Abstract
Metabolic syndrome is a disorder characterized by abdominal obesity, hypertension, increased triglycerides, decreased HDL cholesterol and increased blood glucose. Accumulating evidence strongly indicates that insulin resistance and an increased amount of abdominal fat are the pathogenic factors for the characteristics of metabolic syndrome. The metabolic syndrome is characterized by an increased risk for the development of cardiovascular disease and type 2 diabetes mellitus. Studies indicate that sleep apnea may be a manifestation of the metabolic syndrome. It has also been suggested that the metabolic syndrome or "syndrome X" should also comprise obstructive sleep apnea and should then be called syndrome "Z". It appears that obstructive sleep apnea and the metabolic syndrome are characterized by the same pathophysiologic environment, which increases the risk for the development of cardiovascular disease. The increased amount of visceral fat and the accompanying insulin resistance seem to be the main characteristics responsible for the development of obstructive sleep apnea and the metabolic syndrome.
Keywords: metabolic syndrome, sleep apnea, obstructive sleep apnea, obesity
The metabolic syndrome is a disorder characterized by abdominal obesity, arterial hypertension, increased blood triglycerides, decreased HDL cholesterol and increased blood glucose1,2. Accumulating evidence indicates that insulin resistance and an increased amount of abdominal fat may be the pathogenic factors responsible for the constellation of symptoms of the metabolic syndrome3-5. The metabolic syndrome is characterized by an increased risk for the development of cardiovascular disease and type 2 diabetes mellitus6,7. Recent studies show that sleep apnea may be a manifestation of the metabolic syndrome8,9. It has been suggested that the metabolic syndrome "syndrome X" may include obstructive sleep apnea and must then be called "syndrome Z"10.
The metabolic syndrome
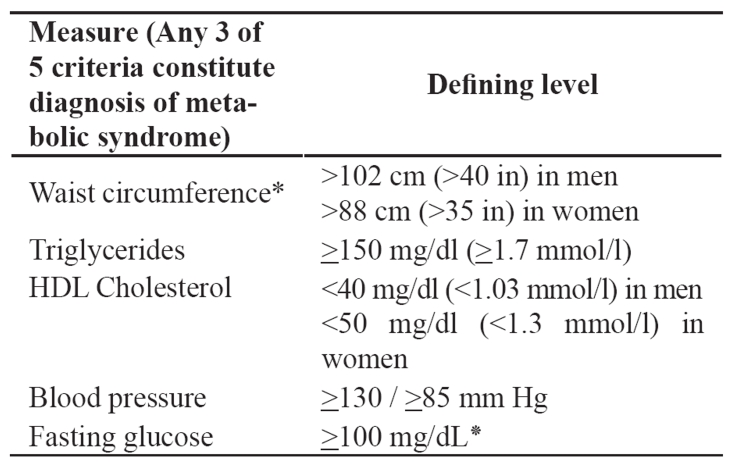
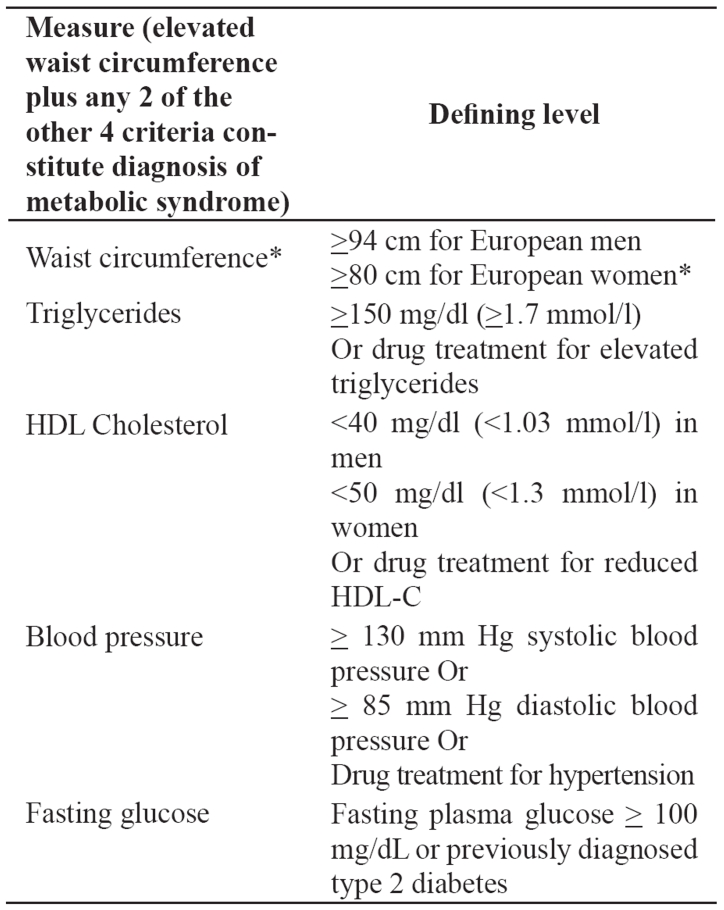
The metabolic syndrome is correlated with an increased risk for cardiovascular disease and type 2 diabetes mellitus6,7. The National Cholesterol Education Program’s Adult Treatment Panel III report (ATP III) identified the metabolic syndrome as a multiplex risk factor for cardiovascular disease that deserves more clinical attention11,12. ATP III viewed cardiovascular disease as the primary clinical outcome of metabolic syndrome. Most individuals who develop cardiovascular disease have multiple risk factors. In 1988, Reaven13 noted that several risk factors, namely dyslipidemia, hypertension and hyperglycemia cluster together. He called this clustering Syndrome X and he recognized it as a multiplex risk factor for cardiovascular disease. Reaven13 and subsequently others postulated that insulin resistance underlies Syndrome X. Other researchers, as well as ATP III use the term metabolic syndrome for this clustering of metabolic risk factors. Although ATP III identified cardiovascular disease as the primary clinical outcome of the metabolic syndrome, most people with this syndrome have insulin resistance, which confers an increased risk for type 2 diabetes. Abdominal obesity is the form of obesity strongly associated with the metabolic syndrome. It presents clinically as increased waist circumference. Atherogenic dyslipidemia is a characteristic of the metabolic syndrome and manifests in routine lipoprotein analysis by raised triglycerides and low concentrations of HDL cholesterol. A more detailed analysis usually reveals other lipoprotein abnormalities, such as increased remnant lipoproteins, elevated apolipoprotein