Abstract
Alport syndrome (AS) is the most common hereditary nephritis often associated with extrarenal manifestations. It was first described by Alport on 1927. There is a primary disorder in collagen type IV which is the main component of the basement membranes. Alport syndrome is more frequently inherited as an X-linked and less commonly as an autosomal dominant or autosomal recessive trait. We describe the case of a 3-year-old boy with the X-linked variant of AS. The diagnosis was at first speculated from the child's detailed family history and was finally confirmed by a skin biopsy. Skin biopsy is an efficient and less invasive method for the X-linked variant of the AS diagnosis.
Keywords: Alport syndrome, collagen type IV, collagen genes, renal biopsy, skin biopsy
Alport syndrome (AS) is an inherited disorder of the glomerular basement membrane characterized of a combination of usually progressive glomerular disease, sensoryneural hearing loss and ocular abnormalities1. Studies carried out since the 1980s have established AS as a primary basement membrane disorder arising from mutations in genes encoding the chains of the type IV collagen. In approximately 80 percent of patients, the disorder is inherited as an X-linked trait, arising from mutations in the gene encoding the a5 chain of the collagen type IV on the X chromosome. In this variant women are heterozygous carriers of the disease mutation. Almost all carriers have some degree of haematuria, but only a minority develop renal failure1. Autosomal recessive inheritance accounts for about 15 percent and only 5 percent of patients have autosomal dominant disease. The clinical and histology features in the above two forms are similar to those of X-linked disease, although failure of renal function tends to occur less progressively in patients with the autosomal dominant type of the disease2,3. The basement's membrane disorder in the autosomal variant appears as a result of mutations in genes encoding the a3 and a4 chains of the collagen type IV. Haematuria is the main clinical manifestation of AS. Renal or skin biopsy is the diagnostic procedure of choice1–4. Genetic analysis of collagen genes is necessary for the molecular diagnosis of the syndrome5. There is no specific treatment for Alport syndrome. Renal transplantation is the treatment of choice for patients with end stage renal failure6.
Case report
A 3-year-old boy was presented to our clinic for evaluation of microscopic haematuria detected incidentally at the age of one year. Since then in repeated urine tests (every 1-2 months) microscopic haematuria (15-20 red cells per high-power field) persisted. No fever, abdominal pain, dysouria or other symptoms were referred.
The patient was the first child of phenotypically healthy parents, born with caesarian section. From the family history, the mother had microscopic haematuria (10-20 red blood cells per high-power field). This finding was detected for the first time in an incidental urine test during her pregnancy and since then persisted. His father and sister were phenotypically healthy and never had haematuria. Studying the child's pedigree we found that there were relatives of the mother's side with end-stage-renal failure (3 women with late onset and a man at the age of 35 years).
At his admission according to physical examination the child was in perfect condition, with normal psychomotor development and growth. His weight and height were between 25th and 50th percentile and the blood pressure was below the 50th percentile.The results of physical examination revealed no pathologic findings. In repeated urine tests there was a persistent microscopic haematuria (15-30 red cells per high power field). Red cells in urine at the phase-contrast microscopy test were dysmorphic and the G1 cells were >5% in all tested specimens. These findings suggested that haematuria was of glomerural origin. Laboratory workup including haematology, chemistry, immunology and clotting tests was normal with the exception of haematuria.
Considering that the child's mother had microscopic haematuria and four relatives of the mother's side had developed end stage renal failure, Alport syndrome seemed to be the most probable diagnosis of our case, especially the X-linked variant. Based on the fact that this type of hereditary nephritis may have extrarenal manifestations such as sensorineural deafness or ocular abnormalities we performed audiometry and ophthalmologic evaluation that appeared normal. The absence of extrarenal manifestations did not exclude the diagnosis of AS, since our patient was only 3 years old.
We performed skin biopsy instead of renal for AS diagnosis. The skin tissue was studied by immunofluorescence. After incubation of the skin tissue with monoclonal antibody against a5 chain of collagen type IV the patient showed no staining for the a5 chain.
Following these the patient was dismissed in an overall satisfactory condition with the instructions to monitor renal function (blood pressure, plasma creatinine, 24 hours urine collection for protein) on a yearly basis and to perform audiometry and ophthalmologic evaluation once every two years.
