Abstract
Ischaemic diabetic foot ulcers pose a significant problem which is associated with a high likelihood of amputation. With the advent of endovascular surgery, the management of lower limb arterial lesions in the diabetic population has become more appealing. Coronary 0.014 monorail guide wires, appropriate sized angioplasty balloons and stents, and subintimal recanalisation, are all useful adjuncts and techniques to achieve revascularization. This article reviews the modern endovascular management of the diabetic foot.
Keywords: diabetic foot, endovascular interventions
According to the World Health Organization and the International Working Group on the Diabetic Foot, "diabetic foot" is defined as the foot of diabetic patients with ulceration, infection and / or destruction of the deep tissues, associated with neurological abnormalities and various degrees of peripheral vascular disease in the lower limb1. Approximately 5% of diabetic patients give a history of foot ulcers, whereas about 15% are bound to experience this complication during their lifetime2. The prevalence of foot ulcers in patients with diabetes in developed countries is approximately 4–10%3. The most feared complication of the diabetic foot problems is amputation. About 40–70% of all non–traumatic lower limb amputations are performed in the diabetic population, and 85% of these are preceded by a foot ulcer. The most common cause of amputation is ischaemia and infection: gangrene or non–healing foot ulcer is the cause of amputations in 50–70% and infection in 20–50% of patients4. There are two main types of ulcer: neuropathic and ischaemic. In patients with diabetes, pure ischemic ulcers are less common and the vast majority of ulcers are either pure neuropathic or mixed neuroischaemic1–5. The latter group is the focus of this article.
Epidemiology–Aetiology
Peripheral arterial disease (PAD) is almost 3 times more frequent in diabetics compared with age–, and sex– matched individuals. Arterial lesions are more diffuse, frequently bilateral, and tend to involve arteries below the knee level. Ischaemia may be responsible in almost 50% of diabetic ulcer cases.
Clinical evaluation
Diabetic patients with PAD may be asymptomatic (Fontaine stage I) or suffering from intermittent claudication (Fontaine stage II). More severe symptoms may include rest pain (Fontaine stage III) and ulceration / gangrene (stage IV)6. Critical limb ischaemia (CLI) is defined by the presence of ischemic skin lesions, either ulcers or gangrene, and symptoms of rest pain of more than two weeks that require regular analgesics. The diagnosis of CLI should be confirmed by non–invasive means.
The presentation of a diabetic patient with foot or leg pain should be addressed with careful history taking and clinical assessment. This will help differentiating between neuropathic, claudication or ischaemic rest pain and guide appropriate treatment6. Neuropathic pain is usually located in the leg and shin, feels like numbness or burning sensation, is worse at night, and is relieved with exercise. The foot is warm and pulses are easily palpable. Intermittent claudication usually involves the calf or the thigh (depending on the level of occlusion), and is a cramp–like pain that occurs with exercise and is relieved with rest. Foot pulses are weak or impalpable. Finally, ischaemic rest pain most typically occurs at night (when the limb is no longer in a dependent position) but in severe cases can be continuous6. The pain is localized in the distal part of the foot or in the vicinity of an ischaemic ulcer or gangrenous toe. The pain often wakes the patients at night and forces them to rub the foot, get up, or take a short walk around the room. Partial relief may be obtained by the dependent position, whereas elevation and cold increase the severity of the pain. Often, patients sleep with their ischemic leg dangling over the side of the bed, or sitting in an armchair; as a consequence ankle and foot oedema develop. In severe cases, sleep becomes impossible because pain sets in after only a short period of supine rest, causing in many patients a progressive further decline of their general physical and psychological condition.
The appearance of a foot ulcer in a diabetic should also follow a diagnostic algorithm which will allow the treating physician or surgeon to reach the appropriate diagnosis and order the correct treatment. Most common types and characteristics of foot and leg ulcers are summarised in Table 16.
Table 1. Differential diagnosis of common leg and foot ulcers.