B, small LDL particles and small HDL particles. All of these abnormalities have been implicated as being independently atherogenic. Elevated blood pressure strongly associates with obesity and commonly occurs in insulin-resistant persons. Insulin resistance is present in the majority of people with the metabolic syndrome. A proinflammatory state, recognized clinically by elevated C-reactive protein (CRP), is commonly present in individuals with the metabolic syndrome14–16. Multiple mechanisms seem to contribute to elevated CRP. One cause is obesity, because excess adipose tissue releases inflammatory cytokines that may elicit higher CRP levels17. The metabolic syndrome is characterized by a prothrombotic state, increased plasminogen activator inhibitor-118–20 and fibrinogen19–22. Fibrinogen, an acute-phase reactant like CRP, rises in response to a high-cytokine state. Thus, prothrombotic and proinflammatory states may be metabolically interconnected. The Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults-ATP III established criteria11,12 for the diagnosis of the syndrome (Table 1). Adults with 3 or more criteria, including abdominal obesity, arterial hypertension, increased blood triglycerides, decreased HDL cholesterol and increased blood glucose are considered as having the syndrome. Clinically, the aim is the recognition of individuals with increased risk for the development of cardiovascular disease. These people need increased surveillance. The World Health Organization (WHO) also recommended clinical criteria23 for the diagnosis of the metabolic syndrome, agreeing, on the core components of obesity, hyperglycemia, dyslipidemia and hypertension. The ATP III criteria for the metabolic syndrome have been widely used in both clinical practice and epidemiological studies. The American Heart Association and the National Heart, Lung, and Blood Institute affirmed the overall utility and validity of the ATP III criteria and proposed that they continue to be used with minor modifications and clarifications24. These modifications and clarifications refer to waist circumference, triglyceride, HDLC levels, blood pressure and elevated fasting glucose. In particular, lower levels for waist circumference have been introduced for cases or groups prone to insulin resistance. Triglyceride, HDL-C levels and blood pressure are considered abnormal when there is drug treatment for them. Additionally, blood pressure is considered as elevated if it exceeds the threshold for either systolic or diastolic blood pressure and the threshold for counting elevated fasting glucose was reduced from <110 mg/dl to >100 mg/dl in accordance with the American Diabetes Association's (ADA's) revised definition of impaired fasting glucose. The International Diabetes Federation (IDF) proposed a set of clinical criteria that are similar to those of the updated ATP III criteria (Table 2)25. In fact, thresholds are identical for triglycerides, HDL-C, blood pressure, and plasma glucose. The major difference is that the IDF proposed that waist circumference thresholds should be different for different ethnic groups. This suggestion is consistent with emerging information on the variable relationship between waist circumference and metabolic risk factors in different populations. Abdominal obesity is highly correlated with and easier to measure than other indicators of insulin resistance. The IDF therefore concluded that abdominal obesity incorporates both concepts of obesity and insulin resistance as being the 2 major underlying risk factors of the metabolic syndrome. Thus, they included increased waist circumference a required element for diagnosing the metabolic syndrome.