Discussion
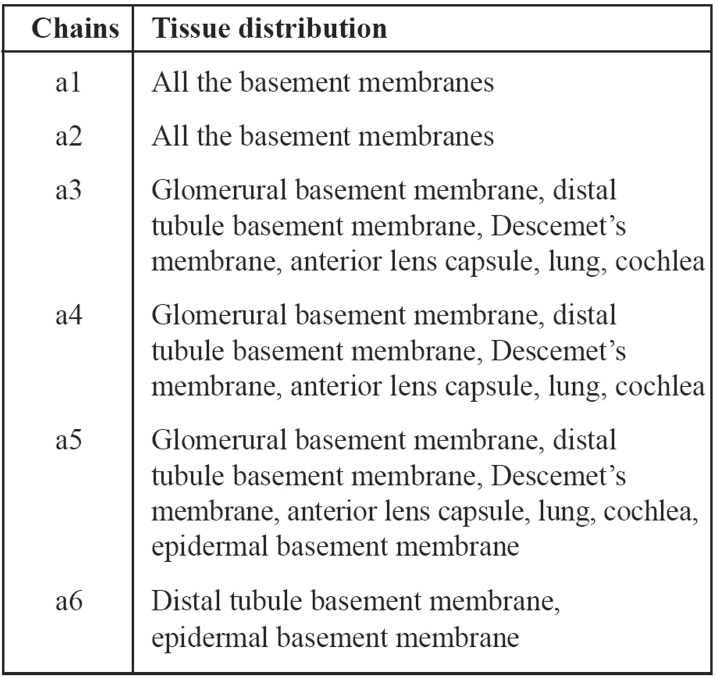
Type IV collagen is the main structural component of basement membranes. It consists of 6 chains a1-a6. The epidermal basement membrane consists of a1, a2, a5 and a6 chains. The glomerural basement membrane consists of a1, a2, a3, a4 and a5 chains, whereas no a6 chains are present (Table 1). The chains a3, a4 and a5 form a network of collagen in the glomerular basement membrane. An abnormality even in one of the above chains could limit the network formation and lead to the persistence of the a1 and a2 fetal isoforms. Persistence of these fetal isoforms confers an increased susceptibility to proteolytic attack by collagenases and cathepsins7. Proteolytic injury over a prolonged period may be responsible for the progressive glomerural basement membrane splitting and eventual glomerulosclerosis that is characteristic of AS. It is possible that the cysteine-rich alpha-3, -4 and -5 chains increase the resistance of the glomerular basement membrane to proteolytic attack of enzymes at the site of filtration. When the a5 chain which is responsible for the X-linked variant of the Alport's syndrome is absent from the glomerural basement membrane it is absent from the basement membrane underlying the epidermis as well. This is the reason why a skin biopsy may confirm the diagnosis of the X-linked variant of the syndrome as it happened in the case we report8.
Table 1. Tissue distribution of the a1-a6 chains of collagen type IV.
The clinical manifestations of the syndrome are similar in all the hereditary variants, although in the autosomal dominant variant renal deterioration occurs later. The initial renal manifestation of hereditary nephritis is asymptomatic microscopic haematuria. which has an early onset in boys1. In this case we report the microscopic haematuria was found incidentally at the age of one year. Recurrent episodes of gross haematuria are not unusual especially after respiratory infections during the first two decades of life. Progressive renal failure occurs with time. The males usually develop end stage renal failure before the age of 40 years. However females who are carriers of the X-linked trait develop less usually progressive renal disease than males and if this is the case, it occurs in older age than in males9. Renal function tests of our patient's mother were normal.
A variety of extrarenal manifestations may be seen. The most common is sensorineural deafness, which is bilateral, occurs by late childhood or early adolescence, begins in the high tones and progresses over time to frequencies in the range of conversational speech. From the ocular abnormalities the anterior lenticonus is a regular conical protrusion of the central portion of the lens into the anterior chamber1. Our patient had no extrarenal manifestation yet.
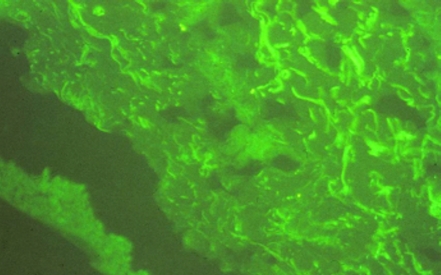
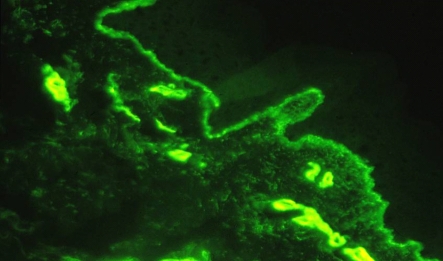
Skin biopsy studied by immunofluorescence is the diagnostic procedure of choice for the X-linked variant of the syndrome. After incubation of the skin tissues with monoclonal antibodies against a5 epidermal chain, the male patients show negative staining of the a5 chain, while the women carriers show a mosaic staining pattern4. This finding in women carriers is explained by the Lyon hypothesis. Results of skin biopsy in our patient are shown in Figures 1, 2. However, about 20 percent of patients with AS (autosomal variant) exhibit normal staining of the skin for the a5 chain. In this case it is necessary to perform renal biopsy. Molecular genetic testing can confirm or exclude the diagnosis of Alport's syndrome. Over 300 mutations have been described in the COL4A5 gene10.