Paraclinical evaluation
The initial noninvasive testing includes calculation of the ankle–brachial pressure index (ABPI) using a handheld Doppler. An ABPI of 0.9–1.2 should be considered as normal, whereas an ABPI between 0.5 and 0.89 is indicative of peripheral arterial disease, usually causing intermittent claudication, or, in more severe cases, early rest pain. An ABPI of < 0.5 is usually consistent with more severe occlusive disease, i.e. Fontaine stages III or IV, ABPI > 1.2 or inability of measuring an index indicates incompressible arteries resulting from medial calcification, a condition very often seen in diabetics6. Loss of the triphasic waveform in the Doppler examination may point towards occlusive disease in such cases. Segmental pressures, toe pressures (measured by a pneumatic toe cuff), transcutaneous oximetry (normal values of transcutaneous oxygen pressure, TcPO2, are between 40 and 70 mm Hg), segmental plythesmography, and triplex ultrasonography are other useful noninvasive tests. However, all but the latter are available only in dedicated specialised vascular laboratories. In the presence of CLI, the absolute ankle systolic pressure is below 50 mmHg or a toe pressure less than 30 mmHg in incompressible arteries, as defined by the 2nd European Consensus Document on Chronic Critical Leg Ischaemia (1992)1–6.
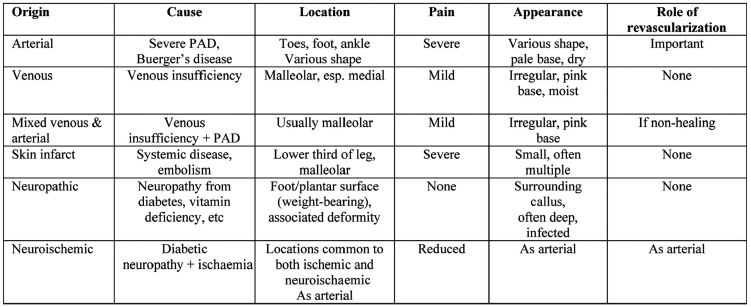
If triplex ultrasonography identifies a circulatory component to the diabetic foot problem, then a more detailed imaging technique should follow. These are digital subtraction arteriography (DSA), computed tomography angiography (CT angio), and magnetic resonance angiography (MRA). DSA is invasive and both DSA and CTA require the administration of potentially nephrotoxic contrast media. This can lead to worsening of renal function, particularly in diabetics with pre–existing renal impairment and those taking oral metformin. Nowadays, our unit relies heavily on MRA to guide the subsequent endovascular intervention or vascular surgery (Figure 1).
Figure 1. MRA of the lower limb arterial system. There is a normal arterial supply to the right leg, but a flush occlusion of the left superficial femoral artery. The popliteal artery is reconstituted through collaterals originating from the profunda femoris artery.
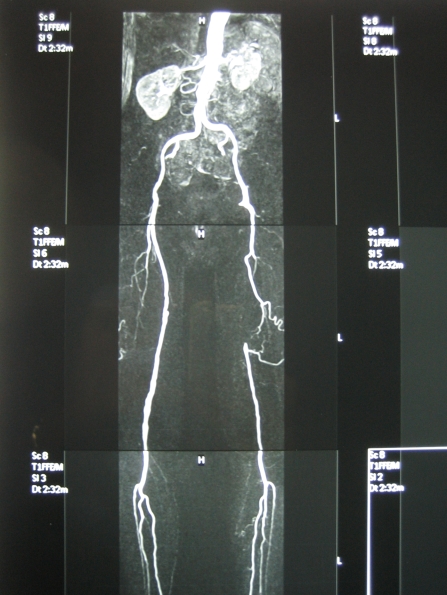
It would appear that the distribution of lower extremity arterial lesions in diabetics may be slightly different than non–diabetics, with the former presenting with more distal lesions, particularly, below the knee, in the crural and pedal arteries (Figure 2). Interestingly, there are few good studies to document this. Strandness et al, in 1961, suggested that approximately two thirds of diabetic patients have arterial lesions below the knee7. Another study by King et al showed diabetics to have profunda femoris artery lesions more frequently than non–diabetics8. A recent multicentre Italian trial studied 417 diabetics with 2893 arterial lesions9. Only 1% of them were located in the iliac arteries, whereas 74% were below the knee. In fact, two thirds of the below the knee lesions were occlusions, and approximately one third had all three crural arteries occluded. The most frequent angiographic picture (36%) was found to be 2 occluded crural arteries and multiple stenoses of the third tibial/peroneal artery or of the superficial femoral artery.
