Abstract
Perfusion to exercising skeletal muscle is regulated to match O2 delivery to the O2 demand, but this regulation might be compromised during or approaching maximal whole-body exercise as muscle blood flow for a given work rate is blunted. Whether muscle perfusion is restricted when there is an extreme metabolic stimulus to vasodilate during supramaximal exercise remains unknown. To examine the regulatory limits of systemic and muscle perfusion in exercising humans, we measured systemic and leg haemodynamics, O2 transport, and 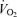 , and estimated non-locomotor tissue perfusion during constant load supramaximal cycling (498 ± 16 W; 110% of peak power; mean ± s.e.m.) in addition to both incremental cycling and knee-extensor exercise to exhaustion in 13 trained males. During supramaximal cycling, cardiac output (
, and estimated non-locomotor tissue perfusion during constant load supramaximal cycling (498 ± 16 W; 110% of peak power; mean ± s.e.m.) in addition to both incremental cycling and knee-extensor exercise to exhaustion in 13 trained males. During supramaximal cycling, cardiac output ( ), leg blood flow (LBF), and systemic and leg O2 delivery and
), leg blood flow (LBF), and systemic and leg O2 delivery and 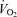 reached peak values after 60–90 s and thereafter levelled off at values similar to or ∼6% (P < 0.05) below maximal cycling, while upper body blood flow remained unchanged (∼5.5 l min−1). In contrast,
reached peak values after 60–90 s and thereafter levelled off at values similar to or ∼6% (P < 0.05) below maximal cycling, while upper body blood flow remained unchanged (∼5.5 l min−1). In contrast,  and LBF increased linearly until exhaustion during one-legged knee-extensor exercise accompanying increases in non-locomotor tissue blood flow to ∼12 l min−1. At exhaustion during cycling compared to knee-extensor exercise,
and LBF increased linearly until exhaustion during one-legged knee-extensor exercise accompanying increases in non-locomotor tissue blood flow to ∼12 l min−1. At exhaustion during cycling compared to knee-extensor exercise,  , LBF, leg vascular conductance, leg O2 delivery and leg
, LBF, leg vascular conductance, leg O2 delivery and leg  for a given power were reduced by 32–47% (P < 0.05). In conclusion, locomotor skeletal muscle perfusion is restricted during maximal and supramaximal whole–body exercise in association with a plateau in
for a given power were reduced by 32–47% (P < 0.05). In conclusion, locomotor skeletal muscle perfusion is restricted during maximal and supramaximal whole–body exercise in association with a plateau in 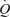 and limb vascular conductance. These observations suggest that limits of cardiac function and muscle vasoconstriction underlie the inability of the circulatory system to meet the increasing metabolic demand of skeletal muscles and other tissues during whole-body exercise.
and limb vascular conductance. These observations suggest that limits of cardiac function and muscle vasoconstriction underlie the inability of the circulatory system to meet the increasing metabolic demand of skeletal muscles and other tissues during whole-body exercise.
During exercise, an important function of the cardiovascular system is to supply O2 to the active muscles according to the demand. Studies in humans and animals which have examined small muscle mass exercise and submaximal whole-body exercise indicate that skeletal muscle perfusion is tightly regulated to preserve a close match between O2 demand and supply (Andersen & Saltin, 1985; Rowell et al. 1986; Koskolou et al. 1997; Delp & Laughlin, 1998; González-Alonso et al. 2002). This precise regulation, however, might be compromised during maximal whole-body exercise, as leg blood flow (LBF) for a given work rate appears to be blunted during incremental and constant maximal cycling in parallel to a plateau or a drop in cardiac output ( ) and vascular conductance (Knight et al. 1992; González-Alonso & Calbet, 2003; Mortensen et al. 2005). Whether a mismatch between muscle perfusion and metabolism is a general feature of exercise at maximal effort remains to be conclusively established.
) and vascular conductance (Knight et al. 1992; González-Alonso & Calbet, 2003; Mortensen et al. 2005). Whether a mismatch between muscle perfusion and metabolism is a general feature of exercise at maximal effort remains to be conclusively established.