Table 1. Adult Treatment Panel III (ATP III) clinical criteria of the metabolic syndrome11,12.
* The American Diabetes Association has established a cutpoint of ≥100 mg/dL, above which persons have either prediabetes (impaired fasting glucose) or diabetes. This new cutpoint should be applicable for identifying the lower boundary to define elevated blood glucose as one criterion for the metabolic syndrome.
Table 2. The 2005 International Diabetes Federation diagnostic criteria25 of the metabolic syndrome.
* South Asian and South-East Asian men ≥ 90cm, women ≥ 80cm, Japanese men ≥ 85cm, women ≥ 90cm.
Steadily increasing evidence strongly indicates that insulin resistance may be the common pathogenic factor for the characteristics of the metabolic syndrome and explain the constellation of symptoms1–5. Within the context of the metabolic syndrome, researchers have suggested that insulin resistance may be the common factor responsible for the development of type 2 diabetes mellitus and atherosclerosis6,7,11,12.
Teleological views have given rise to the hypothesis that the metabolic syndrome is a remnant of evolutionary development under the pressure of a "feast-or-famine" existence26. The theory holds that one or more "thrifty" genes emerged that act to conserve energy during times of famine. Examples of these include reducing thermogenesis or inhibiting pregnancy and lactation. The genotype also should enable the maximal storage of energy during times of plenty in the form of adipose tissue rather than glycogen since this type of energy storage provides more sustenance during periods of starvation27. The "thrifty" genes thus afford a survival advantage when the food supply is highly variable. However, this theory holds that the survival advantages of the genotype become liabilities when energy supplies are abundant and remain so. The result is metabolic syndrome and impaired survival. Several putative mediators have been identified in support of this theory. One of them is leptin, which is a 167-amino acid protein produced by adipocytes and a variety of other tissues. Leptin has been shown to suppress hypothalamic neuropeptide Y in mice28, and, since neuropeptide Y stimulates appetite and thermogenesis, it is involved in an useful negative feedback mechanism. The ob gene has been identified as encoding for leptin, and the db gene as encoding for a hypothalamic leptin receptor in mice, and similar genes have been located in humans29. Interestingly, mice that are heterogenous for defective ob or db genes live longer when fasted than do normal mice, thus demonstrating the kind of survival advantage necessary for a "thrifty" gene29. Other possible mediators of the "thrifty" genotype are insulin receptor substrates (particularly insulin receptor substrate-1), phosphoinositide 3-kinase, hormone sensitive lipase, endothelial lipoprotein lipase, mitochondrial uncoupling proteins, tumor necrosis factor–α, glycogen synthase, and others29,30. The evolutionary pressure of a feast-or-famine existence lasting millennia opposed to the constant abundance of (at most) the last few hundred years, gives rise to metabolic syndrome quite often.
Sleep apnea
Sleep apnea refers to the temporary absence or cessation of breathing during sleep31. Airflow must be absent for a period of time longer than the usual inter-breath interval. This is traditionally defined as 10 seconds for adults, and 8 seconds, or more than two times the normal respiratory cycle time for infants. Airflow, in and out of the lungs, can stop for several reasons. In central apnea, no effort to breathe is made. In obstructive apnea32, there is ventilatory effort, but no airflow because the upper airway is closed. In typical mixed apnea, there is initially no ventilatory effort, but an obstructive sleep apnea pattern is evident when effort resumes. Dividing the total number of apneas during a recording period by the total sleep time yields the apnea index, the average number of apneas per hour of sleep. A variety of other indices can be measured, including the apnea/hypopnea index, which is the number of apneas plus hypopneas per hour of sleep. The obstructive sleep apnea syndrome is defined by an apnea/hypopnea index of 5 or higher in association with excessive daytime somnolence33.