Figure 1. The monoclonal antibody against the a5 chain has no reaction because of the chain's absence from the skin tissue.
Figure 2. The monoclonal antibody against the a1 chain has a positive reaction with the epidermal and vascular basement membrane.
There is no specific treatment for AS. Renal transplantation is the treatment of choice for end stage renal failure in individuals with AS6.
It should be noted that in the presented case a detailed family history suggested strongly the possibility of AS (X-linked variant). For this reason and although there were no extrarenal manifestations we performed skin biopsy which was the proper test to confirm the diagnosis. Skin biopsy is an easy and less invasive method compared to renal biopsy that may give us the opportunity to diagnose similar problems as well.
References
- 1.Kashtan CE. Alport syndrome. An inherited disorder of renal, ocular, and cochlear basement membranes. Medicine (Baltimore) 1999;78:338–360. doi: 10.1097/00005792-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Pescucci C, Francesca M. Autosomal-dominant Alport syndrome: natural history of a disease due to COL4A3 or COL4A4 gene. Kidney Int. 2004;65:1598–1603. doi: 10.1111/j.1523-1755.2004.00560.x. [DOI] [PubMed] [Google Scholar]
- 3.Tazon Vega B, Badenas C, Ars E, et al. Autosomal recessive Alport's syndrome and benign familial hematuria are collagen type IV diseases. Am J Kidney Dis. 2003;42:952–959. doi: 10.1016/j.ajkd.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Massella L, Onetti Muda A, Faraggiana T, Bette C, Renieri A, Rizzoni G. Epidermal basement membrane alpha 5 (IV) expression in females with Alport syndrome and severity of renal disease. Kidney Int. 2003;64:1787–1791. doi: 10.1046/j.1523-1755.2003.00251.x. [DOI] [PubMed] [Google Scholar]
- 5.Inoue Y, Nishio H, Shirakawa T, et al. Detection of mutations in the COL4A5 gene in over 90% of male patients with X-linked Alport's syndrome by RT-PCR and direct sequencing. Am J Kidney Dis. 1999;34:854–862. doi: 10.1016/S0272-6386(99)70042-9. [DOI] [PubMed] [Google Scholar]
- 6.Byrne MC, Budisavlijevic MN, Fan Z, Self SE, Ploth DW. Renal transplant in patients with Alport's syndrome. Am J Kidney Dis. 2002;39:769–775. doi: 10.1053/ajkd.2002.31997. [DOI] [PubMed] [Google Scholar]
- 7.Kalluri R, Shield CF, Todd P, Hudson BG, Neilson EG. Isoform switching of type IV collagen is developmentally arrested in X- linked Alport syndrome leading to increased susceptibility of renal basement membranes to endoproteolysis. J Clin Invest. 1997;99:2470–2478. doi: 10.1172/JCI119431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muda AO, Massella L, Giannakakis K, Renieri A, Rizzoni G, Faraggiana T. Confocal microscopy of the skin in the diagnosis of X-linked Alport syndrome. J Invest Dermatol. 2003;121:208–211. doi: 10.1046/j.1523-1747.2003.12322.x. [DOI] [PubMed] [Google Scholar]
- 9.Jais JP, Knebelmamm B, Giatras I, et al. X-linked Alport syndrome: natural history in 195 families and genotype - phenotype correlations in males. J Am Soc Nephrol. 2000;11:649–657. doi: 10.1681/ASN.V114649. [DOI] [PubMed] [Google Scholar]
- 10.Martin P, Heiskari N, Zhou J, et al. High mutation detection rate in the COL4A5 collagen gene in suspected alport syndrome using PCR and direct DNA sequencing. J Am Soc Nephrol. 1998;9:2291–2301. doi: 10.1681/ASN.V9122291. [DOI] [PubMed] [Google Scholar]