A disparity between O2 supply and demand in the active muscles due to restrictions in blood flow could limit maximal O2 uptake ( ) independently of any concomitant effects of systemic haemodynamics or central motor drive because of the inevitable reductions in muscle metabolism and force production when the compensatory adjustments in muscle O2 extraction are exhausted (Hogan et al. 1996; Saltin & Calbet, 2006; Lanza et al. 2006; Noakes & Marino, 2007). An unsolved question is whether the circulatory system reaches its regulatory capacity when humans perform maximal exercise such that muscle perfusion,
) independently of any concomitant effects of systemic haemodynamics or central motor drive because of the inevitable reductions in muscle metabolism and force production when the compensatory adjustments in muscle O2 extraction are exhausted (Hogan et al. 1996; Saltin & Calbet, 2006; Lanza et al. 2006; Noakes & Marino, 2007). An unsolved question is whether the circulatory system reaches its regulatory capacity when humans perform maximal exercise such that muscle perfusion,  , vascular conductance and thus O2 delivery do not increase further when exercise is performed at an intensity above
, vascular conductance and thus O2 delivery do not increase further when exercise is performed at an intensity above  (supramaximal exercise). There are three possible scenarios during supramaximal exercise: (i) muscle blood flow and vascular conductance and thus muscle O2 supply are restrained via increases in sympathetic vasoconstrictor activity overriding metabolic vasodilatation to defend blood pressure regulation (Joyner et al. 1992; Calbet et al. 2004; Rowell, 2004), (ii) vasoconstriction restricts muscle perfusion in response to a levelling off or decline in
(supramaximal exercise). There are three possible scenarios during supramaximal exercise: (i) muscle blood flow and vascular conductance and thus muscle O2 supply are restrained via increases in sympathetic vasoconstrictor activity overriding metabolic vasodilatation to defend blood pressure regulation (Joyner et al. 1992; Calbet et al. 2004; Rowell, 2004), (ii) vasoconstriction restricts muscle perfusion in response to a levelling off or decline in 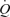 , rather than a pressure error signal, and (iii) muscle perfusion is closely coupled to the metabolic demand via augmented metabolic vasodilator activity offsetting sympathetic activation (i.e. functional sympatholysis; Rosenmeier et al. 2004). The similar
, rather than a pressure error signal, and (iii) muscle perfusion is closely coupled to the metabolic demand via augmented metabolic vasodilator activity offsetting sympathetic activation (i.e. functional sympatholysis; Rosenmeier et al. 2004). The similar 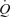 and
and  during supramaximal and maximal cycling suggests that perfusion to active muscles might be limited during high-intensity whole-body exercise (Mitchell et al. 1958; Åstrand & Saltin, 1961; Nummela & Rusko, 1995; Perrey et al. 2002; Thomas et al. 2005; Brink-Elfegoun et al. 2007; Hawkins et al. 2007). To date, no study has compared leg and systemic haemodynamic responses to supramaximal and maximal exercise to gain insight into the regulatory limits of muscle and systemic perfusion in exercising humans and their influence on
during supramaximal and maximal cycling suggests that perfusion to active muscles might be limited during high-intensity whole-body exercise (Mitchell et al. 1958; Åstrand & Saltin, 1961; Nummela & Rusko, 1995; Perrey et al. 2002; Thomas et al. 2005; Brink-Elfegoun et al. 2007; Hawkins et al. 2007). To date, no study has compared leg and systemic haemodynamic responses to supramaximal and maximal exercise to gain insight into the regulatory limits of muscle and systemic perfusion in exercising humans and their influence on  .
.
