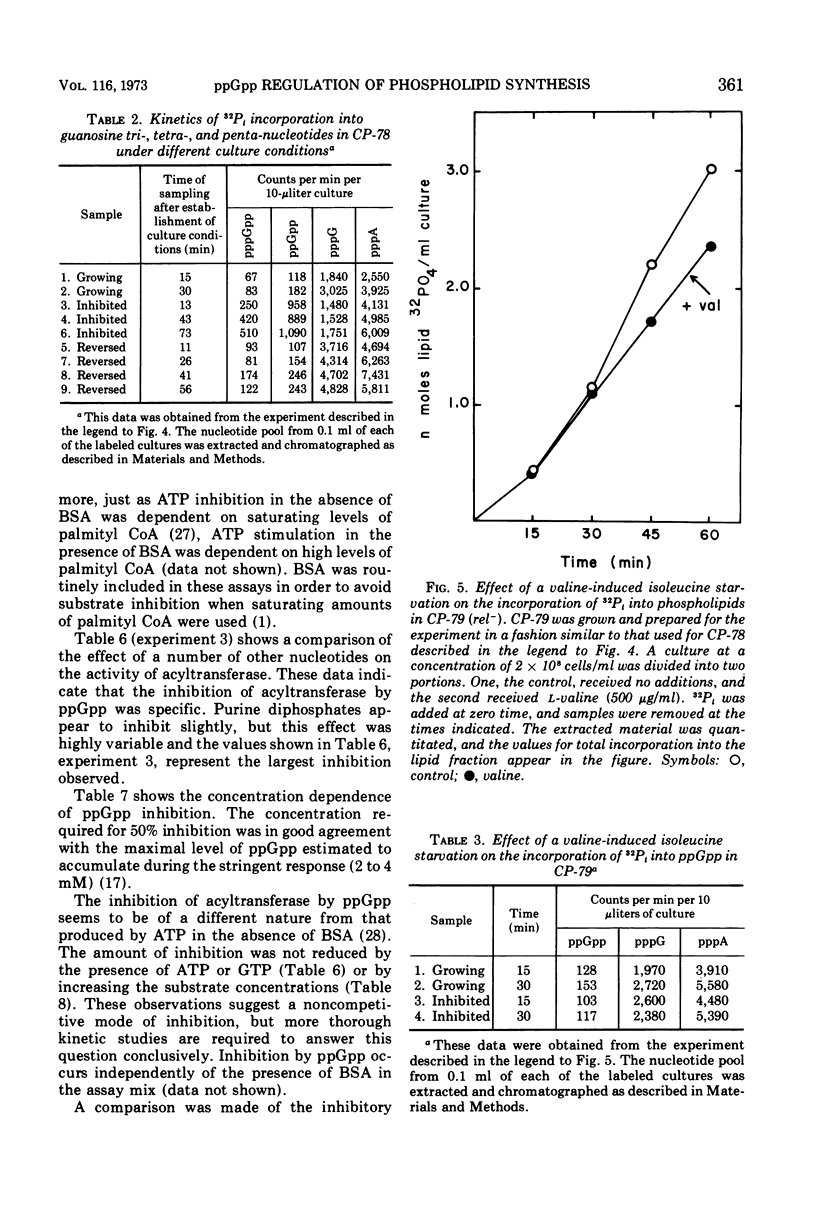
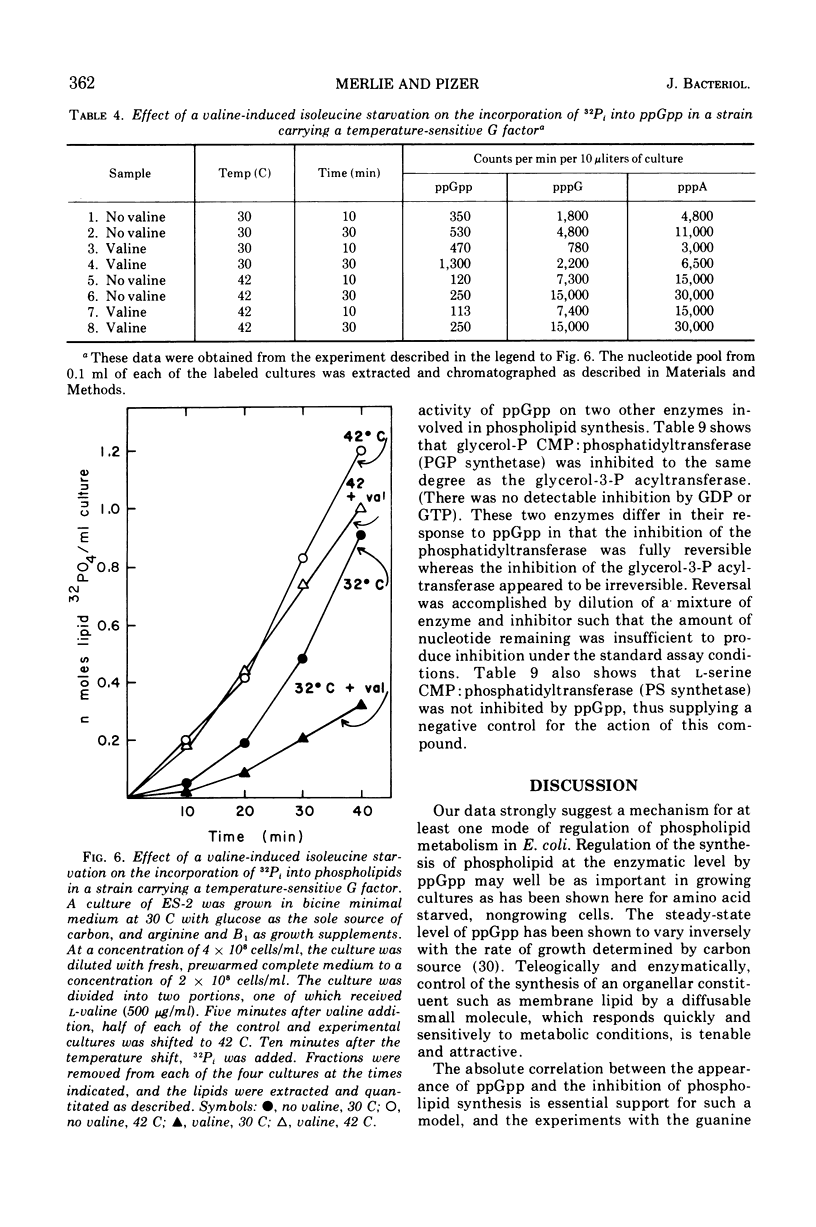
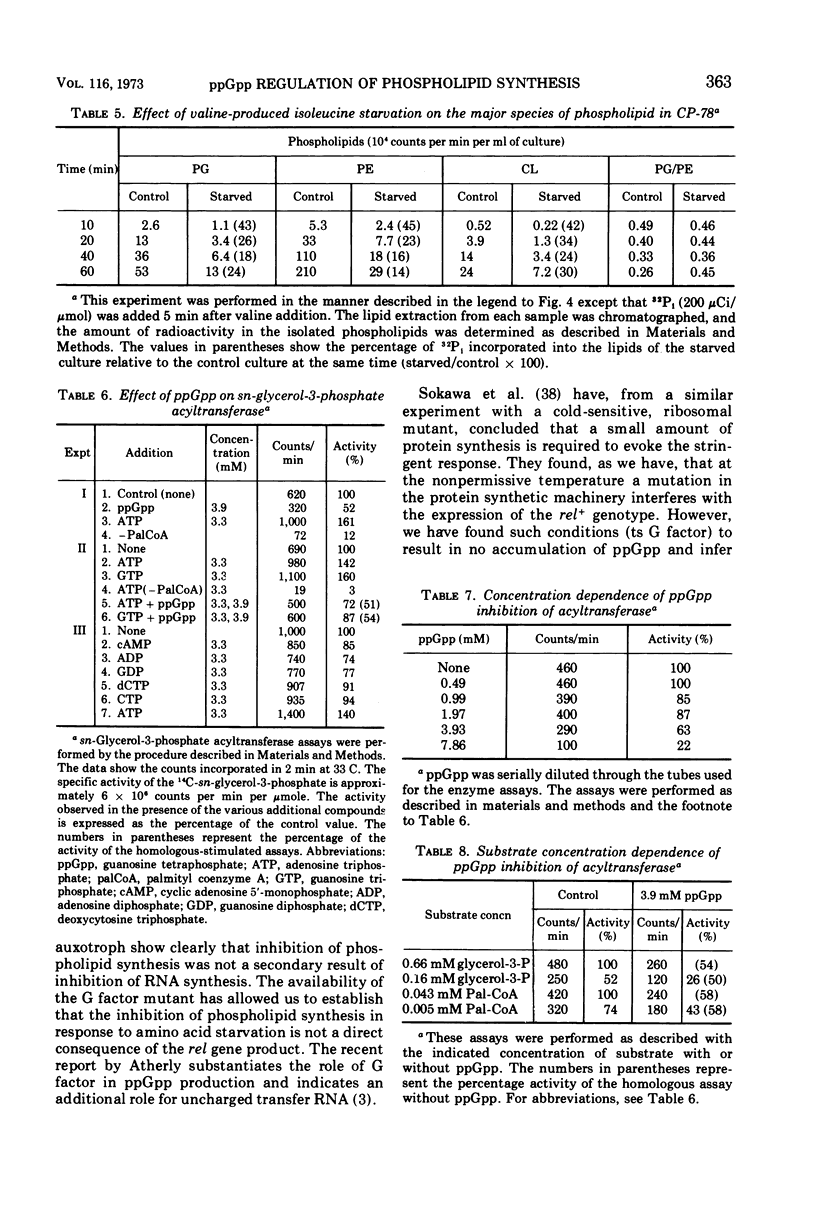
Phospholipid synthesis has been reported to be subject to stringent control in Escherichia coli. We present evidence that demonstrates a strict correlation between guanosine tetraphosphate accumulation and inhibition of phospholipid synthesis. In vivo experiments designed to examine the pattern of phospholipid labeling with 32P-inorganic phosphate and 32P-sn-glycerol-3-phosphate suggest that regulation must occur at the glycerol-3-phosphate acyltransferase step. Assay of phospholipid synthesis by cell-free extracts and semipurified preparations revealed that guanosine tetraphosphate inhibits at least two enzymes specific for the biosynthetic pathway, sn-glycerol-3-phosphate acyltransferase as well as sn-glycerol-3-phosphate phosphatidyl transferase. These findings provide a biochemical basis for the stringent control of lipid synthesis as well as regulation of steady-state levels of phospholipid in growing cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ailhaud G. P., Vagelos P. R. Palmityl-acyl carrier protein as acyl donor for complex lipid biosynthesis in Escherichia coli. J Biol Chem. 1966 Aug 25;241(16):3866–3869. [PubMed] [Google Scholar]
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherly A. G. Temperature-sensitive relaxed Phenotype in a stringent strain of Escherichia coli. J Bacteriol. 1973 Jan;113(1):178–182. doi: 10.1128/jb.113.1.178-182.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Ballesta J. P., Schaechter M. Dependence of the rate of synthesis of phosphatidylethanolamine and phosphatidylglycerol on the rate of growth of Escherichia coli. J Bacteriol. 1972 Apr;110(1):452–453. doi: 10.1128/jb.110.1.452-453.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Cashel M., Kalbacher B. The control of ribonucleic acid synthesis in Escherichia coli. V. Characterization of a nucleotide associated with the stringent response. J Biol Chem. 1970 May 10;245(9):2309–2318. [PubMed] [Google Scholar]