Figure 2. Diabetic patients usually present with more distal arterial lesions, usually below the knee (a), and often have diseased profunda femoris artery (b).
Therapeutic strategies: vascular versus endovascular surgery
Patients with diabetic foot, identified as having circulatory insufficiency of the lower extremity, are potential candidates for revascularization. The decision to proceed to intervention and the type of it (including a revascularization procedure or a major amputation) depends on the location of the arterial lesion (proximal versus distal), the adequacy of run–off arteries, the local expertise and resources, the magnitude of the proposed intervention, the comorbidities, particularly cardiac, respiratory and renal disease, and, last but not least, what is the best for the patient. Common sense, above all, should guide such decisions. For example, there is no point offering a major intervention to a patient with extensive gangrene, however, this can lower the level of amputation in order to get away with a below rather than an above–the–knee amputation. The former has a higher chance of survival, rehabilitation and subsequent independent mobilization with a prosthetic limb than the latter. Finally, any decision for a local amputation or debridement should be preceded by, or combined with, a revascularization procedure.
Once the decision to proceed to revascularisation has been taken, the choice of the procedure should be discussed. Open surgical procedures in the aorto–iliac segment include aorto–iliac endarterectomy, aorto–biiliac or aorto–bi–femoral bypass, and extraanatomical procedures, such as an axillo–femoral or femoro–femoral bypass. Common femoral bifurcation disease, quite common in diabetics, has been traditionally treated with femoral endarterectomy and patch profundoplasty. In patients with femoropopliteal disease, a vein or prosthetic femoro–popliteal bypass may be performed, whereas, in case of more distal disease, a femoro–distal bypass is required by means of a femoro–tibial, femoro–peroneal, or femoro–pedal bypass. These open surgical procedures are major and lengthy operations requiring general or regional anaesthesia and diabetics pose a major cardiovascular risk.
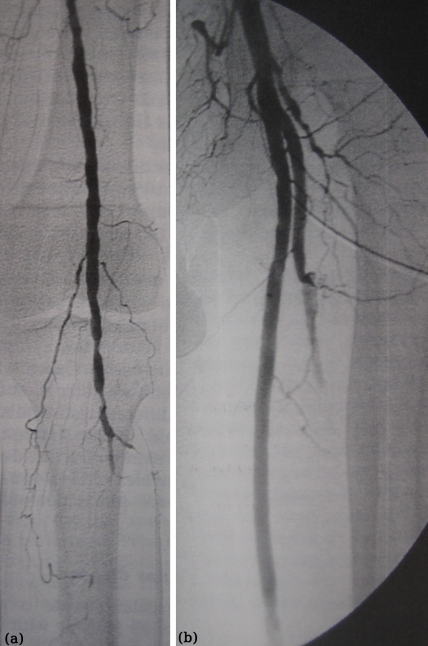
The advent of endovascular surgery has resulted in achieving percutaneous revascularisation via a remote access. Thus, isolated aortic disease can be treated by aortic stenting via the femoral or, rarely, the brachial route. Combined aortic and iliac disease can be treated by aortic and bilateral iliac stents or by raising the aortic bifurcation with biliateral iliac stenting. The latter can be accomplished by the "kissing" balloon and stent technique (Figure 3). The same technique is also indicated in case of common iliac ostial disease, unilateral or bilateral. Isolated common iliac disease can be treated by balloon angioplasty and/or stenting, obtaining access either via the ipsilateral groin or the contraleteral route using a crossover technique over the aortic bifurcation. External iliac artery lesions can be treated either from the ipsilateral or, more frequently, the contraleteral groin.
Figure 3 (a-c). The "kissing-stent" technique is indicated for the aortic bifurcation or common iliac artery stenoses in order to avoid a "spill-over" effect. Ballooning of only one iliac stenosis may compromise flow to the other leg (a). In such cases, two balloon expandable stents are being positioned in the aortic bifurcation and inflated simultaneously, i.e. "kissing" each other. Note the "waist" of the stent-balloons at the origin of both common iliacs at low inflation pressure (3-4 Atm) (b) disappearing at high pressure (8 Atm) (c).