The main aims of this study were to determine (1) whether systemic and locomotor muscle blood flow are restrained during supramaximal compared to maximal exercise despite an extreme metabolic stimulus to vasodilate, (2) whether a limited O2 supply prior to exhaustion impairs leg 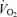 during supramaximal and maximal cycling, and (3) whether
during supramaximal and maximal cycling, and (3) whether  for a given work rate is restricted during supramaximal and maximal cycling compared to maximal incremental knee-extensor exercise. To accomplish these aims, we measured leg and systemic haemodynamics during incremental and supramaximal cycling as well as during incremental knee-extensor exercise in trained male subjects. We hypothesized that locomotor limb muscle perfusion is restrained during supramaximal cycling despite greater metabolic stimulus to vasodilate and that O2 delivery and
for a given work rate is restricted during supramaximal and maximal cycling compared to maximal incremental knee-extensor exercise. To accomplish these aims, we measured leg and systemic haemodynamics during incremental and supramaximal cycling as well as during incremental knee-extensor exercise in trained male subjects. We hypothesized that locomotor limb muscle perfusion is restrained during supramaximal cycling despite greater metabolic stimulus to vasodilate and that O2 delivery and  per unit of power are restrained during intense cycling compared to maximal knee-extensor exercise. Further, we hypothesized that the restricted active muscle blood flow during high-intensity whole-body exercise is associated with a plateau in limb muscle vascular conductance.
per unit of power are restrained during intense cycling compared to maximal knee-extensor exercise. Further, we hypothesized that the restricted active muscle blood flow during high-intensity whole-body exercise is associated with a plateau in limb muscle vascular conductance.
Methods
Thirteen trained male subjects with a mean (±s.d.) age of 28 ± 6 years, body weight of 84.7 ± 9.9 kg, height of 188 ± 8 cm and  of 4.7 ± 0.2 l min−1 (56.7 ± 9.8 ml kg−1 min−1) participated in this study. The subjects were informed of any risks and discomforts associated with the experiments before providing their written consent to participate. The study was approved by the Ethics Committee of Copenhagen and Frederiksberg communities (KF j. no. 01-320) and conducted in accordance with the guidelines of the Declaration of Helsinki.
of 4.7 ± 0.2 l min−1 (56.7 ± 9.8 ml kg−1 min−1) participated in this study. The subjects were informed of any risks and discomforts associated with the experiments before providing their written consent to participate. The study was approved by the Ethics Committee of Copenhagen and Frederiksberg communities (KF j. no. 01-320) and conducted in accordance with the guidelines of the Declaration of Helsinki.
On the first two visits to the laboratory, the subjects performed incremental one-legged knee-extensor exercise and incremental and supramaximal cycling (Excalibur, Lode, the Netherlands) to determine  , peak power and time to exhaustion. On the day of the experiment, the subjects arrived at the laboratory after a light breakfast. Catheters were placed into the brachial artery and an anticubital vein, with the latter catheter being advanced to the right atrium (n = 10) or pulmonary artery (n = 3). The 10 subjects with right atrium catheters participated in a study by González-Alonso et al. (2006) and were examined two days after reinfusion of 0.84 ± 0.16 l (range 0.66–1.02 l) of packed red blood cells. In all subjects, a third catheter was inserted into the femoral vein ∼2 cm from the inguinal ligament to allow for blood sampling and measurements of LBF by the constant-infusion thermodilution method (Andersen & Saltin, 1985; González-Alonso et al. 2000).
, peak power and time to exhaustion. On the day of the experiment, the subjects arrived at the laboratory after a light breakfast. Catheters were placed into the brachial artery and an anticubital vein, with the latter catheter being advanced to the right atrium (n = 10) or pulmonary artery (n = 3). The 10 subjects with right atrium catheters participated in a study by González-Alonso et al. (2006) and were examined two days after reinfusion of 0.84 ± 0.16 l (range 0.66–1.02 l) of packed red blood cells. In all subjects, a third catheter was inserted into the femoral vein ∼2 cm from the inguinal ligament to allow for blood sampling and measurements of LBF by the constant-infusion thermodilution method (Andersen & Saltin, 1985; González-Alonso et al. 2000).
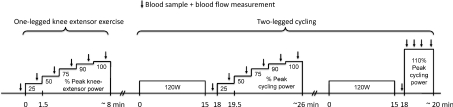
Following 30 min of rest, the subjects performed incremental one-legged knee-extensor exercise to exhaustion, followed by incremental and supramaximal two-legged cycling to exhaustion separated by 60 min of recovery (Fig. 1). The cycling trials were preceded by 15 min of submaximal exercise (120 W; < 50% ) and 3 min of rest. During incremental knee-extensor exercise and cycling, the workload was increased every 1.5 min to elicit 25%, 50%, 75%, 90% and 100% of peak power, whereas during supramaximal cycling the workload was increased during the first 20 s of exercise to elicit 110% of peak power (498 ± 16 W). The order of the two cycling trials was randomised.