Obstructive apneas and hypopneas are terminated by an arousal, a transient partial or complete return to awake physiology, this not necessarily being the case with central apneas and hypopneas. Arousals resulting in sleep fragmentation appear to be the primary cause of daytime hypersomnolence34. They may play a role, along with hypoxemia, in the long-term cardiovascular consequences of sleep apnea. The frequency of events that result in arousals becomes an important descriptor of sleep-disordered breathing. Morbidity may be related to a patient's autonomic response to the obstructive events. It is evident that people who spend all of their sleep time apneic and who fall asleep driving have an important medical problem potentially associated with significant morbidity and mortality.
Sleep apnea and the cardiovascular system
Patients with obstructive sleep apnea experience repetitive hemodynamic oscillations during the night35. Changes in systemic arterial blood pressure, pulmonary arterial blood pressure, heart rate and cardiac function occur in association with sleep state and respiration. These hemodynamic changes may be impressive, with post-apneic systolic arterial pressure exceeding 300 mmHg in patients who are normotensive while awake during the day. Because of these extreme changes after upper airway obstruction during sleep, investigations have attempted to examine the relationship of obstructive sleep apnea to cardiovascular morbidity and mortality.
Multiple studies have shown that obstructive sleep apnea may be an independent risk factor for systemic arterial hypertension36–39. In multiple studies the relationship between obstructive sleep apnea and systemic arterial hypertension was found to be independent from body mass index, age and cholesterol level. It has also been observed that the management of obstructive sleep apnea may lead to a decrease in arterial pressure40,41.
Patients with obstructive sleep apnea may be in an increased risk for the development of congestive heart failure, myocardial infarction and stroke42. This increased risk for cardiovascular morbidity and specifically myocardial infarction or cerebrovascular disease is under investigation.
Obstructive sleep apnea may be associated with cardiac arrhythmias. The most prominent and significant rhythm disturbances associated with obstructive sleep apnea include extreme bradycardia and ventricular asystole lasting longer than 10 seconds43–45. When present, bradycardia and asystole in patients with obstructive sleep apnea appear to be the result of enhanced vagal tone and not of structural disease of the conduction system45,46. Activation of the parasympathetic nervous system in this setting can be the result of multiple physiologic abnormalities including hypoventilation, hypoxemia, respiratory acidosis and vigorous inspiratory effort against a closed airway. Therapy with nasal continuous positive airway pressure abolishes all episodes of ventricular asystole in most such patients. In a study of 10 patients with sleep apnea induced asystole it was found that the effective nasal continuous positive airway pressure restored normal cardiac rhythm in eight47.
Thus, it appears that the obstructive sleep apnea may be a risk factor for the development of systemic arterial hypertension and may lead to the development of coronary artery and cerebrovascular disease.
Metabolic syndrome and sleep apnea
Morbid obesity is correlated to the syndrome of hypoventilation48–50. The syndrome of obesity hypoventilation may be accompanied by obstructive sleep apnea and may lead to significant clinical problems50. The syndrome of obesity and hypoventilation is characterized by findings from the history and physical examination. Patients suffer from sleepiness and they sleep during the day when they are not involved in any specific activity. Patients with coexisting sleep apnea snore so heavily that their snoring is characterized as heroic by their partners. Morbid obesity is the main physical finding. Other findings are the plethoric facies, the short and thich neck, the small oropharynx, rales, cyanosis and symptoms of right cardiac insufficiency, such as increased pressure in the jugular veins, hepatomegaly and pedal oedema.
Patients with the syndrome of obesity hypoventilation have by definition alveolar hypoventilation, hyperkapnia and hypoxemia when they are awake and breathe room air. Obstructive sleep apnea is frequently observed in these patients51. Obstructive sleep apnea may contribute to the development of systemic arterial hypertension in obese patients52 through activation of the sympathetic nervous system, blood leptin increase, insulin resistance, angiotensin II and aldosterone increase, oxidative and inflammatory stress and endothelial dysfunction. If obstructive sleep apnea exists the patients should be treated by different measures such as the application of positive pressure in the airway.