The management of common femoral artery disease remains surgical at large, but can be frequently combined with endovascular procedures, proximal or distal. Multifocal disease, involving also the common femoral and profunda femoris arteries could also be treated with the kissing balloon angioplasty technique using a crossover approach and two 0.014 guidewires and appropriate sized balloons inflated across the origins of the profunda and superficial femoral arteries.
The endovascular treatment of stenotic and occlusive disease of the superficial femoral artery remains one of the biggest challenges in modern vascular practice. More than four decades after the first superficial femoral artery angioplasty, we still do not have a durable solution for long lesions10. The length of the stenosis or occlusion is the most important predictor of success of a stand–alone conventional percutaneous transluminal angioplasty (PTA). Risk factors which negatively affect patency are the type and number of lesions treated, total occlusions, poor run–off and critical ischaemia. Diabetes itself is not a consistent risk factor for poor outcome across published studies.
The usefulness of PTA is frequently limited by elastic recoil and high rates of flow–limiting dissection. Stent placement may improve radiological and clinical results in such cases, but stenting may also stimulate myointimal hyperplasia which, in turn, may compromise the mid–term patency results. Others favour primary stenting, even for long lesions, and claim satisfactory results. To date, the successful introduction of drug–eluting stents in the coronary circulation has not been duplicated in the superficial femoral artery region11. Covered stents or stent–grafts have also been used for occlusive lesions but their mid– and long–term results are awaited. Collective data on the results of balloon angioplasty and/or stenting in both diabetic and non–diabetic patients are presented in Table 2.
Table 2. Collective 1-, 3- and 5-year patency rates after PTA and PTA with stenting for stenoses and occlusions, respectively6,11.
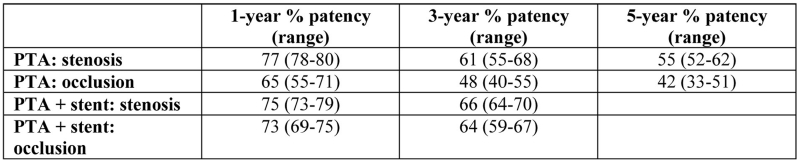
Subintimal angioplasty is another useful technique in the management of diabetic patients with occlusive disease in the superficial femoral artery, particularly long lesions. In principle, an intentional subintimal plane is initiated proximal to the lesion in order to bypass the entire diseased area and to exit into a disease–free segment just distal to the lesion10, 12. Once the wire passes back into the true lumen distal to the occlusion, the whole length of the dissection is then dilated with a balloon. Thus, an extralumimal neolumen is created (Figure 4). Theoretically, this new lumen between the intima and media is free from atheroma and, consequently more likely to remain open in the long term. Finally, other endovascular adjuncts include cutting balloon or laser–assisted angioplasty, cryoplasty, brachytherapy, atherectomy techniques and remote endarterectomy.
Figure 4 (a-f). Subintimal angioplasty of the superficial femoral artery. The occlusion (a) is being traversed subintimally with a guidewire loop (b). Re-entry back into the true lumen with the tip of the wire lying in the anterior tibial artery (c). The"neolumen" is being dilated with a balloon (d, e) with a satisfactory result (f).