) and 3 min of rest. During incremental knee-extensor exercise and cycling, the workload was increased every 1.5 min to elicit 25%, 50%, 75%, 90% and 100% of peak power, whereas during supramaximal cycling the workload was increased during the first 20 s of exercise to elicit 110% of peak power (498 ± 16 W). The order of the two cycling trials was randomised.
Figure 1. Sequence of the experimental protocol.
Subjects first performed incremental one-legged knee-extensor exercise to exhaustion. Subsequently, they performed either incremental or constant supramaximal cycling to exhaustion following a 15 min warm-up (120 W) and 3 min of seated rest. The order of the two cycling trials was randomized and they were separated by 60 min of rest. The incremental knee-extensor and cycling trials were separated by 30 min of rest. Blood samples and blood flow measurements were obtained at rest and during exercise.
During both incremental knee-extension and incremental cycling, blood samples (1–5 ml) were drawn simultaneously from the brachial artery, right atrium or pulmonary artery, and femoral artery before the start of exercise, after 0.75 min on each workload and before exhaustion, while LBF was measured before the start of exercise, after 1 min at each workload and before exhaustion. During supramaximal cycling, blood samples were drawn before the start of exercise, after 30 s, after 60 s and before exhaustion, while LBF was measured before the start of exercise, after 45 s, after 75 s and before exhaustion.
Exercise was performed at ∼22°C with a fan directed against the back of the subjects. Pulmonary  was measured online (Quark b2 system, Cosmed, Italy). Heart rate (HR) was obtained from an electrocardiogram while arterial pressue, pulmonary artery mean pressure (PAMP) and central venous pressure were monitored with transducers (Pressure Monitoring Kit, Baxter, Deerfield, IL, USA) positioned at the level of the heart and connected to a haemodynamic monitor (Dialogue 2000, Danica Elektronic, Copenhagen, Denmark). Blood gas variables and haemoglobin, glucose and lactate concentrations were measured using an ABL725 analyser (Radiometer, Copenhagen, Denmark) and were corrected to the temperature of the femoral vein.
was measured online (Quark b2 system, Cosmed, Italy). Heart rate (HR) was obtained from an electrocardiogram while arterial pressue, pulmonary artery mean pressure (PAMP) and central venous pressure were monitored with transducers (Pressure Monitoring Kit, Baxter, Deerfield, IL, USA) positioned at the level of the heart and connected to a haemodynamic monitor (Dialogue 2000, Danica Elektronic, Copenhagen, Denmark). Blood gas variables and haemoglobin, glucose and lactate concentrations were measured using an ABL725 analyser (Radiometer, Copenhagen, Denmark) and were corrected to the temperature of the femoral vein.  was calculated using the Fick principle, assuming negligible differences in blood oxygenation between the right atrium and pulmonary artery in the 10 subjects with the catheter in the right atrium (Baratt-Boyes & Wood, 1957). In three subjects a pulmonary artery catheter was used. Stroke volume (SV) was the quotient between
was calculated using the Fick principle, assuming negligible differences in blood oxygenation between the right atrium and pulmonary artery in the 10 subjects with the catheter in the right atrium (Baratt-Boyes & Wood, 1957). In three subjects a pulmonary artery catheter was used. Stroke volume (SV) was the quotient between  and HR and systemic vascular conductance was that between
and HR and systemic vascular conductance was that between  and perfusion pressure. To estimate perfusion pressure, mean arterial pressure (MAP) was corrected for the hydrostatic pressure, whereas pulse pressure was the difference between systolic and diastolic pressure. For systemic O2 delivery,
and perfusion pressure. To estimate perfusion pressure, mean arterial pressure (MAP) was corrected for the hydrostatic pressure, whereas pulse pressure was the difference between systolic and diastolic pressure. For systemic O2 delivery,  was multiplied by the arterial O2 content, whereas systemic O2 extraction was the ratio between the systemic arterio-venous O2 difference and the arterial O2 content. Leg muscle mass was calculated from whole-body dual-energy X-ray absorptiometry scanning (Prodigy, GE Healthcare, Chalfont St. Giles, UK) whereas quadriceps femoris muscle mass was calculated using the antropometric method (Andersen & Saltin, 1985).