Studies have been performed suggesting that sleep apnea may be a manifestation of the metabolic syndrome8,9. It has also been proposed that the metabolic syndrome or "syndrome X" might include obstructive sleep apnea and then might be called "syndrome Z"10. The mechanisms which contribute to the development of cardiovascular disease in the metabolic syndrome and obstructive sleep apnea are similar. Patients with obstructive sleep apnea appear to suffer from the disorders which characterize the metabolic syndrome53,54. Thus, patients with obstructive sleep apnea have hypertension, high fasting blood glucose levels, increased waist circumference, low HDL cholesterol and high triglycerides and many other characteristics, including sympathetic activation, endothelial dysfunction, systemic inflammation, hypercoagulation and insulin resistance. A positive relationship was observed between the apnea/hypopnea index, body weight, body mass index, skinfold thickness, lipid percentage in whole body weight, blood glucose levels, uric acid, fibrinogen levels and leptin levels in men examined for possible apnea hypopnea55. The relationship between the apnea/hypopnea index and leptin levels disappeared when it was corrected for factors, which are indices of obesity. The exogenous administration of testosterone exacerbates obstructive sleep apnea while hormone replacement therapy with oestrogens in postmenopausal women may protect from obstructive sleep apnea56.
In a recent study it was found that the prevalence of the metabolic syndrome according to the ATP-III criteria is almost 40% greater in patients with obstructive sleep apnea57. It is not clear whether the syndrome of obstructive sleep apnea is observed as part of the basic pathophysiology of the metabolic syndrome or whether the syndrome of obstructive sleep apnea through repetitive night hypoxemia and other mechanisms induces the appearance of the characteristics of the metabolic syndrome. The size of the risk for the development of cardiovascular disease that can be attributed to the coexistence of the metabolic syndrome and obstructive sleep apnea may be cumulative, synergic or smaller.
It appears that the successful management of obstructive sleep apnea with the application of positive pressure in the airways decreases arterial blood pressure58 increases insulin sensitivity56,59 and improves testicular function in man56. Thus, it appears that the successful management of obstructive sleep apnea may decrease morbidity and mortality from cardiovascular diseases.
A study that included fifty four pacients with CAD and SAS showed that the patients who accepted therapy for the obstructive sleep apnea finally had one third of the risk for the development of a major incident from the cardiovascular system and especially from the coronary arteries compared to patients who did not recieve therapy for obstructive sleep apnea60.
Obese men with sleep apnea had higher plasma leptin levels and higher levels of inflammatory cytokines, such as tumor necrosis factor–α and interleukin-6, which promote the development of daytime sleepiness and insulin resistance than obese men without apnea and normal weight men61. In this study it was found that obese men with sleep apnea had statistically significantly higher amount of abdominal fat than obese men without sleep apnea. It was shown that apnea indices correlated positively with the amount of abdominal fat. In the same study, in the group of men with sleep apnea, a higher degree of insulin resistance was observed compared to obese men without sleep apnea. Higher levels of tumor necrosis factor–α and interleukin-6 have been detected in patients with disorders inducing sleepiness during the day62,63. It has been suggested that these cytokines are responsible for the development of sleepiness during the day.
In another study patients with obstructive sleep apnea the successful management of apnea decreased plasma leptin levels64 that correlated with the change in the apnea/ hypopnea index. Parish et al65 found that the prevalence of metabolic syndrome and hypertension was significantly greater in patients with obstructive sleep apnea compared to those without obstructive sleep apnea. In another study it was observed that obstructive sleep apnea was correlated with hypertension, dyslipidemia and hyperglycemia66. The authors concluded that obstructive sleep apnea may predispose even not obese patients to the development of metabolic syndrome.
It appears that obstructive sleep apnea and metabolic syndrome are characterized by the same pathophysiologic environment which increases the risk for the development of cardiovascular diseases54,67. The metabolic syndrome may be the final common pathway connecting sleep apnea with cardiovascular diseases26. Currently there is an epidemic of obesity resulting in an increase of the prevalence of the metabolic syndrome and obstructive sleep apnea increases51. The effect of this increase on the development of cardiovascular diseases may be very significant.