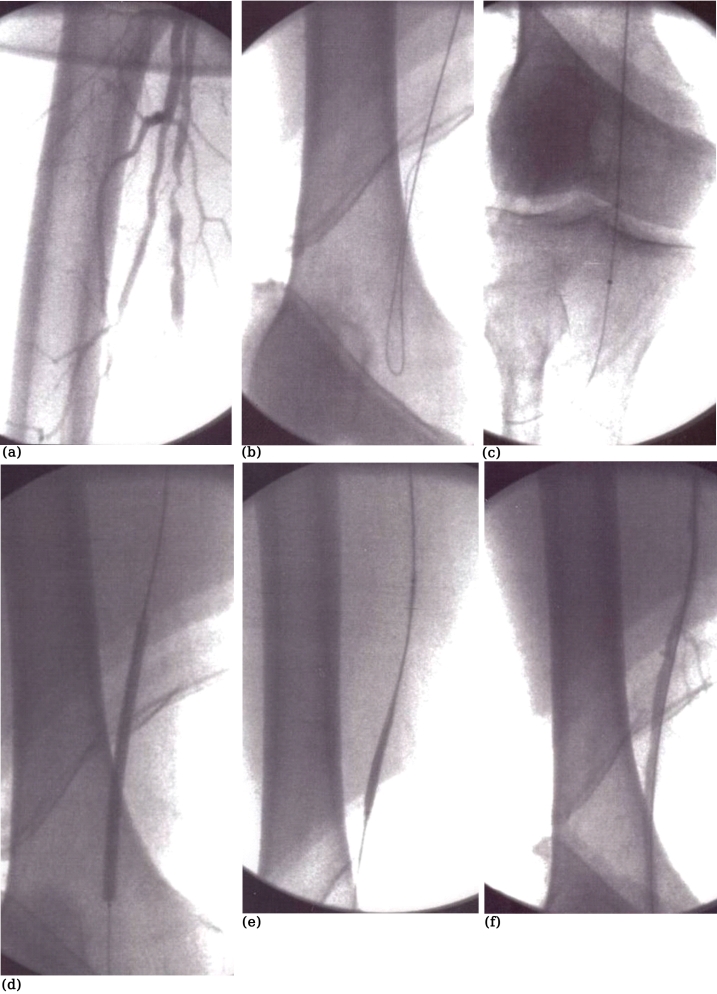
Distal disease, i.e. infrapopliteal lesions, is a particular problem in diabetics12–15. Some would consider revascularisation not an option in diabetics with CLI and would opt for a primary amputation. Others would go to great extent and perform femorodistal bypasses. To our opinion, endovascular therapy is very appealing in such cases, and we would consider this as a first option. Transluminal angioplasty certainly has its place and may be useful for stenotic lesions. However, in most cases, diabetics with CLI have one, two, or, even, three occluded crural arteries12–15. Subintimal recanalization is most useful in these cases. The number of patent run–off crural vessels after the angioplasty and the length of occlusion are significant risk factors for reocclusion of infrainguinal subintimal angioplasty in patients with CLI. Trying to recanalize more than one run–off vessels could raise the patency12–14. In fact, diabetics have no worse outcome than non–diabetics, and therefore, diabetes should not deter one from offering revascularisation to patients presenting with diabetic foot13–15. Overall 1–year patency after subintimal angioplasty of tibial arteries in patients with CLI is around 50%. This ranges from 81% in those with >1 patent run–off arteries to only 25% in those with one patent artery. Loss of patency, however, does not equal limb loss or ulcer recurrence. Finally, stenting may also have a role in the infrapopliteal level. Fine guidewires (0.014) and rapid exchange catheters, balloons and stents may improve the management of such difficult lesions and patients. Ostial lesions in the proximity of the popliteal trifurcation may also require "kissing" techniques to ensure patency of all neighbouring arteries (Figure 5).
Figure 5 (a-d). A stenotic lesion in the distal tibio-peroneal trunk (a). This has been treated with the "kissing balloon" technique using two 0.014 guidewires (b) and two coronary balloons 3mm x 4cm being inflated simultaneously in the distal tibio-peroneal trunk and the proximal part of the posterior tibial and peroneal arteries (c) with a satisfactory end-result (d).
In conclusion, the management of the diabetic foot demands a multidisciplinary approach. Once ischaemic ulceration has developed, aggressive management can achieve excellent results with a significant reduction of amputation and re–ulceration rates similar to those seen in non–diabetics. A recipe for success includes a combination of revascularisation, surgical debridement and appropriate antibiotics.
Endovascular surgery is the first choice, even though this depends on the local resources and expertise. An endovascular intervention can be easily repeated, is well–tolerated by the patient, is associated with low rates of serious complications, and has, perhaps, a lower cost. Finally, in contrast to the occlusion of a femoro–distal bypass for CLI, loss of angioplasty patency does not necessarily mean limb loss or ulcer recurrence.
