Abstract
Recent studies have suggested that spinal G-protein-coupled, inwardly rectifying K+ (GIRK) channels play an important role in thermal nociception and the analgesic actions of morphine and other agents. In this study, we show that spinal GIRK channels are activated by an endogenous neurotransmitter using whole-cell patch-clamp recordings from substantia gelatinosa (SG) neurones in adult rat spinal cord slices. Although repetitive stimuli applied to the dorsal root did not induce any slow responses, ones focally applied to the spinal dorsal horn produced slow inhibitory postsynaptic currents (IPSCs) at a holding potential of −50 mV in about 30% of the SG neurones recorded. The amplitude and duration of slow IPSCs increased with the number of stimuli and decreased with removal of Ca2+ from the external Krebs solution. Slow IPSCs were associated with an increase in membrane conductance; their polarity was reversed at a potential close to the equilibrium potential for K+, calculated from the Nernst equation. Slow IPSCs were blocked by addition of GDP-β-S into the patch-pipette solution, reduced in amplitude in the presence of Ba2+, and significantly suppressed in the presence of an antagonist of GIRK channels, tertiapin-Q. Somatostatin produced an outward current in a subpopulation of SG neurones and the slow IPSC was occluded during the somatostatin-induced outward current. Moreover, slow IPSCs were significantly inhibited by the somatostatin receptor antagonist cyclo-somatostatin. These results suggest that endogenously released somatostatin may induce slow IPSCs through the activation of GIRK channels in SG neurones; this slow synaptic transmission might play an important role in spinal antinociception.
G-protein-coupled, inwardly rectifying K+ (GIRK) channels are expressed in the central and peripheral nervous systems (CNS and PNS, respectively), where they are mainly involved in slow postsynaptic inhibitory signalling via G-protein-coupled receptors (GPCRs) (Dascal, 1997; Sadja et al. 2003). There are four distinct mammalian genes encoding GIRK channels (termed GIRK1–GIRK4 or Kir3.1–Kir3.4); neuronal GIRK channels are comprised of homotetramers or heterotetramers containing GIRK1–GIRK3 subunits (Koyrakh et al. 2005). GIRK channels are coupled to a variety of GPCRs, including opioid, adrenergic, muscarinic and dopaminergic receptors (Mark & Herlitze, 2000). Upon GPCR stimulation, the α subunit of the coupled G-protein replaces its bound GDP with GTP, which causes dissociation of the βγ dimer from the α subunit; the βγ dimer then directly binds and activates GIRK channels (Clapham & Neer, 1993; Jan & Jan, 1997; Logothetis et al. 2007). GIRK channels contribute to regulation of the resting membrane potential and excitability of neurones (Hille, 1992). In addition, the activation of GIRK channels is regulated by several intracellular signal pathways, such as inositol phosphates and protein kinases (Sadja et al. 2003; Logothetis et al. 2007). GIRK1, 2 and 3 are highly abundant in the brain; the expression of GIRK1 and 2 is enriched in the superficial layers of the dorsal horn, especially the substantia gelatinosa (SG), in the spinal cord (Marker et al. 2005). GIRK1 and 2 knock-out mice exhibited thermal hyperalgesia in the tail-flick test and displayed decreased analgesic responses following intrathecal administration of morphine (Marker et al. 2004). These findings suggest that spinal GIRK channels play an important role in thermal nociception and the analgesic actions of morphine and other agents.
Superficial layers of the spinal dorsal horn receive nociceptive information from the viscera, skin and other organs through fine myelinated Aδ and unmyelinated C primary afferent fibres (Kumazawa & Perl, 1978; Cervero & Iggo, 1980; Sugiura et al. 1986; Yoshimura & Jessell, 1989). Nociceptive information is modulated by a variety of endogenous systems in the spinal dorsal horn and then transferred to the higher centres (Willis & Coggeshall, 2004). Immunohistochemical studies have revealed that most neurotransmitters are expressed at relatively high concentrations in the superficial dorsal horn, particularly the SG (Cervero & Iggo, 1980; Willis & Coggeshall, 2004). These neurotransmitters derive from primary afferent fibres, neurones intrinsic to the dorsal horn, and axon terminals whose cell bodies are located in the brainstem. A large number of studies have suggested that several of these neurotransmitters contribute to the inhibition of nociceptive transmission in the spinal cord. Electrophysiological findings have provided evidence that the exogenous application of several neurotransmitters, such as opioids, adenosine, noradrenaline, serotonin (5-HT) and dopamine, directly hyperpolarizes the membranes of SG neurones (Yoshimura & North, 1983; Ito et al. 2000; Sonohata et al. 2004; Liu et al. 2004; Tamae et al. 2005; Fujita & Kumamoto, 2006). However, it is poorly understood how endogenous substances function to inhibit nociceptive transmission in the spinal dorsal horn. In the present study, using whole-cell patch-clamp recordings from adult rat spinal cord slices, we show that spinal GIRK channels are activated by an endogenous neurotransmitter to inhibit the excitability of SG neurones.
Methods
All experimental procedures involving the use of animals were approved by the Ethics Committee on Animal Experiments, Saga University, and were in accordance with the UK Animals (Scientific Procedures) Act of 1986 and associated guidelines.
Spinal cord slice preparation
The methods used to obtain adult rat spinal cord slice preparations have been previously described (Nakatsuka et al. 2000). In brief, male adult Sprague–Dawley rats (6–8 weeks of age, 200–300 g) were deeply anaesthetized with urethane (1.2 g kg−1, intraperitoneal), and then lumbosacral laminectomy was performed. The lumbosacral spinal cord (L1–S3) was removed and placed in pre-oxygenated Krebs solution at 1–3°C. Immediately after the removal of the spinal cord, the rats were given an overdose of urethane and then killed by exsanguination. The pia-arachnoid membrane was removed after cutting all the ventral and dorsal roots near the root entry zone, except for the L5 dorsal root on one side. The spinal cord was mounted on a microslicer and then a 600-μm-thick transverse slice through the dorsal root was cut. The slice was placed on nylon mesh in the recording chamber, which had a volume of 0.5 ml, and then perfused at a rate of 10–15 ml min−1 with Krebs solution saturated with 95% O2 and 5% CO2, and maintained at 36 ± 1°C. The Krebs solution contained (mm): 117 NaCl, 3.6 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3 and 11 glucose (pH = 7.4).