was multiplied by the arterial O2 content, whereas systemic O2 extraction was the ratio between the systemic arterio-venous O2 difference and the arterial O2 content. Leg muscle mass was calculated from whole-body dual-energy X-ray absorptiometry scanning (Prodigy, GE Healthcare, Chalfont St. Giles, UK) whereas quadriceps femoris muscle mass was calculated using the antropometric method (Andersen & Saltin, 1985).
Statistical analysis
A one-way repeated measures ANOVA was used to test differences within and between the three trials. Following a significant F-test, pair-wise differences were identified using Tukey's honestly significant difference (HSD) post hoc procedure. To compare the haemodynamic response between incremental and supramaximal cycling, peak and exhaustion values were compared using one-way repeated measures ANOVA with Tukey's HSD post hoc procedure. The significance level was set at P < 0.05 and data are presented as means ± s.e.m. unless otherwise indicated.
Results
Supramaximal and maximal cycling
Work rate during supramaximal cycling was 498 ± 16 W and the time to exhaustion was 2.1 ± 0.1 min. During supramaximal cycling, 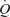 , LBF, and systemic and leg O2 delivery and
, LBF, and systemic and leg O2 delivery and 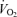 increased for 60 s and thereafter plateaued (Figs 2 and 3). In contrast, the systemic and leg a–v O2 difference kept increasing until exhaustion (P < 0.05). The plateau in
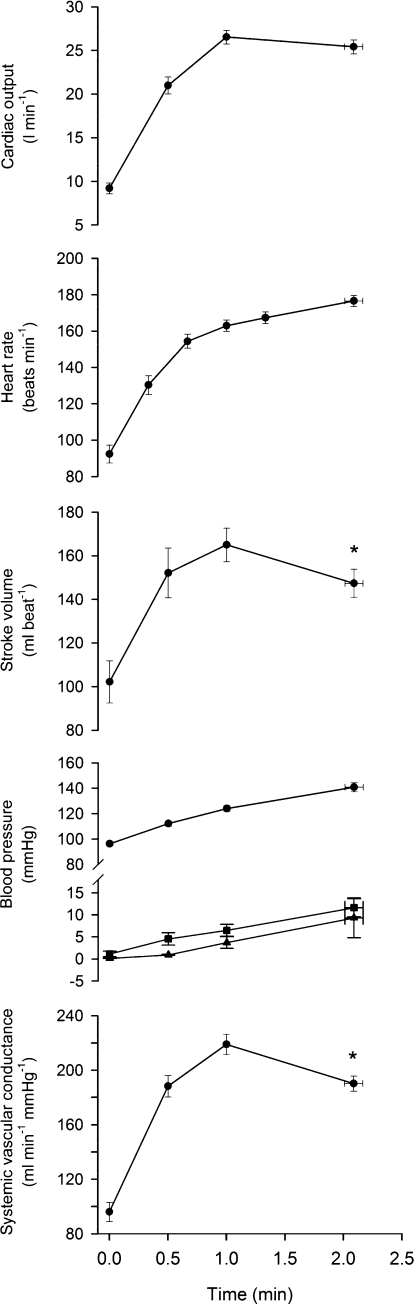
increased for 60 s and thereafter plateaued (Figs 2 and 3). In contrast, the systemic and leg a–v O2 difference kept increasing until exhaustion (P < 0.05). The plateau in  was associated with a fall in SV (18 ± 4 ml beat−1) because HR increased to 177 ± 4 beat min−1 (P < 0.05). CVP, PAMP and MAP increased progressively, reaching 12 ± 2, 9 ± 5 and 141 ± 4 mmHg, respectively, upon exhaustion. Systemic and leg vascular conductance increased for 60 s, but declined before exhaustion (P < 0.05). Femoral venous temperature increased from 37.6°C at the start of exercise to 38.2°C at exhaustion.
was associated with a fall in SV (18 ± 4 ml beat−1) because HR increased to 177 ± 4 beat min−1 (P < 0.05). CVP, PAMP and MAP increased progressively, reaching 12 ± 2, 9 ± 5 and 141 ± 4 mmHg, respectively, upon exhaustion. Systemic and leg vascular conductance increased for 60 s, but declined before exhaustion (P < 0.05). Femoral venous temperature increased from 37.6°C at the start of exercise to 38.2°C at exhaustion.