- Cashel M., Lazzarini R. A., Kalbacher B. An improved method for thin-layer chromatography of nucleotide mixtures containing 32P-labelled orthophosphate. J Chromatogr. 1969 Mar 11;40(1):103–109. doi: 10.1016/s0021-9673(01)96624-5. [DOI] [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Chang Y. Y., Kennedy E. P. Biosynthesis of phosphatidyl glycerophosphate in Escherichia coli. J Lipid Res. 1967 Sep;8(5):447–455. [PubMed] [Google Scholar]
- Cronan J. E., Jr, Ray T. K., Vagelos P. R. Selection and characterization of an E. coli mutant defective in membrane lipid biosynthesis. Proc Natl Acad Sci U S A. 1970 Mar;65(3):737–744. doi: 10.1073/pnas.65.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donini P. Amino acid control over deoxyribonucleic acid synthesis in Escherichia coli infected with T-even bacteriophage. J Bacteriol. 1970 Jun;102(3):616–627. doi: 10.1128/jb.102.3.616-627.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin G., Donini P. Synthesis of guanosine 5'-diphosphate, 2'-(or 3'-) diphosphate and related nucleotides in a variety of physiological conditions. J Biol Chem. 1971 Jul 10;246(13):4371–4373. [PubMed] [Google Scholar]
- Gallant J., Harada B. The control of ribonucleic acid synthesis in Escherichia coli. 3. The functional relationship between purine ribonucleoside triphosphate pool sizes and the rate of ribonucleic acid accumulation. J Biol Chem. 1969 Jun 25;244(12):3125–3132. [PubMed] [Google Scholar]
- Gallant J., Irr J., Cashel M. The mechanism of amino acid control of guanylate and adenylate biosynthesis. J Biol Chem. 1971 Sep 25;246(18):5812–5816. [PubMed] [Google Scholar]
- Gallant J., Margason G., Finch B. On the turnover of ppGpp in Escherichia coli. J Biol Chem. 1972 Oct 10;247(19):6055–6058. [PubMed] [Google Scholar]