Patch-clamp recordings from SG neurones
Blind whole-cell patch-clamp recordings were made from SG neurones using patch-pipette electrodes with a resistance of 5–10 MΩ (Nakatsuka et al. 2000). The composition of the patch-pipette solution used was as follows (mm): 135 potassium gluconate, 5 KCl, 0.5 CaCl2, 2 MgCl2, 5 EGTA, 5 Hepes and 5 ATP-Mg (pH = 7.2). Guanosine-5′-O-(2-thiodiphosphate) (GDP-β-S) was added at a concentration of 2 mm to the patch-pipette solution when necessary. Signals were acquired using a patch-clamp amplifier (Molecular Devices; Axon Instruments, Foster City, CA, USA). Data were digitized using an A/D converter (Digidata 1322, Molecular Devices), stored and analysed with a personal computer using the pCLAMP data acquisition program (Version 8.2, Molecular Devices). Focal stimulation was given via a monopolar silver wire electrode (50 μm diameter), positioned in the dorsal horn within 200 μm of the recorded SG neurones (Fig. 1A). Orthodromic stimulation of the dorsal root, which had a length of 8–12 mm, was performed using a suction electrode (Fig. 1A). The minimum stimulus intensities required to activate Aβ, Aδ and C afferent fibres were 6 μA, 12 μA and 120 μA, respectively, with durations of 0.1 ms, as previously reported (Nakatsuka et al. 1999, 2000). To examine changes in membrane conductance during slow inhibitory postsynaptic currents (IPSCs), voltage steps (duration: 400 ms) from a holding potential (VH) of −50 mV to potentials ranging from −50 to −140 mV, in steps of 10 mV, were given to SG neurones.
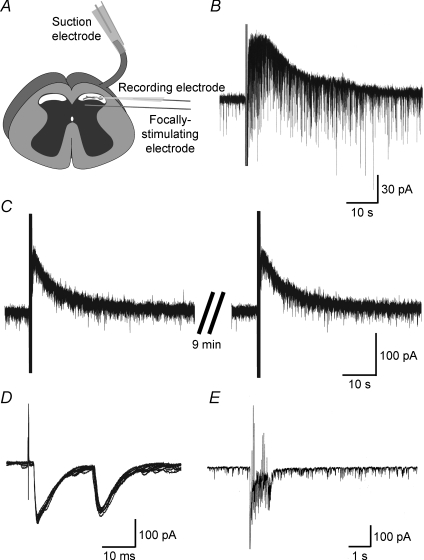
Figure 1. Synaptic responses evoked by intraspinal or primary afferent stimulation.
A, schematic diagram of a spinal cord slice preparation in which electrically evoked synaptic responses were examined. Focal stimulation was given via a monopolar silver wire electrode positioned near the recorded neurone to activate the fibres of interneurones, or descending fibres. Orthodromic stimulation of the dorsal root was performed using a suction electrode to activate primary afferent fibres. B, a slow IPSC elicited by repetitive stimuli at 20 Hz for 1 s (stimulus strength and duration: 0.6 mA and 0.4 ms, respectively), focally applied to the dorsal horn with a monopolar electrode. C, repeated repetitive stimuli, focally applied to the dorsal horn at 10 min intervals, produced a similar slow IPSC without any decrease in amplitude and duration. D and E, synaptic responses elicited by a single stimulus or repetitive stimuli (D: at 0.1 Hz for 100 s; E: at 20 Hz for 1 s; stimulus strength and duration: 0.5 mA and 0.1 ms, respectively) applied to the dorsal root using a suction electrode. The single stimulus produced Aδ and C primary afferent-mediated EPSCs (D: 10 EPSCs are superimposed), while tetatus stimulus did not elicit any slow synaptic responses (E). The holding potential (VH) used was −50 mV (B, C) or −70 mV (D, E).
Application of drugs
Drugs were dissolved in Krebs solution and applied by perfusion via a three-way stopcock without any change in the perfusion rate or temperature. The time necessary for the solution to flow from the stopcock to the surface of the spinal cord slice was approximately 20 s. The drugs used in this study were somatostatin, cyclo(7-aminoheptanoyl-Phe-d-Trp-Lys-Thr[Bzl]) (cyclo-somatostatin), barium chloride dehydrate, tertiapin-Q, endomorphin-1, naloxone hydrochloride, d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP), CGP 35348, methiothepin, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), dl-2-amino-5-phosphonopentanoic acid (AP5), GDP-β-S (Sigma, St Louis, MO, USA), yohimbine hydrochloride, sulpiride (Wako, Osaka, Japan), CGP 52432 (Tocris, Ellisville, MO, USA) and WAY100635 (synthesized at Asahi Chemical Industry, Japan). CNQX and DPCPX were first dissolved in dimethyl sulphoxide (DMSO) at 1000 times the concentrations to be used. The other drugs were first dissolved in distilled water at 1000 times the concentrations to be used, and then these drugs were diluted to the final concentration in Krebs solution immediately before use. The osmotic pressure of nominally Ca2+-free, high-Mg2+ (5 mm) Krebs solution was adjusted by lowering the Na+ concentration.
Statistical analysis
All numerical data were expressed as means ± s.e.m. Statistical significance was determined as P < 0.05 using the Student's paired t test to compare the amplitudes of slow IPSCs. In electrophysiological data, n refers to the number of neurones studied.
Results
SG neurones were viable for up to 24 h in slices perfused with pre-oxygenated Krebs solution. However, all the recordings described here were obtained within 12 h after the dissection. Whole-cell patch-clamp recordings were stable for up to 4 h. All neurones had resting membrane potentials more negative than −50 mV. A holding membrane potential of −50 mV was used unless otherwise mentioned.
Slow synaptic responses evoked by intraspinal but not primary afferent stimulation
Slow synaptic responses were evoked by repetitive stimuli at 20 Hz for 1 s (stimulus strength and duration: 0.3–1.0 mA and 0.4 ms, respectively), focally applied near to the recorded neurone via a monopolar electrode. A slow IPSC was induced in 94 out of the 289 neurones examined (Fig. 1B). When measured in some neurones, the average peak amplitude and duration of the slow IPSCs were 62 ± 4 pA and 58.6 ± 3.6 s, respectively (n = 73). When repetitive stimuli were focally applied repeatedly at 5–10 min intervals, they produced similar slow IPSCs with almost the same amplitude (Fig. 1C). We next tested whether the slow IPSCs were generated by repetitive stimuli applied to the dorsal root. As shown in Fig. 1D, the majority of SG neurones exhibited Aδ- or C-fibre-evoked fast excitatory synaptic responses to a single stimulus (stimulus strength and duration: 0.5 mA and 0.1 ms, respectively) at a VH of −70 mV (n = 12). In contrast to repetitive stimuli focally applied to the dorsal horn, no slow synaptic response was generated by repetitive stimuli at 20 Hz for 1 s (stimulus strength and duration: 0.5 mA and 0.1 ms, respectively), applied to the dorsal root with a suction electrode in all 12 neurones recorded (Fig. 1E).