References
- 1.International Working Group on the Diabetic Foot. International consensus on the diabetic foot. International Working Group on the Diabetic Foot, The Netherlands; 1999. pp. 20–96. [Google Scholar]
- 2.Reiber GE, Boyko E, Smith DG. Lower extremity ulcers and amputations in individuals with diabetes. In: Harris MI, editor. Diabetes in America. 2nd edition. Bestheda: National Institute of Health; 1995. pp. 407–429. National Institute of Health Publication No 95-1468. [Google Scholar]
- 3.Boulton AJ. The diabetic foot: from art to science. The 18th Camillo Golgi lecture. Diabetologia. 2004;47:1343–1353. doi: 10.1007/s00125-004-1463-y. [DOI] [PubMed] [Google Scholar]
- 4.Tentolouris N, Al-Sabbacgh NS, Walker MG, Boulton AJ, Jude EB. Mortality in diabetic and non-diabetic patients after amputations performed from 1990 to 1995: a 5-year follow-up study. Diabetes Care. 2004;27:1598–1604. doi: 10.2337/diacare.27.7.1598. [DOI] [PubMed] [Google Scholar]
- 5.Reiber GE, Ledoux WR. Epidemiology of diabetic foot ulcers and amputations: evidence for prevention. In: Williams B, Herman W, Kinmonth AL, Warehan NJ, editors. The evidence-base diabetes care. Chichester: John Wiley; 2002. pp. 641–655. [Google Scholar]
- 6.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33:S1–S75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Strandness DE, Priest RE, Gibbons RE, Seattle MD. Combined clinical and pathological study of diabetic and nondiabetic peripheral artery disease. Diabetes. 1961;13:366–372. doi: 10.2337/diab.13.4.366. [DOI] [PubMed] [Google Scholar]
- 8.King TA, De Palma RG, Rhodes RS. Diabetes mellitus and atherosclerotic involvement of the profunda femoris artery. Surg Gynecol Obstet. 1984;159:553–556. [PubMed] [Google Scholar]
- 9.Graziani L, Silvestro A, Bertone V, et al. Vascular involvement in diabetic subjects with ischemic foot ulcer: a new morphologic categorization of disease severity. Eur J Vasc Endovasc Surg. 2007;33:453–460. doi: 10.1016/j.ejvs.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Karkos CD, Rancic Z, Svetlikov A, et al. Superficial femoral artery occlusive disease : open surgery and endotechniques. In: Branchereau A, Jacobs M, editors. Open surgery versus endovascular procedures. Oxford: Paris Consultants Ltd 2007; 2007. pp. 189–203. EVC 2007. [Google Scholar]
- 11.Duda SH, Bosiers M, Lammer J, et al. Drug-eluting and bare nitinol stents for the treatment of atherosclerotic lesions in the superficial femoral artery : long-term results from the SIROCCO trial. J Endovasc Ther. 2006;13:701–710. doi: 10.1583/05-1704.1. [DOI] [PubMed] [Google Scholar]
- 12.Karkos CD, Karamanos DG, Papadimitriou DN, et al. Current Therapeutic Options in the Management of Superficial Femoral Artery Occlusive Disease. Acta Chir Belg. 2007;107:605–615. doi: 10.1080/00015458.2007.11680134. [DOI] [PubMed] [Google Scholar]
- 13.Lazaris AM, Salas C, Tsiamis AC, et al. Factors Affecting Patency of Subintimal Infrainguinal Angioplasty in Patients with Critical Lower Limb Ischemia. Eur J Vasc Endovasc Surg. 2006;32:668–674. doi: 10.1016/j.ejvs.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Lazaris AM, Tsiamis AC, Fishwick G, Bolia A, Bell PRF. Clinical Outcome of Primary Infrainguinal Subintimal Angioplasty in Diabetic Patients With Critical Lower Limb Ischemia. J Endovasc Ther. 2004;11:447–453. doi: 10.1583/03-1159.1. [DOI] [PubMed] [Google Scholar]
- 15.Awad S, Karkos CD, Serrachino-Inglott F, et al. The Impact of Diabetes on Current Revascularisation Practice and Clinical Outcome in Patients with Critical Lower Limb Ischaemia. Eur J Vasc Endovasc Surg. 2006;32:51–99. doi: 10.1016/j.ejvs.2005.12.019. [DOI] [PubMed] [Google Scholar]