Conclusion
Obstructive sleep apnea and the metabolic syndrome are characterized by the same pathophysiologic environment which increases the risk for the development of cardiovascular diseases. The increased amount of abdominal fat and the accompanying insulin resistance are the main characteristics responsible for the pathophysiology of obstructive sleep apnea and the metabolic syndrome.
References
- 1.Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. 1995;75:473–486. doi: 10.1152/physrev.1995.75.3.473. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia and atherosclerotic cardiovascular disease. . Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 3.Zavaroni I, Bonora E, Pagliana M, et al. Risk factors for coronary artery disease in healthy persons with hyperinsulinemia and normal glucose tolerance. N Engl J Med. 1989;320:702–706. doi: 10.1056/NEJM198903163201105. [DOI] [PubMed] [Google Scholar]
- 4.Ferrannini E, Haffner SM, Mitchell BD, Stern MP. Hyperinsulinaemia: the key feature of a cardiovascular and metabolic syndrome. Diabetologia. 1991;34:416–422. doi: 10.1007/BF00403180. [DOI] [PubMed] [Google Scholar]
- 5.Laakso M, Sarlund H, Mykkanen L. Insulin resistance is associated with lipid and lipoprotein abnormalities in subjects with varying degrees of glucose tolerance. Arteriosclerosis. 1990;10:223–231. doi: 10.1161/01.atv.10.2.223. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 7.Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM. San Antonio Heart Study. The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care. 2003;26:3153–3159. doi: 10.2337/diacare.26.11.3153. [DOI] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev. 2005;9:211–224. doi: 10.1016/j.smrv.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Coughlin S, Calverley P, Wilding J. Sleep disordered breathing - a new component of syndrome x? Obes Rev. 2001;2:267–274. doi: 10.1046/j.1467-789x.2001.00045.x. [DOI] [PubMed] [Google Scholar]
- 10.Wilcox I, McNamara SG, Collins FL, Grunstein RR, Sullivan CE. "Syndrome Z": the interaction of sleep apnoea, vascular risk factors and heart disease. Thorax. 1998;53(Suppl 3):S25–S28. [PMC free article] [PubMed] [Google Scholar]
- 11.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 12.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 13.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 14.Pradhan AD, Cook NR, Buring JE, Manson JE, Ridker PM. Creactive protein is independently associated with fasting insulin in nondiabetic women. Arterioscler Thromb Vasc Biol. 2003;23:650–655. doi: 10.1161/01.ATV.0000065636.15310.9C. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin T, Abbasi F, Lamendola C, et al. Differentiation between obesity and insulin resistance in the association with C-reactive protein. Circulation. 2002;106:2908–2912. doi: 10.1161/01.cir.0000041046.32962.86. [DOI] [PubMed] [Google Scholar]
- 16.Festa A, D'Agostino R, Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 17.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arteriosc Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 18.Potter van Loon BJ, Kluft C, Radder JK, Blankenstein MA, Meinders AE. The cardiovascular risk factor plasminogen activator inhibitor type 1 is related to insulin resistance. Metabolism. 1993;42:945–949. doi: 10.1016/0026-0495(93)90005-9. [DOI] [PubMed] [Google Scholar]
- 19.Haffner SM, D'Agostino R, Jr, Mykkanen L, et al. Insulin sensitivity in subjects with type 2 diabetes. Relationship to cardiovascular risk factors: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 1999;22:562–568. doi: 10.2337/diacare.22.4.562. [DOI] [PubMed] [Google Scholar]
- 20.Festa A, D'Agostino R, Jr, Mykkanen L, et al. Relative contribution of insulin and its precursors to fibrinogen and PAI-1 in a large population with different states of glucose tolerance. The Insulin Resistance Atherosclerosis Study (IRAS) Arteriosc Thromb Vasc Biol. 1999;19:562–568. doi: 10.1161/01.atv.19.3.562. [DOI] [PubMed] [Google Scholar]
- 21.Ernst E, Resch KL. Fibrinogen as a cardiovascular risk factor: a meta-analysis and review of the literature. Ann Intern Med. 1993;118:956–963. doi: 10.7326/0003-4819-118-12-199306150-00008. [DOI] [PubMed] [Google Scholar]