Characterization of slow IPSCs evoked by intraspinal stimulation
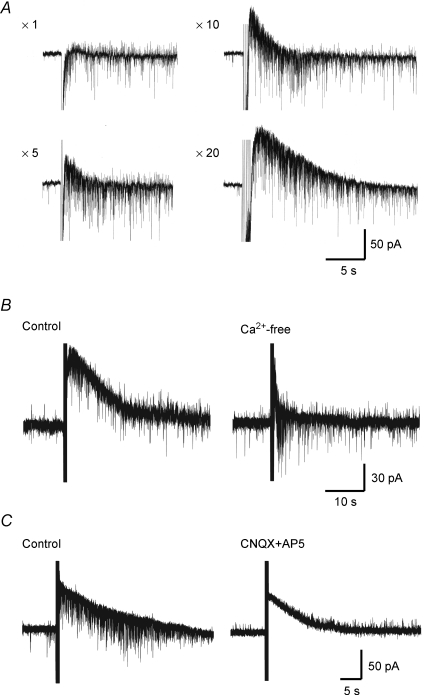
As illustrated in Fig. 2A, the amplitude and duration of slow IPSCs increased with the number of focal stimuli at 20 Hz (n = 3). We examined whether the slow IPSCs were dependent on extracellular Ca2+. The average amplitude of slow IPSCs was 15 ± 6 pA in a Ca2+-free bath solution; this value was significantly smaller than that in a normal bath solution (54 ± 4 pA, n = 4; Fig. 2B). On the other hand, the slow IPSCs were not affected by glutamate receptor antagonists. The average amplitude of the slow IPSCs was 48 ± 8 pA in the presence of CNQX (10 μm) and AP5 (50 μm), and this value was not significantly different from that in the absence of CNQX and AP5 (48 ± 8 pA, n = 4; Fig. 2C).
Figure 2. A slow IPSC mediated by endogenous neurotransmitters other than glutamate released by intraspinal stimulation.
A, dependence of slow IPSCs on the number of stimuli given. Slow IPSCs elicited by various numbers (shown on the left of each trace) of stimuli at 20 Hz, given to the dorsal horn. Note that the slow IPSCs increased in amplitude and duration with an increase in the number of stimuli. B, dependence of slow IPSCs on extracellular Ca2+ concentration. Slow IPSCs produced by repetitive stimuli focally applied to the dorsal horn in normal (left) and nominally Ca2+-free Krebs solution (right). Note that the slow IPSCs were largely decreased in amplitude and duration in a Ca2+-free solution. C, effect of glutamate receptor antagonists on slow IPSCs. Slow IPSCs were produced by repetitive stimuli focally applied to the dorsal horn in the absence (left) and presence of CNQX (10 μm) and AP5 (50 μm) (right). Note that glutamate receptor antagonists did not significantly change the amplitude and duration of slow IPSCs. VH = −50 mV.
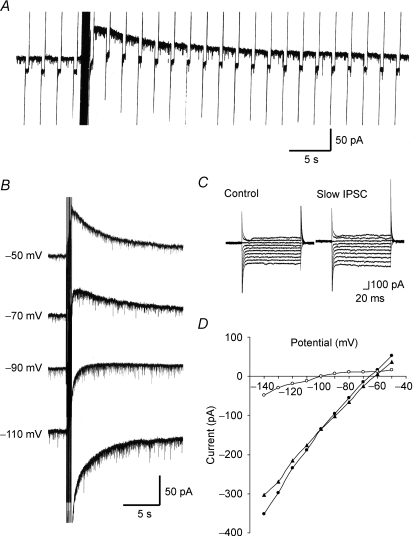
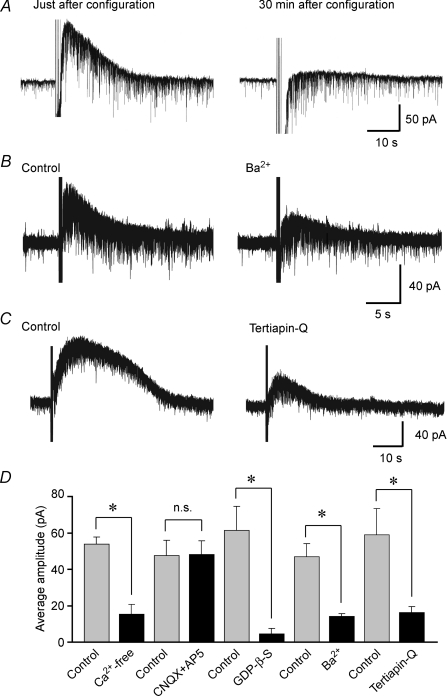
When voltage pulses with an amplitude of 20 mV and a duration of 400 ms at 0.5 Hz were applied to neurones, the membrane conductance increased during slow IPSCs (n = 3; Fig. 3A). To identify the ionic mechanisms underlying slow IPSCs evoked by repetitive stimuli focally applied to the dorsal horn, slow IPSCs were evoked at various VH values. When the VH was closer to −90 mV, the amplitude and duration of the slow IPSCs became smaller (n = 3; Fig. 3B). To investigate more accurately the dependence of slow IPSCs on membrane potential, a voltage step (duration, 400 ms) from a VH of −70 mV to potentials ranging from −50 to −140 mV, in steps of 10 mV, was given to SG neurones before and just after repetitive stimuli were focally applied to the dorsal horn (Fig. 3C). Figure 3D demonstrates the relationships between the membrane potential and the steady current at the end of its pulse before (filled triangles) and during (filled circles) the slow IPSCs. The net current of slow IPSCs (open circles), estimated from the difference between the two currents, exhibited a clear reverse and inward rectification. The average reversal potential was −95.3 ± 2.1 mV (n = 4). This potential was close to the equilibrium potential (−97.3 mV) for K+, as calculated from the Nernst equation using the K+ concentrations (3.6 and 140 mm, respectively) of normal Krebs and patch-pipette solutions. The slope conductance of the inward current (1.93 ± 0.29 nS, at −110 mV) was significantly larger than that of the outward current (0.65 ± 0.16 nS, at −70 mV; n = 4). To examine the involvement of G-proteins in slow IPSCs, GDP-β-S (2 mm), a non-hydrolysable analogue of GDP that competitively inhibits G-protein-mediated actions, was added to the patch-pipette solution. Although slow IPSCs were clearly evoked by repetitive stimuli focally applied to the dorsal horn just after establishing the whole-cell configuration with pipettes containing GDP-β-S, they were markedly reduced when repetitive stimuli were again applied 30 min later (Fig. 4A). The average amplitude of slow IPSCs was 62 ± 13 pA just after establishing the whole-cell configuration; this value was significantly larger than that obtained 30 min later (5 ± 3 pA, n = 4; Fig. 4A). Slow IPSCs were significantly suppressed in amplitude by the K+ channel blocker Ba2+ (500 μm). The average amplitude of slow IPSCs was 14 ± 2 pA in the presence of Ba2+; this value was significantly smaller than that in the absence of Ba2+ (47 ± 7 pA, n = 6; Fig. 4B). We further investigated the effect of a GIRK channel blocker, tertiapin-Q (Jin & Lu, 1998), on the slow IPSCs evoked by repetitive focal stimuli applied to the dorsal horn. The slow IPSCs were significantly reduced in amplitude by tertiapin-Q (0.1 μm), administered 5 min prior to the repetitive focal stimuli. The average amplitude of slow IPSCs was 16 ± 3 pA in the presence of tertiapin-Q; this value was significantly smaller than that in the absence of tertiapin-Q (59 ± 15 pA, n = 5; Fig. 4C). Figure 4D summarizes the effects of Ca2+-free Krebs solution, glutamate receptor antagonists, Ba2+, GDP-β-S and tertiapin-Q on the slow IPSCs. These results suggest that the slow IPSCs are due to a direct activation of spinal GIRK channels.
