Background
Cardiac fibroblasts have been implicated in arrhythmia initiation and maintenance affecting electrical propagation through slow, discontinuous conduction. Fibroblasts are unexcitable cells, their resting membrane potential is more depolarized than that of myocytes and their membrane resistance is higher. These characteristics suggest that, if coupled to myocytes, fibroblasts may function as current sinks and/or sources for electrical charge and as short- and long-range conductors. Abnormal accumulation of fibroblasts occurs in the heart under pathological conditions and ageing, which may alter normal cardiac function. Most clinical, experimental and numerical studies have regarded fibroblasts and fibrosis as electrically insulating obstacles. However, recent suggest that when coupled to cardiac myocytes, fibroblasts could influence impulse propagation not only by acting as passive transducers but also by influencing excitability of neighbouring myocytes (Miragoli et al. 2007).
Compared to the atria and ventricles, the sinoatrial (SA) node contains a relatively higher amount of fibroblasts, which can be electrically coupled to pacemaker cells. Electrical coupling through gap junctions in the SA node is important for achieving pacemaker synchronization (Jalife, 1984). Pacemaker cells in the intact SA node beat at a common frequency but when isolated they show large variations in intrinsic beating frequency. Functional electrical coupling of SA nodal pacemaker cells through connexins forming gap junctions has been demonstrated in several species. However, the connexin isotypes expressed in the SA node vary somewhat depending on the species. Cx43, the most abundant connexin in the heart, has also been found in the SA node of rabbit, hamster and guinea pig although it is less abundant than other connexins. Interestingly, Cx43 was not detected in rat, cow or human nodal tissue. Recently, Kreuzberg et al. (2005) demonstrated that the mouse SA node expresses Cx30.2. Further studies using Cx30.2-knock-out mice suggest that Cx30.2 provides higher intercellular resistance and slower conductance due to a relatively small unitary conductance (∼9 pS). Nevertheless, the role of gap junctions in heterocellular tissue in the SA node remains unknown. The study by Fahrenbach et al. (2007) provides indirect evidence toward addressing this question. They investigated the mechanism through which non-excitable cells influence the spontaneous activity of pacemaker cells using heterocellular monolayers of HL-1 cells, a cell line derived from murine atrial myocyte tumour lineage AT-1, and fibroblasts that were isolated from neonatal rat and mouse ventricular tissue. The influence of fibroblasts on frequency of spontaneous activity, conduction velocity and interbeat interval variability were studied by co-culturing fibroblasts and myocytes at varying ratios.
Results
Fahrenbach et al. (2007) found that increasing the ratio of fibroblasts to myocytes reduced the frequency of spontaneous activity and the conduction velocity, concomitant with the increased interbeat interval variability. In addition, myocyte resting membrane potentials were depolarized in the presence of fibroblasts when compared to homocellular myocyte monolayers (−42 versus −65 mV, respectively). Hence, the results of Fahrenbach et al. (2007) support the observations of Miragoli et al. (2007) by demonstrating that an increase in non-excitable fibroblasts in cardiac cell cultures can modulate electrical properties of cardiac myocytes, including pacemaker activity.
In an effort to determine the basis of heterocellular coupling in their monolayers, Fahrenbach et al. (2007) also conducted immunostaining studies. The authors demonstrated expression of Cx40, Cx43 and Cx45 between fibroblasts and HL-1 cells, and functional coupling was confirmed using calcein-AM-dye-transfer experiments.
To further investigate the role of intercellular communication via gap junctions in the heterocellular monolayers, they co-cultured the fibroblasts derived from Cx43-deficient mice (Cx43−/−) with HL-1 cells. Under these conditions, increasing the ratio of fibroblasts did not reduce conduction velocity as dramatically as was seen with the WT fibroblasts. In contrast, the frequency of spontaneous activity was further reduced and the interbeat variability was increased when coupling was reduced between fibroblasts and HL-1 cells.
From the foregoing, the investigators concluded that fibroblasts can modulate pacemaker excitability by two mechanisms: the first mechanism is dependent on heterocellular coupling, in which fibroblasts depolarize the cardiomyocytes, and thus inactivate the sodium current (INa), which results in slowed propagation. The authors also proposed that when heterocellular coupling is absent/reduced, the more physical separation of cardiomyocytes by fibroblasts induces bradycardia through a reduction in an intrinsic entrainment mechanism, which has been proposed earlier to explain the synchronous activation of cardiac pacemaker cells (Jalife, 1984).
Discussion
The study by Fahrenbach et al. (2007) nicely demonstrates that the electrical interaction between fibroblasts and myocytes in co-cultured monolayers reduces the spontaneous beating frequency of the latter and increases interbeat interval variability in a ratio-dependent but coupling-independent manner. In addition, it provides valuable confirmatory evidence that fibroblasts act as obstacles to electrical propagation, and that when differentiated in culture can act as current sinks due to electrotonic coupling (Miragoli et al. 2007). Moreover, the authors make use of an appropriate system that allows for the study of electrical propagation patterns without the confounding factors introduced by thick 3D preparations.