Figure 2. Systemic haemodynamics during supramaximal cycling.
Cardiac output, heart rate, stroke volume, arterial (•), pulmonary (▴) and central venous (▪) pressure, and systemic vascular conductance during constant load supramaximal cycling to exhaustion. Data are means ± s.e.m. for 8 subjects, except heart rate and arterial blood pressure (n = 12) and pulmonary blood pressure (n = 3). *Different from 60 s, P < 0.05.
Figure 3. Systemic and leg haemodynamics during supramaximal and incremental cycling.
Cardiac output, systemic O2 delivery, systemic a–v O2 difference, systemic  , leg blood flow, leg O2 delivery, leg a–v O2 difference and leg
, leg blood flow, leg O2 delivery, leg a–v O2 difference and leg  during supramaximal and incremental cycling to exhaustion. Data are means ± s.e.m. for 12 subjects, except cardiac output, systemic O2 delivery and systemic a–v O2 difference (n = 8). *Lower than incremental cycling, P < 0.05.
during supramaximal and incremental cycling to exhaustion. Data are means ± s.e.m. for 12 subjects, except cardiac output, systemic O2 delivery and systemic a–v O2 difference (n = 8). *Lower than incremental cycling, P < 0.05.
During supramaximal compared with incremental cycling,  reached similar peak values (27.0 ± 0.9 versus 27.6 ± 1.1 l min−1, respectively), but was lower during supramaximal cycling upon exhaustion (25.4 ± 0.8 versus 26.9 ± 1.1 l min−1; P < 0.05; Fig. 3). Consequently, at exhaustion systemic
reached similar peak values (27.0 ± 0.9 versus 27.6 ± 1.1 l min−1, respectively), but was lower during supramaximal cycling upon exhaustion (25.4 ± 0.8 versus 26.9 ± 1.1 l min−1; P < 0.05; Fig. 3). Consequently, at exhaustion systemic  was also lower (4.4 ± 0.2 versus 4.7 ± 0.2 l min−1; P < 0.05) despite systemic O2 extraction reaching similar values (81 ± 2%versus 82 ± 2%). In the exercising limbs, LBF and leg O2 delivery reached similar values. However, leg
was also lower (4.4 ± 0.2 versus 4.7 ± 0.2 l min−1; P < 0.05) despite systemic O2 extraction reaching similar values (81 ± 2%versus 82 ± 2%). In the exercising limbs, LBF and leg O2 delivery reached similar values. However, leg  was lower compared to incremental cycling (1.80 ± 0.17 versus 1.93 ± 0.15 l min−1, P < 0.05) because of a reduced leg a–v O2 difference (181 ± 4 versus 188 ± 3 ml l−1, P < 0.05).
was lower compared to incremental cycling (1.80 ± 0.17 versus 1.93 ± 0.15 l min−1, P < 0.05) because of a reduced leg a–v O2 difference (181 ± 4 versus 188 ± 3 ml l−1, P < 0.05).
Knee-extensor exercise compared to cycling
Peak power during incremental one-legged knee-extensor exercise and two-legged cycling was 104 ± 3 and 450 ± 16 W, respectively. Therefore, the peak power developed by each leg during incremental and supramaximal cycling was 225 ± 8 and 249 ± 8 W, respectively. The power produced by the leg muscles during cycling was therefore 2.1- to 2.3-fold greater than the 104 ± 3 W produced by the quadriceps muscles alone during knee-extensor exercise (Fig. 4). The muscle mass of the leg was 3.9-fold higher than that of the quadriceps (11.7 ± 0.5 versus 3.0 ± 0.1 kg).