Figure 3. Identification of the ionic mechanisms underlying slow IPSCs evoked by intraspinal stimulation.
A, a slow IPSC produced by repetitive stimuli focally applied to the dorsal horn, during the production of which a voltage pulse with an amplitude of 20 mV and a duration of 400 ms was applied at 0.5 Hz to the neurone. Note that the slow IPSC was associated with an increase in membrane conductance. VH = −50 mV. B, slow IPSCs produced by a repetitive stimulation given to the dorsal horn, recorded at various VH values. C, membrane currents in response to voltage pulses from −50 to −140 mV in steps of 10 mV before (left) and just after repetitive stimulation (right). D, membrane currents, which were plotted against membrane potentials; these were obtained before (▴) and just after a repetitive stimulation (•). The current–voltage relationship for net slow IPSCs (○) was estimated from the difference between the current responses before and just after a repetitive stimulation. Note that the polarity of the slow IPSC was reversed at about −100 mV, close to the equilibrium potential for K+, indicating an involvement of K+ channels.
Figure 4. Characterization of slow IPSCs evoked by intraspinal stimulation.
A, effect of the intracellular injection of GDP-β-S on slow IPSCs. Slow IPSCs were produced by repetitive stimuli focally applied to the dorsal horn just after (left) and 30 min (right) after establishing the whole-cell configuration using a patch-pipette solution containing GDP-β-S (2 mm). Note that the intracellular injection of GDP-β-S reduced the amplitude of slow IPSCs. B, effect of the K+ channel blocker Ba2+ on slow IPSCs. Repetitive stimuli were focally applied to the dorsal horn in the absence (left) and presence of Ba2+ (500 μm; right). Note that the slow IPSCs were largely decreased in amplitude and duration in the presence of Ba2+. C, effect of the GIRK channel blocker tertiapin-Q on slow IPSCs. Repetitive stimuli were focally applied to the dorsal horn in the absence (left) and presence of tertiapin-Q (0.1 μm; right). Note that the slow IPSCs were markedly decreased in amplitude and duration in the presence of tertiapin-Q. D, the average amplitudes of the slow IPSCs in normal and nominally Ca2+-free Krebs solution (n = 4), in the absence and presence of CNQX and AP5 (n = 4), Ba2+ (n = 6), tertiapin-Q (n = 5), as well as just after and 30 min after establishing the whole-cell configuration using a patch-pipette solution containing GDP-β-S (n = 4). *P < 0.05; n.s., not significant. VH = −50 mV.
Identification of the neurotransmitter mediating the slow IPSCs
Electrophysiological findings have provided evidence that several neurotransmitters, such as opioids, adenosine, noradrenaline, 5-HT and dopamine, directly induce an outward current at −50 mV in SG neurones (Yoshimura & North, 1983; Ito et al. 2000; Sonohata et al. 2004; Liu et al. 2004; Tamae et al. 2005; Fujita & Kumamoto, 2006). To identify whether these neurotransmitters mediate the slow IPSC elicited by repetitive stimuli focally applied to the dorsal horn, we examined the effects of antagonists of the receptors activated by these neurotransmitters on the slow IPSCs. Slow IPSCs were not affected by CTAP (1 μm), a μ-opioid receptor antagonist (data not shown). The average amplitude of the slow IPSCs was 40 ± 13 pA in the presence of CTAP, and this value was not significantly different from that in the absence of CTAP (42 ± 14 pA, n = 4; Table 1). Naloxone (1 μm), a non-specific opioid receptor antagonist, also did not affect the slow IPSCs (data not shown). The average amplitude of the slow IPSCs was 45 ± 5 pA in the presence of naloxone, and this value was not significantly different from that in the absence of naloxone (52 ± 5 pA, n = 11; Table 1). As well as opioid receptor antagonists, the slow IPSCs were not affected by selective antagonists of adenosine A1 receptor (DPCPX), GABAB receptor (CGP 35348, CGP 52432), α2-adrenoceptor (yohimbine), 5-HT1 receptor (WAY100635), 5-HT1A receptor (methiothepin), or dopamine D2-like receptor (sulpiride). Table 1 gives the average amplitudes of the slow IPSCs in the absence and presence of DPCPX (1 μm), CGP 35348 (50 μm), CGP 52432 (50 μm), yohimbine (1 μm), WAY100635 (10 μm), methiothepin (10 μm) and sulpiride (30 μm).
Table 1.
Effects of neurotransmitter-receptor antagonists on the slow IPSC
| − | + | ||||
|---|---|---|---|---|---|
| Receptor | Antagonist | Conc., μM | Amplitude, pA | Amplitude, pA | No. of Cells |
| Opioid | Naloxone | 1 | 52 ± 5 | 45 ± 5 | 11 |
| μ-Opioid | CTAP | 1 | 42 ± 14 | 40 ± 13 | 4 |
| Adenosine A1 | DPCPX | 1 | 51 ± 9 | 49 ± 9 | 6 |
| GABAB | CGP 35348 | 50 | 42 ± 2 | 42 ± 1 | 4 |
| GABAB | CGP 52432 | 50 | 54 ± 8 | 54 ± 8 | 6 |
| α2-Adrenergic | Yohimbine | 1 | 54 ± 8 | 47 ± 8 | 10 |
| 5-HT1 | Way100635 | 10 | 53 ± 13 | 54 ± 12 | 4 |
| 5-HT1A | Methiotepine | 10 | 52 ± 10 | 51 ± 9 | 5 |
| Dopamine D2-like | Sulpiride | 30 | 59 ± 9 | 60 ± 8 | 4 |
| Somatostatin | Cyclo-somatostatin | 5 | 69 ± 13 | 39 ± 11* | 5 |
− and +: the absence and presence of antagonists, respectively. Values are means ± s.e.m.
P < 0.05.