Although relevant to the propagation of the cardiac impulse in the diseased ventricular myocardium, care should be taken before attempting to extrapolate these results to the SA node. HL-1 cell activation depends significantly on the rapid sodium current, which in the SA nodal region has been shown to be relevant only to the periphery. It is well-known that the L-type calcium current (ICa-L) is responsible for the upstroke in the centre of the SA node, where the dominant pacemaker region lies. Also, evidence shows that INa, although present in the newborn rabbit SA node, disappears after 40 days of birth.
Mutual entrainment, as a mechanism for firing synchronization in the SA node, involves a ‘democratic’ process in which each pacemaker cell contributes to an aggregate signal (Jalife, 1984). This implies that the cells must be coupled in order to achieve a consensus frequency of activation. Conversely, pacemaker cell isolation (the physical separation of cardiomyocytes by Cx43-deficient fibroblasts) would interfere with synchronization since the ability of neighbouring pacemaker cells to mutually entrain through electrotonic coupling would be compromised. Thus, one would expect that if two individual pacemaker cells are separated by Cx43-deficient fibroblasts, each cell would pace at its own independent intrinsic frequency, which may be faster or slower than when the cells are coupled through functional connexins, rather than undergoing bradycardia. Thus, the interpretation that separation of cardiomyocytes by fibroblasts induces bradycardia through a reduction in an intrinsic entrainment mechanism needs re-evaluation (Fig. 1).
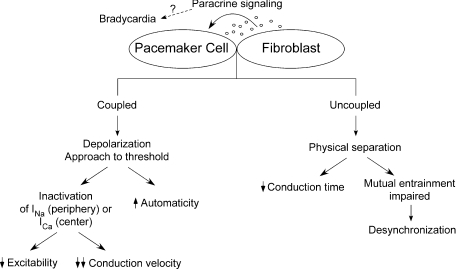
Figure 1. Schematic illustrating possible ways of how fibroblasts can affect pacemaking.
Heterocellular electrical coupling may lead to depolarization-induced automaticity or a reduction in excitability and conduction velocity by inactivating inward currents (i.e. INa and ICa). If uncoupled, fibroblasts would act primarily as insulating barriers between pacemaker cells. As a consequence, conduction time increases as the electrical wavefront encounters non-excitable ‘obstacles’. Moreover, mutual entrainment would be impaired leading to desynchronization. Paracrine signalling may be one of the mechanisms of fibroblast-induced bradycardia.
Deletion of the gene responsible for Cx43 in the mouse heart resulted in the significant conduction delay in the ventricles (Gutstein et al. 2001). However, the pacemaker activity in Cx43−/− mice remains unknown. In the studies of Fahrenbach et al. (2007) the role of electrical coupling in heterocellular tissue was studied using fibroblasts derived from Cx43−/− mice. The authors found that intercellular coupling through gap junctions is involved in a reduction in conduction velocity in the presence of fibroblasts. One should keep in mind, however, that Cx43 is absent or less abundant in the central SA nodal region of some species including human (Opthof, 1994). In particular, gap junctions expressed in mouse SA node tissue are mainly Cx30.2 and Cx45 that have a relatively small unitary conductance compared to other cardiac connexins (i.e. Cx40 and Cx43). Cx30.2-deficient mice demonstrated an accelerated conduction velocity (Kreuzberg et al. 2005), suggesting that electrical coupling via Cx30.2 provides high-resistance which shortens the space constant. Thus, caution should be exerted when interpreting these results that use HL-1 cells (derived from mice atrial tissue).
The role of cardiac fibroblasts in influencing the electrophysiological properties in the normal and pathophy siologically compromised myocardium is an important topic to study. Thus, these studies provide new insights of the role of heterocellular electrical coupling via gap junctions expressed in non-excitable cells. Furthermore, the work by Fahrenbach et al. (2007) represents an excellent start in our attempt to understand the complex and multiple ways in which the initiation and propagation of the cardiac electrical impulse is modulated via fibroblasts.
Acknowledgments
This work was supported by NHLBI Grants PO1-HL039707, RO1-HL070074 and RO1-HL080159.
References
- Fahrenbach JP, Mejia-Alvarez R, Banach K. The relevance of non-excitable cells for cardiac pacemaker function. J Physiol. 2007;585:565–578. doi: 10.1113/jphysiol.2007.144121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–339. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalife J. Mutual entrainment and electrical coupling as mechanisms for synchronous firing of rabbit sino-atrial pace-maker cells. J Physiol. 1984;356:221–243. doi: 10.1113/jphysiol.1984.sp015461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzberg MM, Sohl G, Kim JS, Verselis VK, Willecke K, Bukauskas FF. Functional properties of mouse connexin30.2 expressed in the conduction system of the heart. Circ Res. 2005;96:1169–1177. doi: 10.1161/01.RES.0000169271.33675.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res. 2007;101:755–758. doi: 10.1161/CIRCRESAHA.107.160549. [DOI] [PubMed] [Google Scholar]
- Opthof T. Gap junctions in the sinoatrial node: immunohistochemical localization and correlation with activation pattern. J Cardiovasc Electrophysiol. 1994;5:138–143. doi: 10.1111/j.1540-8167.1994.tb01153.x. [DOI] [PubMed] [Google Scholar]