- Glaser M., Bayer W. H., Bell R. M., Vagelos P. R. Regulation of macromolecular biosynthesis in a mutant of Escherichia coli defective in membrane phospholipid biosynthesis. Proc Natl Acad Sci U S A. 1973 Feb;70(2):385–389. doi: 10.1073/pnas.70.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden N. G., Powell G. L. Stringent and relaxed control of phospholipid metabolism in Escherichia coli. J Biol Chem. 1972 Oct 25;247(20):6651–6658. [PubMed] [Google Scholar]
- Goldfine H. Use of a filter-paper disk assay in the measurement of lipid biosynthesis. J Lipid Res. 1966 Jan;7(1):146–149. [PubMed] [Google Scholar]
- Harshman R. B., Yamazaki H. Formation of ppGpp in a relaxed and stringent strain of Escherichia coli during diauxie lag. Biochemistry. 1971 Oct 12;10(21):3980–3982. doi: 10.1021/bi00797a027. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc Natl Acad Sci U S A. 1973 May;70(5):1564–1568. doi: 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Cashel M. The regulation of purine utilization in bacteria. V. Inhibition of purine phosphoribosyltransferase activities and purine uptake in isolated membrane vesicles by guanosine tetraphosphate. J Biol Chem. 1972 Nov 10;247(21):7067–7072. [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. II. BIOSYNTHESIS OF PHOSPHOLIPIDS IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1720–1726. [PubMed] [Google Scholar]
- Kito M., Lubin M., Pizer L. I. A mutant of Escherichia coli defective in phosphatidic acid synthesis. Biochem Biophys Res Commun. 1969 Feb 21;34(4):454–458. doi: 10.1016/0006-291x(69)90403-3. [DOI] [PubMed] [Google Scholar]
- Kito M., Pizer L. I. Phosphatidic acid synthesis in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1321–1327. doi: 10.1128/jb.97.3.1321-1327.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito M., Pizer L. I. Purification and regulatory properties of the biosynthetic L-glycerol 3-phosphate dehydrogenase from Escherichia coli. J Biol Chem. 1969 Jun 25;244(12):3316–3323. [PubMed] [Google Scholar]