Recently, it has been demonstrated that somatostatin activates G-protein-coupled K+ channels, resulting in postsynaptic hyperpolarization in a subpopulation of SG neurones (Kim et al. 2002; Jiang et al. 2003). Therefore, we investigated whether the slow IPSCs were occluded by the somatostatin-induced outward current. Superfusing somatostatin (1 μm) generated an outward current in 5 out of 13 SG neurones recorded. A slow IPSC was produced by repetitive stimuli focally applied to the dorsal horn in all neurones in which somatostatin generated an outward current. During the somatostatin-induced outward current, the slow IPSC was reversibly reduced in amplitude (Fig. 5A). The average amplitude of the slow outward currents was 16 ± 3 pA during the action of somatostatin, and this value was significantly smaller than that before the action of somatostatin (68 ± 15 pA, n = 5; Fig. 5C). On the other hand, endomorphin-1 (1 μm) also induced an outward current in 5 out of 10 SG neurones examined, as previously reported (Fujita & Kumamoto, 2006), but the slow IPSCs were not significantly occluded by the endomorphin-1-induced outward current (Fig. 5B). Slow IPSCs were produced by repetitive stimulation in 4 out of 5 neurones in which endomorphin-1 generated an outward current. The average amplitude of the slow outward currents was 62 ± 8 pA during the action of endomorphin-1, and this value was not significantly different from that before the action of endomorphin-1 (70 ± 5 pA, n = 4; Fig. 5C). We further investigated the effect of an antagonist of somatostatin receptors, cyclo-somatostatin (Heppelmann & Pawlak, 1999), on the slow IPSCs induced by repetitive stimuli focally applied to the dorsal horn (Fig. 6A). The slow IPSCs were significantly reduced in amplitude by cyclo-somatostatin (5 μm) administered 5 min prior to the repetitive focal stimuli in a reversible manner, where this drug by itself did not affect holding membrane currents. The average amplitude of slow IPSCs was 39 ± 11 pA in the presence of cyclo-somatostatin; this value was significantly smaller than that in the absence of cyclo-somatostatin (69 ± 13 pA, n = 5; Fig. 6B; Table 1).
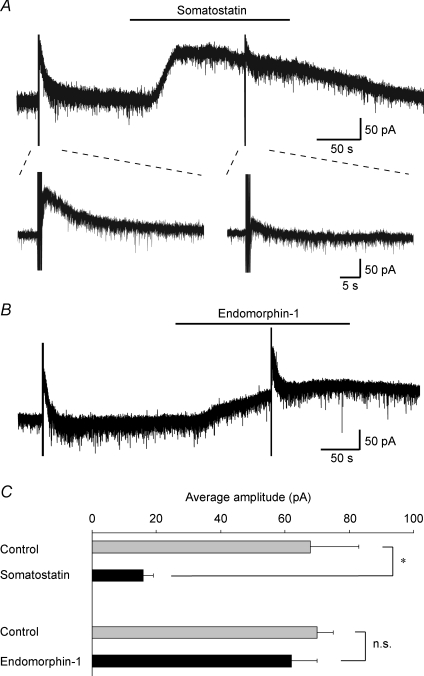
Figure 5. Slow IPSCs were occluded during outward currents induced by somatostatin but not endomorphin-1.
A, bath-applied somatostatin (1 μm) produced an outward current (upper). Slow IPSCs (lower left) were occluded during the somatostatin-induced outward current (lower right). The lower traces are shown in an expanded time scale before and during the application of somatostatin. B, bath-applied endomorphin-1 (1 μm) produced an outward current, during the production of which the slow IPSCs were not affected. C, the average amplitudes of the slow IPSCs before and during the application of somatostatin (n = 5) or endomorphin-1 (n = 4) *P < 0.05; n.s., not significant. VH = −50 mV.
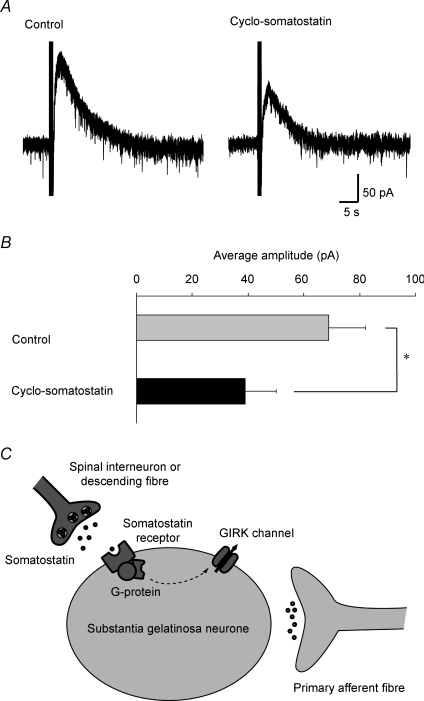
Figure 6. Effect of a somatostatin receptor antagonist on slow IPSCs and possible involvement of somatostatin in the slow IPSC.
A, effect of the somatostatin receptor antagonist cyclo-somatostatin on slow IPSCs. Repetitive stimuli were focally applied to the dorsal horn in the absence (left) and presence of cyclo-somatostatin (5 μm; right). Note that the slow IPSCs were inhibited by cyclo-somatostatin. B, the average amplitudes of the slow IPSCs before and during the application of cyclo-somatostatin (n = 5). *P < 0.05. VH = −50 mV. C, schematic diagram showing the activation of GIRK channels by somatostatin in SG neurones. Endogenous somatostatin released from spinal interneurones or descending fibres, but not from primary afferent fibres, activates GIRK channels via G-protein-coupled somatostatin receptors.
Discussion
In the present study, we investigated whether a slow synaptic current could be elicited by stimulation applied to the dorsal horn or the dorsal root by using whole-cell patch-clamp recordings from SG neurones. Slow synaptic responses were not generated by repetitive stimuli applied to the dorsal root, whereas slow IPSCs could be evoked by repetitive focal stimuli applied to the dorsal horn in about 30% of the neurones examined. The polarity of IPSCs was reversed at a potential close to the equilibrium potential for K+, calculated from the Nernst equation. Slow IPSCs were blocked by the addition of GDP-β-S into the patch-pipette solution and suppressed in the presence of an antagonist of GIRK channels, tertiapin-Q. Moreover, slow IPSCs were occluded during the somatostatin-induced outward current and inhibited by the somatostatin receptor antagonist, cyclo-somatostatin. In the present study, we first demonstrated that spinal GIRK channels could be functionally activated by an endogenous neurotransmitter.
Slow excitatory synaptic responses have been extensively studied in the spinal dorsal horn (Urbán & Randić, 1984; De Koninck & Henry, 1991; Yajiri et al. 1997). By contrast, slow inhibitory synaptic responses in the spinal dorsal horn are poorly understood. In the present study, slow IPSCs increased in amplitude and duration with an increase in the number of stimuli. In addition, slow IPSCs were largely decreased in amplitude and duration in a Ca2+-free solution. These results indicate that the slow IPSCs are generated by an endogenous neurotransmitter released from axon terminals whose cell bodies are located in the spinal dorsal horn or the brainstem. When examined by applying a voltage pulse to the neurone during the production of slow IPSCs, the membrane conductance increased. Moreover, slow IPSCs showed a reversal potential of about −95 mV, and were completely blocked by the addition of GDP-β-S to the patch-pipette solution. The perfusion of a non-selective K+ channel blocker, Ba2+, also inhibited slow IPSCs. These findings suggest that the slow IPSCs elicited by repetitive focal stimuli applied to the dorsal horn are mediated by the activation of G-protein-activated K+ channels in postsynaptic SG neurones.