- 22.Imperatore G, Riccardi G, Iovine C, Rivellese AA, Vaccaro O. Plasma fibrinogen: a new factor of the metabolic syndrome. A population-based study. Diabetes Care. 1998;21:649–654. doi: 10.2337/diacare.21.4.649. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Report of a WHO consultation. Part 1: Diagnosis and classification of diabetes mellitus. Geneva: World Health Organization; 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. [Google Scholar]
- 274.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 25.Alberti K, Zimmet P, Shaw J. The metabolic syndrome-a new world-wide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 26.Brown LK. A waist is a terrible thing to mind. Central obesity, the metabolic syndrome, and sleep apnea hypopnea syndrome. Chest. 2002;122:774–778. doi: 10.1378/chest.122.3.774. [DOI] [PubMed] [Google Scholar]
- 27.Neel JV. Diabetes mellitus: a "thrifty" genotype rendered detrimental by "progress"? Am J Hum Genet. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman J, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 29.Groop L, Orho-Melander M. The dysmetabolic syndrome. J Intern Med. 2001;250:105–120. doi: 10.1046/j.1365-2796.2001.00864.x. [DOI] [PubMed] [Google Scholar]
- 30.Abate N. Obesity and cardiovascular disease. Pathogenetic role of the metabolic syndrome and therapeutic implications. J Diab Complic. 2000;14:154–174. doi: 10.1016/s1056-8727(00)00067-2. [DOI] [PubMed] [Google Scholar]
- 31.Lugaresi E, Mondini S, Zucconi M, Montagna P, Cirignotta F. Staging of heavy snorers' disease. A proposal. Bull Eur Physiopathol Respir. 1983;19:590–594. [PubMed] [Google Scholar]
- 32.Wright J, Johns R, Watt I, Melville A, Sheldon T. Health effects of obstructive sleep apnoea and the effectiveness of continuous positive airways pressure: a systematic review of the research evidence. Br Med J. 1997;314:851–860. doi: 10.1136/bmj.314.7084.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flemons WW. Clinical practice. Obstructive sleep apnea. N Engl J Med. 2002;347:498–504. doi: 10.1056/NEJMcp012849. [DOI] [PubMed] [Google Scholar]
- 34.Horstmann S, Hess CW, Bassetti C, Gugger M, Mathis J. Sleepiness-related accidents in sleep apnea patients. Sleep. 2000;23:383–389. [PubMed] [Google Scholar]
- 35.Podszus T, Mayer J, Penzel T, Peter JH, von Wichert P. Nocturnal hemodynamics in patients with obstructive sleep apnea. Eur J Respir Dis Suppl. 1986;146:435–442. [PubMed] [Google Scholar]
- 36.Hla KM, Young TB, Bidwell T, Palta M, Skatrud JB, Dempsey J. Sleep apnea and hypertension. A population-based study. Ann Intern Med. 1994;120:382–388. doi: 10.7326/0003-4819-120-5-199403010-00005. [DOI] [PubMed] [Google Scholar]
- 37.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. Br Med J. 2000;320:479–482. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 39.Richert A, Ansarin K, Baran AS. Sleep apnea and hypertension: pathophysiologic mechanisms. Semin Nephrol. 2002;22:71–77. [PubMed] [Google Scholar]
- 40.Guilleminault C, Simmons FB, Motta J, et al. Obstructive sleep apnea syndrome and tracheostomy. Long-term follow-up experience. Arch Intern Med. 1981;141:985–988. [PubMed] [Google Scholar]
- 41.Mayer J, Becker H, Brandenburg U, Penzel T, Peter JH, von Wichert P. Blood pressure and sleep apnea: results of long-term nasal continuous positive airway pressure therapy. Cardiology. 1991;79:84–92. doi: 10.1159/000174864. [DOI] [PubMed] [Google Scholar]
- 42.Shahar E, Whitney CW, Redline S, et al. Sleep disordered breathing and cardiovascular disease: cross sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 43.Tilkian AG, Guilleminault C, Schroeder JS, Lehrman KL, Simmons FB, Dement WC. Sleep induced apnea syndrome. Prevalence of cardiac arrhythmias and their reversal after tracheostomy. Am J Med. 1977;63:348–358. doi: 10.1016/0002-9343(77)90272-8. [DOI] [PubMed] [Google Scholar]
- 44.Miller WP. Cardiac arrhythmias and conduction disturbances in the sleep apnea syndrome. Prevalence and significance. Am J Med. 1982;73:317–321. doi: 10.1016/0002-9343(82)90716-1. [DOI] [PubMed] [Google Scholar]