Figure 4. Systemic and leg haemodynamics and 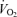 versus power during cycling and knee-extensor exercise.
versus power during cycling and knee-extensor exercise.
Cardiac output, blood flow, vascular conductance and  at the systemic and exercising leg level during incremental and supramaximal cycling and incremental knee-extensor exercise plotted against power output. Note that systemic
at the systemic and exercising leg level during incremental and supramaximal cycling and incremental knee-extensor exercise plotted against power output. Note that systemic  during incremental cycling increased linearly with power output (slope 9.4 ml min−1 W−1). At the level of the exercising limb muscles, leg
during incremental cycling increased linearly with power output (slope 9.4 ml min−1 W−1). At the level of the exercising limb muscles, leg  increased linearly until ∼100 W (slope 12.2 and 10.2 ml min−1 W−1 for knee-extensor exercise and cycling, respectively), becoming attenuated thereafter. Data are means ± s.e.m. for 12 subjects, except for cardiac output and systemic vascular conductance during cycling and one-legged blood flow, one-legged vascular conductance and leg
increased linearly until ∼100 W (slope 12.2 and 10.2 ml min−1 W−1 for knee-extensor exercise and cycling, respectively), becoming attenuated thereafter. Data are means ± s.e.m. for 12 subjects, except for cardiac output and systemic vascular conductance during cycling and one-legged blood flow, one-legged vascular conductance and leg  during supramaximal cycling (n = 8) and cardiac output and systemic vascular conductance during knee-extensions (n = 6).
during supramaximal cycling (n = 8) and cardiac output and systemic vascular conductance during knee-extensions (n = 6).
In all exercises, there was an increase in haemoglobin concentration with both work rate and over time, resulting in an elevated arterial O2 content in spite of a declining arterial O2 saturation during incremental and supramaximal cycling (Tables 1–3). During incremental cycling, 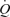 increased linearly until 88 ± 1% of peak power and then plateaued (Fig. 4) in association with a drop in SV (14 ± 2 ml beat−1). MAP increased from 100 ± 2 mmHg at rest to 139 ± 3 mmHg at peak power whereas CVP increased from −2 ± 1 to 5 ± 2 mmHg. Thus, the increase in systemic vascular conductance was attenuated at high cycling intensities reaching a plateau above ∼80% of peak power. In the exercising legs, LBF and vascular conductance increased curvilinearly, being attenuated at intensities above 50% of peak power (LBF: 63 versus 17 ml min−1 W−1 at ≤50%versus ≥50% peak power, respectively; P < 0.05). Likewise, the rise in leg
increased linearly until 88 ± 1% of peak power and then plateaued (Fig. 4) in association with a drop in SV (14 ± 2 ml beat−1). MAP increased from 100 ± 2 mmHg at rest to 139 ± 3 mmHg at peak power whereas CVP increased from −2 ± 1 to 5 ± 2 mmHg. Thus, the increase in systemic vascular conductance was attenuated at high cycling intensities reaching a plateau above ∼80% of peak power. In the exercising legs, LBF and vascular conductance increased curvilinearly, being attenuated at intensities above 50% of peak power (LBF: 63 versus 17 ml min−1 W−1 at ≤50%versus ≥50% peak power, respectively; P < 0.05). Likewise, the rise in leg  was blunted above 50% of peak power (10.5 versus 5.1 ml min−1 W−1 at ≤50%versus ≥50% peak power, respectively; P < 0.05). In contrast, during incremental knee-extensor exercise,
was blunted above 50% of peak power (10.5 versus 5.1 ml min−1 W−1 at ≤50%versus ≥50% peak power, respectively; P < 0.05). In contrast, during incremental knee-extensor exercise, 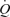 , LBF and leg
, LBF and leg 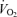 increased linearly until exhaustion (r2 = 0.993–0.996, P < 0.01), reaching peak values of 19.7 ± 0.6, 8.0 ± 0.5 and 1.3 ± 0.1 l min−1, respectively. During incremental knee-extensor exercise, MAP increased from 93 ± 1 mmHg at rest to 149 ± 3 mmHg at peak power, CVP increased from 1 ± 1 to 8 ± 2 mmHg and PAMP increased from 0 ± 1 to 4 ± 2 mmHg. At exhaustion during incremental knee-extensor exercise and cycling, femoral venous temperature reached 37.5 and 38.8°C, respectively.