Since the proposal of the gate-control theory by Melzack & Wall (1965), it has been thought that a modulation of synaptic transmission in the SG plays an important role in regulating nociceptive transmission. A variety of endogenous neurotransmitters released from neurones intrinsic to the dorsal horn and descending neurones arising from the brainstem, presynaptically or postsynaptically, can inhibit synaptic transmission in the SG (Willis & Coggeshall, 2004). μ-Opioid receptors have been detected in the superficial layers of the spinal cord, particularly the SG (Besse et al. 1990; Rahman et al. 1998). Opioids open one or more K+ channels by activating μ-opioid receptors, and thus hyperpolarize membranes of a subset of SG neurones (Yoshimura & North, 1983; Fujita & Kumamoto, 2006). Furthermore, several lines of evidence suggest that GIRK channels are coupled with μ-opioid receptors (Kobayashi et al. 1996), and that K+ currents activated by μ-opioid receptors in SG neurones exhibit GIRK-like characteristics (Schneider et al. 1998). However, the slow IPSCs elicited by repetitive focal stimuli applied to the dorsal horn in the present study were not affected by naloxone or CTAP. As well as opioids, several classical neurotransmitters, such as noradrenaline, 5-HT, dopamine and adenosine, are present in the spinal cord, and have been shown to produce hyperpolarizing responses in a subset of SG neurones (Ito et al. 2000; Sonohata et al. 2004; Liu et al. 2004; Tamae et al. 2005). However, the slow IPSCs were not affected by the adenosine A1 receptor antagonist DPCPX, the GABAB receptor antagonists CGP 35348 and CGP 52432, the α2-adrenoceptor antagonist yohimbine, the 5-HT1 receptor antagonist WAY100636, the 5-HT1A receptor antagonist methiothepin, or the dopamine D2-like receptor antagonist sulpiride, as well as CTAP and naloxone. Thus, opioids and known neurotransmitters are also unlikely to be responsible for the slow IPSCs elicited by repetitive focal stimuli applied to the dorsal horn.
Neuronal GIRK channels are homotetrameric or heterotetrameric complexes formed by GIRK1–3 subunits (Koyrakh et al. 2005). GIRK1 and 2 are expressed in the superficial layers of the spinal dorsal horn, and are enriched in the postsynaptic membranes of SG neurones (Marker et al. 2005). In spite of the broad distribution of GIRK3 mRNA in the brain (Karschin et al. 1996), there is little evidence to support a role for GIRK3 in the formation of GIRK channels in spinal dorsal horn neurones. The level of GIRK3 protein in the spinal cord is much lower than that in the brain (Marker et al. 2004). Therefore, GIRK channels mediating the inhibitory effects in the spinal cord are formed by GIRK1 and/or GIRK2 with little or no contribution from GIRK3. Interestingly, functional GIRK channels formed by GIRK1 and 2 are localized in excitatory interneurones in the SG (Marker et al. 2006). GIRK channels are also found in a subpopulation of SG neurones that responds to a selective agonist of GABAB receptors, but not to a selective agonist of μ-opioid receptors. Based on morphological analysis, C-fibres, but not Aδ-fibres, impinge primarily on GIRK-containing SG neurones (Marker et al. 2006). GIRK1 and 2 knock-out mice, as well as wild-type mice, given intrathecal injections of a selective antagonist of GIRK channels, exhibit thermal hyperalgesia and blunt analgesic responses to intrathecal morphine (Marker et al. 2005). These studies have suggested that spinal GIRK channels play an important role in thermal nociception and the analgesic action of morphine and other agents. Consistent with these findings, the present study using the whole-cell patch-clamp technique has revealed that slow IPSCs elicited by repetitive focal stimuli applied to the dorsal horn are mediated by the activation of GIRK channels in SG neurones. Moreover, slow IPSCs were significantly suppressed in the presence of an antagonist of GIRK channels, tertiapin-Q.
The present finding that somatostatin endogenously released by repetitive stimuli focally applied to the dorsal horn can play an important role in spinal antinociception raises another issue about the origin of the endogenous somatostatin. Somatostatin was originally discovered as a hypothalamic neuroendocrine hormone, which potently inhibits the secretion of growth hormone from the anterior pituitary gland (Brazeau et al. 1973). Somatostatin is widely distributed throughout the CNS, and exhibits a variety of physiological effects through its binding to G-protein-coupled somatostatin receptors (Schindler et al. 1996; Lahlou et al. 2004). In the spinal cord, somatostatin-immunoreactive cell bodies and nerve terminals are predominantly found in superficial layers of the dorsal horn, especially the SG (Mizukawa et al. 1988; Chung et al. 1989). As somatostatin-immunoreactive fibres from the hypothalamus project into the spinal cord (Krisch, 1981), these descending fibres may be a source of endogenous somatostatin. Somatostatin may also be released from primary afferent fibres, because its immunoreactivity is found in primary sensory neurones (Tessler et al. 1986). The immunoreactive somatostatin content of the lumbar dorsal horn is not affected by disruption of descending pathways by spinal transection, but partially reduced by unilateral lumbosacral dorsal rhizotomy, indicating that endogenous somatostatin may arise mainly from the dorsal root and spinal neurones in local segments (Tessler et al. 1986). However, repetitive stimuli applied to the dorsal root did not induce any slow responses in the present study. Therefore, these findings suggest that endogenous somatostatin may be released onto SG neurones from interneurones or descending fibres, but not from primary-afferent fibres, while somatostatin-positive primary-afferent fibres may innervate dorsal horn neurones in other laminae. To date, five somatostatin receptors have been cloned (Lahlou et al. 2004), a few of which are found in the soma and dendrites of SG neurones (Schulz et al. 1998). Recently, it was demonstrated that somatostatin directly hyperpolarizes membranes of a subset of SG neurones through the activation of GIRK channels (Kim et al. 2002). Somatostatin causes a reduction in the number of firings in spinal dorsal horn neurones in vitro (Murase et al. 1982), and the intrathecal administration of somatostatin produces antinociceptive effects in vivo (Mollenholt et al. 1994). In the present study, somatostatin induced an outward current in a subset of SG neurones, as reported previously (Kim et al. 2002; Jiang et al. 2003). The slow IPSCs elicited by repetitive focal stimuli applied to the dorsal horn were not affected by the endomorphin-1-induced outward current, but were significantly occluded by the somatostatin-induced outward current. Furthermore, the slow IPSCs were significantly inhibited by the somatostatin receptor antagonist cyclo-somatostatin. These results suggest that endogenous somatostatin released by repetitive focal stimuli applied to the dorsal horn directly acts on postsynaptic SG neurones to induce slow IPSCs via activation of GIRK channels through somatostatin receptors (Fig. 6C). As a selective antagonist for somatostatin receptors is not commercially available at this time, further investigations will be required to identify which subtype of somatostatin receptor is involved in the slow IPSCs.