- Lazzarini R. A., Cashel M., Gallant J. On the regulation of guanosine tetraphosphate levels in stringent and relaxed strains of Escherichia coli. J Biol Chem. 1971 Jul 25;246(14):4381–4385. [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- Mavis R. D., Vagelos P. R. The effect of phospholipid fatty acid composition in membranous enzymes in Escherichia coli. J Biol Chem. 1972 Feb 10;247(3):652–659. [PubMed] [Google Scholar]
- Pedersen F. S., Lund E., Kjeldgaard N. O. Codon specific, tRNA dependent in vitro synthesis of ppGpp and pppGpp. Nat New Biol. 1973 May 2;243(122):13–15. [PubMed] [Google Scholar]
- Pizer L. I., Merlie J. P. Effect of serine hydroxamate on phospholipid synthesis in Escherichia coli. J Bacteriol. 1973 Jun;114(3):980–987. doi: 10.1128/jb.114.3.980-987.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani E., Srinivasan P. R. Role of the translocation factor G in the regulation of ribonucleic acid synthesis. J Bacteriol. 1973 Mar;113(3):1177–1183. doi: 10.1128/jb.113.3.1177-1183.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokawa J., Sokawa Y., Kaziro Y. Stringent control in Escherichia coli. Nat New Biol. 1972 Dec 20;240(103):242–245. doi: 10.1038/newbio240242a0. [DOI] [PubMed] [Google Scholar]
- Sokawa Y., Nakao E., Kaziro Y. On the nature of the control by RC gene in e. coli: amino acid-dependent control of lipid synthesis. Biochem Biophys Res Commun. 1968 Oct 10;33(1):108–112. doi: 10.1016/0006-291x(68)90263-5. [DOI] [PubMed] [Google Scholar]
- Sy J., Lipmann F. Identification of the synthesis of guanosine tetraphosphate (MS I) as insertion of a pyrophosphoryl group into the 3'-position in guanosine 5'-diphosphate. Proc Natl Acad Sci U S A. 1973 Feb;70(2):306–309. doi: 10.1073/pnas.70.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosa T., Pizer L. I. Effect of serine hydroxamate on the growth of Escherichia coli. J Bacteriol. 1971 Jun;106(3):966–971. doi: 10.1128/jb.106.3.966-971.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow R. M. A consequence of the rel gene during a glucose to lactate downshift in Escherichia coli. The rates of ribonucleic acid synthesis. J Biol Chem. 1971 Aug 10;246(15):4872–4877. [PubMed] [Google Scholar]