- 45.Zwillich C, Devlin T, White D, Douglas N, Weil J, Martin R. Bradycardia during sleep apnea. Characteristics and mechanism. J Clin Invest. 1982;69:1286–1292. doi: 10.1172/JCI110568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grimm W, Hoffmann J, Menz V, et al. Electrophysiologic evaluation of sinus node function and atrioventricular conduction in patients with prolonged ventricular asystole during obstructive sleep apnea. Am J Cardiol. 1996;77:1310–1314. doi: 10.1016/s0002-9149(96)00197-x. [DOI] [PubMed] [Google Scholar]
- 47.Becker H, Brandenburg U, Peter JH, Von Wichert P. Reversal of sinus arrest and atrioventricular conduction block in patients with sleep apnea during nasal continuous positive airway pressure. Am J Respir Crit Care Med. 1995;151:215–218. doi: 10.1164/ajrccm.151.1.7812557. [DOI] [PubMed] [Google Scholar]
- 48.Burwell CS, Robin ED, Whaley RD, Bickelmann AG. Extreme obesity associated with alveolar hypoventilation- -a Pickwickian Syndrome. 1956. Obes Res. 1994;2:390–397. doi: 10.1002/j.1550-8528.1994.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 49.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–1599. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 50.Rochester DF, Arora NS. In: Medical complications of obesity. Mancini M, Lewis B, Contaldo F, editors. London: Academic Press; 1980. pp. 183–190. [Google Scholar]
- 51.Formiguera X, Canton A. Obesity: epidemiology and clinical aspects. Best Pract Res Clin Gastroenterol. 2004;18:1125–1146. doi: 10.1016/j.bpg.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 52.Wolk R, Shamsuzzaman AS, Somers VK. Obesity, sleep apnea, and hypertension. Hypertension. 2003;42:1067–1074. doi: 10.1161/01.HYP.0000101686.98973.A3. [DOI] [PubMed] [Google Scholar]
- 53.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 54.Gami AS, Somers VK. Obstructive sleep apnoea, metabolic syndrome, and cardiovascular outcomes. Eur Heart J. 2004;25:709–711. doi: 10.1016/j.ehj.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Schafer H, Pauleit D, Sudhop T, Gouni-Berthold I, Ewig S, Berthold HK. Body fat distribution, serum leptin, and cardiovascular risk factors in men with obstructive sleep apnea. Chest. 2002;122:829–839. doi: 10.1378/chest.122.3.829. [DOI] [PubMed] [Google Scholar]
- 56.Yee B, Liu P, Phillips C, Grunstein R. Neuroendocrine changes in sleep apnea. Curr Opin Pulm Med. 2004;10:475–481. doi: 10.1097/01.mcp.0000143967.34079.27. [DOI] [PubMed] [Google Scholar]
- 57.Coughlin SR, Mawdsley L, Mugarza JA, Calverley P, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–741. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 58.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomized parallel trial. Lancet. 2002;359:204–210. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 59.Harsch IA, Schahin SP, Radespiel-Troger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–162. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 60.Milleron O, Pilliere R, Foucher A, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25:728–734. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 61.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 62.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 63.Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J Clin Endocrinol Metab. 2004;89:4409–4413. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- 64.Sanner BM, Kollhoser P, Buechner N, Zidek W, Tepel M. Influence of treatment on leptin levels in patients with obstructive sleep apnoea. Eur Respir J. 2004;23:601–604. doi: 10.1183/09031936.04.00067804. [DOI] [PubMed] [Google Scholar]
- 65.Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3:467–472. [PMC free article] [PubMed] [Google Scholar]
- 66.Kono M, Tatsumi K, Saibara T, et al. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome. Chest. 2007;131:1387–1392. doi: 10.1378/chest.06-1807. [DOI] [PubMed] [Google Scholar]
- 67.Svatikova A, Wolk R, Gami AS, Pohanka M, Somers VK. Interactions between obstructive sleep apnea and the metabolic syndrome. Curr Diab Rep. 2005;5:53–58. doi: 10.1007/s11892-005-0068-2. [DOI] [PubMed] [Google Scholar]