Spinal cord stimulation is the most commonly used implantable neurostimulation modality for the management of pain syndromes. In an attempt to determine which neurotransmitters may be influenced by spinal cord stimulation, Meyerson et al. (1985) sampled the CSF for substance P, somatostatin, cholecystokinin, vasoactive intestinal polypeptide, neurotensin and monoamine metabolites in patients with spinal cord stimulation. The only neurotransmitter shown to be influenced by spinal cord stimulation was substance P. It was concluded that the lack of other changes may indicate that pain-related substances are released in very small amounts, rapidly metabolized, and therefore not detected by a single sample of CSF. Another experimental study suggested that spinal cord stimulation may affect GABAergic systems by activating GABA-containing inhibitory interneurones, and facilitated GABA release as a result of spinal cord stimulation may be responsible for the suppression of allodynia in neuropathic pain rat models (Cui et al. 1996). Baba et al. (1994) demonstrated that spinal cord stimulation inhibits nociceptive transmission by reducing neurotransmitter release from primary afferents and by hyperpolarizing the membranes of SG neurones. The decrease in neurotransmitter release from primary afferents was mediated by GABAA receptor-mediated presynaptic inhibition. Moreover, they found that a long-lasting slow inhibitory postsynaptic potential (IPSP) was produced through the activation of K+ channels. Although the neurotransmitter implicated in the slow IPSP by spinal cord stimulation has been uncertain, somatostatin is a possible candidate.
We conclude that endogenous somatostatin released from interneurones or descending fibres, but not from primary-afferent fibres, may induce slow IPSCs by activating GIRK channels in SG neurones. It is suggested that this slow synaptic transmission may play an important role in spinal antinociception. This finding may explain pathological pain sensations such as thermal hyperalgesia.
Acknowledgments
This work was supported by a grant from The General Insurance Association of Japan to T.N. and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to T.N. and K.I.
References
- Baba H, Yoshimura M, Nishi S, Shimoji K. Synaptic responses of substantia gelatinosa neurones to dorsal column stimulation in rat spinal cord in vitro. J Physiol. 1994;478:87–99. doi: 10.1113/jphysiol.1994.sp020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse D, Lombard MC, Zajac JM, Roques BP, Besson JM. Pre- and postsynaptic distribution of μ, δ and κ opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain Res. 1990;521:15–22. doi: 10.1016/0006-8993(90)91519-m. [DOI] [PubMed] [Google Scholar]
- Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Cervero F, Iggo A. The substantia gelatinosa of the spinal cord: a critical review. Brain. 1980;103:717–772. doi: 10.1093/brain/103.4.717. [DOI] [PubMed] [Google Scholar]
- Chung K, Briner RP, Carlton SM, Westlund KN. Immunohistochemical localization of seven different peptides in the human spinal cord. J Comp Neurol. 1989;280:158–170. doi: 10.1002/cne.902800111. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Neer EJ. New roles for G-protein βγ-dimers in transmembrane signalling. Nature. 1993;365:403–406. doi: 10.1038/365403a0. [DOI] [PubMed] [Google Scholar]
- Cui J-G, Linderoth B, Meyerson BA. Effects of spinal cord stimulation on touch-evoked allodynia involve GABAergic mechanisms. An experimental study in the mononeuropathic rat. Pain. 1996;66:287–295. doi: 10.1016/0304-3959(96)03069-2. [DOI] [PubMed] [Google Scholar]
- Dascal N. Signalling via the G protein-activated K+ channels. Cell Signal. 1997;9:551–573. doi: 10.1016/s0898-6568(97)00095-8. [DOI] [PubMed] [Google Scholar]
- De Koninck Y, Henry JL. Substance P-mediated slow excitatory postsynaptic potential elicited in dorsal horn neurons in vivo by noxious stimulation. Proc Natl Acad Sci U S A. 1991;88:11344–11348. doi: 10.1073/pnas.88.24.11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Kumamoto E. Inhibition by endomorphin-1 and endomorphin-2 of excitatory transmission in adult rat substantia gelatinosa neurons. Neuroscience. 2006;139:1095–1105. doi: 10.1016/j.neuroscience.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Heppelmann B, Pawlak M. Peripheral application of cyclo-somatostatin, a somatostatin antagonist, increases the mechanosensitivity of rat knee joint afferents. Neurosci Lett. 1999;259:62–64. doi: 10.1016/s0304-3940(98)00912-4. [DOI] [PubMed] [Google Scholar]
- Hille B. G protein-coupled mechanisms and nervous signaling. Neuron. 1992;9:187–195. doi: 10.1016/0896-6273(92)90158-a. [DOI] [PubMed] [Google Scholar]
- Ito A, Kumamoto E, Takeda M, Takeda M, Shibata K, Sagai H, Yoshimura M. Mechanisms for ovariectomy-induced hyperalgesia and its relief by calcitonin: participation of 5-HT1A-like receptor on C-afferent terminals in substantia gelatinosa of the rat spinal cord. J Neurosci. 2000;20:6302–6308. doi: 10.1523/JNEUROSCI.20-16-06302.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Voltage-gated and inwardly rectifying potassium channels. J Physiol. 1997;505:267–282. doi: 10.1111/j.1469-7793.1997.267bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, Furue H, Katafuchi T, Yoshimura M. Somatostatin directly inhibits substantia gelatinosa neurons in adult rat spinal dorsal horn in vitro. Neurosci Res. 2003;47:97–107. doi: 10.1016/s0168-0102(03)00183-4. [DOI] [PubMed] [Google Scholar]
- Jin W, Lu Z. A novel high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry. 1998;37:13291–13299. doi: 10.1021/bi981178p. [DOI] [PubMed] [Google Scholar]
- Karschin C, Dißmann E, Stühmer W, Karschin A. IRK(1–3) and GIRK(1–4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J Neurosci. 1996;16:3559–3570. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Chung WH, Rhim H, Eun S-Y, Jung SJ, Kim J. Postsynaptic action mechanism of somatostatin on the membrane excitability in spinal substantia gelatinosa neurons of juvenile rats. Neuroscience. 2002;114:1139–1148. doi: 10.1016/s0306-4522(02)00245-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ikeda K, Ichikawa T, Togashi S, Kumanishi T. Effects of sigma ligands on the cloned μ-, δ- and κ-opioid receptors co-expressed with G-protein-activated K+ (GIRK) channel in Xenopus oocytes. Br J Pharmacol. 1996;119:73–80. doi: 10.1111/j.1476-5381.1996.tb15679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyrakh L, Luján R, Colón J, Karschin C, Kurachi Y, Karschin A, Wickman K. Molecular and cellular diversity of neuronal G-protein-gated potassium channels. J Neurosci. 2005;25:11468–11478. doi: 10.1523/JNEUROSCI.3484-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisch B. Somatostatin-immunoreactive fiber projections into the brain stem and the spinal cord of the rat. Cell Tissue Res. 1981;217:531–552. doi: 10.1007/BF00219362. [DOI] [PubMed] [Google Scholar]
- Kumazawa T, Perl ER. Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J Comp Neurol. 1978;177:417–434. doi: 10.1002/cne.901770305. [DOI] [PubMed] [Google Scholar]
- Lahlou H, Guillermet J, Hortala M, Vernejoul F, Pyronnet S, Bousquet C, Susini C. Molecular signaling of somatostatin receptors. Ann N Y Acad Sci. 2004;1014:121–131. doi: 10.1196/annals.1294.012. [DOI] [PubMed] [Google Scholar]
- Liu T, Fujita T, Kawasaki Y, Kumamoto E. Regulation by equilibrative nucleoside transporter of adenosine outward currents in adult rat spinal dorsal horn neurons. Brain Res Bull. 2004;64:75–83. doi: 10.1016/j.brainresbull.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Logothetis DE, Lupyan D, Rosenhouse-Dantsker A. Diverse Kir modulators act in close proximity to residues implicated in phosphoinositide binding. J Physiol. 2007;582:953–965. doi: 10.1113/jphysiol.2007.133157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark MD, Herlitze S. G-protein mediated gating of inward-rectifier K+ channels. Eur J Biochem. 2000;267:5830–5836. doi: 10.1046/j.1432-1327.2000.01670.x. [DOI] [PubMed] [Google Scholar]
- Marker CL, Luján R, Colón J, Wickman K. Distinct populations of spinal cord lamina II interneurons expressing G-protein-gated potassium channels. J Neurosci. 2006;26:12251–12259. doi: 10.1523/JNEUROSCI.3693-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker CL, Luján R, Loh HH, Wickman K. Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of μ- and δ- but not κ-opioids. J Neurosci. 2005;25:3551–3559. doi: 10.1523/JNEUROSCI.4899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker CL, Stoffel M, Wickman K. Spinal G-protein-gated K+ channels formed by GIRK1 and GIRK2 subunits modulate thermal nociception and contribute to morphine analgesia. J Neurosci. 2004;24:2806–2812. doi: 10.1523/JNEUROSCI.5251-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Meyerson BA, Brodin E, Linderoth B. Possible neurohumoral mechanisms in CNS stimulation for pain suppression. Appl Neurophysiol. 1985;48:175–180. doi: 10.1159/000101124. [DOI] [PubMed] [Google Scholar]
- Mizukawa K, Otsuka N, McGeer PL, Vincent SR, McGeer EG. The ultrastructure of somatostatin-immunoreactive cell bodies, nerve fibers and terminals in the dorsal horn of rat spinal cord. Arch Histol Cytol. 1988;51:443–452. doi: 10.1679/aohc.51.443. [DOI] [PubMed] [Google Scholar]
- Mollenholt P, Rawal N, Gordh T, Jr, Olsson Y. Intrathecal and epidural somatostatin for patients with cancer. Analgesic effects and postmortem neuropathologic investigations of spinal cord and nerve roots. Anesthesiology. 1994;81:534–542. doi: 10.1097/00000542-199409000-00004. [DOI] [PubMed] [Google Scholar]
- Murase K, Nedeljkov V, Randić M. The actions of neuropeptides on dorsal horn neurons in the rat spinal cord slice preparation: an intracellular study. Brain Res. 1982;234:170–176. doi: 10.1016/0006-8993(82)90483-8. [DOI] [PubMed] [Google Scholar]
- Nakatsuka T, Ataka T, Kumamoto E, Tamaki T, Yoshimura M. Alteration in synaptic inputs through C-afferent fibers to substantia gelatinosa neurons of the rat spinal dorsal horn during postnatal development. Neuroscience. 2000;99:549–556. doi: 10.1016/s0306-4522(00)00224-4. [DOI] [PubMed] [Google Scholar]
- Nakatsuka T, Park J-S, Kumamoto E, Tamaki T, Yoshimura M. Plastic changes in sensory inputs to rat substantia gelatinosa neurons following peripheral inflammation. Pain. 1999;82:39–47. doi: 10.1016/S0304-3959(99)00037-8. [DOI] [PubMed] [Google Scholar]
- Rahman W, Dashwood MR, Fitzgerald M, Aynsley-Green A, Dickenson AH. Postnatal development of multiple opioid receptors in the spinal cord and development of spinal morphine analgesia. Brain Res Dev Brain Res. 1998;108:239–254. doi: 10.1016/s0165-3806(98)00054-6. [DOI] [PubMed] [Google Scholar]
- Sadja R, Alagem N, Reuveny E. Gating of GIRK channels: details of an intricate, membrane-delimited signaling complex. Neuron. 2003;39:9–12. doi: 10.1016/s0896-6273(03)00402-1. [DOI] [PubMed] [Google Scholar]
- Schindler M, Humphrey PPA, Emson PC. Somatostatin receptors in the central nervous system. Prog Neurobiol. 1996;50:9–47. doi: 10.1016/0301-0082(96)00030-5. [DOI] [PubMed] [Google Scholar]
- Schneider SP, Eckert WA, III, Light AR. Opioid activated postsynaptic, inward rectifying potassium currents in whole cell recordings in substantia gelatinosa neurons. J Neurophysiol. 1998;80:2954–2962. doi: 10.1152/jn.1998.80.6.2954. [DOI] [PubMed] [Google Scholar]
- Schulz S, Schreff M, Schmidt H, Händel M, Przewlocki R, Höllt V. Immunocytochemical localization of somatostatin receptor sst2A in the rat spinal cord and dorsal root ganglia. Eur J Neurosci. 1998;10:3700–3708. doi: 10.1046/j.1460-9568.1998.00386.x. [DOI] [PubMed] [Google Scholar]
- Sonohata M, Furue H, Katafuchi T, Yasaka T, Doi A, Kumamoto E, Yoshimura M. Actions of noradrenaline on substantia gelatinosa neurones in the rat spinal cord revealed by in vivo patch recording. J Physiol. 2004;555:515–526. doi: 10.1113/jphysiol.2003.054932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y, Lee CL, Perl ER. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986;234:358–361. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- Tamae A, Nakatsuka T, Koga K, Kato G, Furue H, Katafuchi T, Yoshimura M. Direct inhibition of substantia gelatinosa neurones in the rat spinal cord by activation of dopamine D2-like receptors. J Physiol. 2005;568:243–253. doi: 10.1113/jphysiol.2005.091843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessler A, Himes BT, Gruber-Bollinger J, Reichlin S. Characterization of forms of immunoreactive somatostatin in sensory neuron and normal and deafferented spinal cord. Brain Res. 1986;370:232–240. doi: 10.1016/0006-8993(86)90478-6. [DOI] [PubMed] [Google Scholar]
- Urbán L, Randić M. Slow excitatory transmission in rat dorsal horn: possible mediation by peptides. Brain Res. 1984;290:336–341. doi: 10.1016/0006-8993(84)90952-1. [DOI] [PubMed] [Google Scholar]
- Willis WD, Jr, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. 3. New York: Kluwer Academic / Plenum Publishers; 2004. [Google Scholar]
- Yajiri Y, Yoshimura M, Okamoto M, Takahashi H, Higashi H. A novel slow excitatory postsynaptic current in substantia gelatinosa neurons of the rat spinal cord in vitro. Neuroscience. 1997;76:673–688. doi: 10.1016/s0306-4522(96)00291-6. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell TM. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989;62:96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, North RA. Substantia gelatinosa neurones hyperpolarized in vitro by enkephalin. Nature. 1983;305:529–530. doi: 10.1038/305529a0. [DOI] [PubMed] [Google Scholar]