increased linearly until exhaustion (r2 = 0.993–0.996, P < 0.01), reaching peak values of 19.7 ± 0.6, 8.0 ± 0.5 and 1.3 ± 0.1 l min−1, respectively. During incremental knee-extensor exercise, MAP increased from 93 ± 1 mmHg at rest to 149 ± 3 mmHg at peak power, CVP increased from 1 ± 1 to 8 ± 2 mmHg and PAMP increased from 0 ± 1 to 4 ± 2 mmHg. At exhaustion during incremental knee-extensor exercise and cycling, femoral venous temperature reached 37.5 and 38.8°C, respectively.
Table 1.
Blood variables at rest and during supramaximal cycling to exhaustion
| Supramaximal cycling (min) | ||||
|---|---|---|---|---|
| Rest | 0.5 | 1.0 | 2.1 ± 0.1 | |
| Haemoglobin (g·l−1) | ||||
| a | 150 ± 2 | 154 ± 2 | 157 ± 3* | 162 ± 3* |
| cv | 153 ± 4 | 156 ± 4* | 162 ± 4* | 167 ± 4* |
| fv | 150 ± 3 | 153 ± 3* | 158 ± 4* | 163 ± 4* |
 (mmHg) (mmHg) | ||||
| a | 115 ± 3 | 101 ± 4* | 94 ± 3* | 102 ± 2* |
| cv | 39 ± 1 | 24 ± 1* | 21 ± 2* | 22 ± 1* |
| fv | 33 ± 2 | 20 ± 1* | 19 ± 1* | 19 ± 1* |
| O2 saturation (%) | ||||
| a | 98.7 ± 0.2 | 97.8 ± 0.3 | 96.6 ± 0.3* | 96.5 ± 0.3* |
| cv | 68.4 ± 2.0 | 34.7 ± 3.2* | 22.9 ± 2.3* | 18.5 ± 1.7* |
| fv | 53.1 ± 3.8 | 25.2 ± 1.6* | 15.8 ± 1.2* | 13.9 ± 1.5* |
| O2 content (ml·l−1) | ||||
| a | 201 ± 3 | 204 ± 3 | 206 ± 4* | 212 ± 3* |
| cv | 141 ± 4 | 74 ± 8* | 50 ± 6* | 37 ± 7* |
| fv | 107 ± 8 | 53 ± 4* | 34 ± 3* | 31 ± 4* |
 (mmHg) (mmHg) | ||||
| a | 38.8 ± 0.8 | 38.5 ± 0.8 | 40.1 ± 0.8 | 38.5 ± 0.8 |
| cv | 45.5 ± 2.3 | 56.5 ± 1.1* | 72.6 ± 2.7* | 88.7 ± 4.1* |
| fv | 54.6 ± 1.0 | 56.9 ± 1.4 | 78.7 ± 2.0* | 93.1 ± 3.2* |
| pH | ||||
| a | 7.39 ± 0.01 | 7.39 ± 0.01 | 7.33 ± 0.01* | 7.28 ± 0.03* |
| cv | 7.33 ± 0.01 | 7.30 ± 0.01 | 7.20 ± 0.02* | 7.09 ± 0.03* |
| fv | 7.31 ± 0.01 | 7.31 ± 0.01 | 7.17 ± 0.01* | 7.07 ± 0.02* |
| Lactate (mmol·l−1) | ||||
| a | 1.6 ± 0.2 | 2.0 ± 0.2 | 4.8 ± 0.3* | 10.3 ± 0.7* |
| cv | 1.6 ± 0.2 | 2.2 ± 0.3 | 5.0 ± 0.6* | 11.0 ± 1.2* |
| fv | 1.3 ± 0.1 | 2.2 ± 0.2 | 5.9 ± 0.3* | 12.2 ± 0.7* |
| Glucose (mmol·l−1) | ||||
| a | 5.7 ± 0.3 | 5.1 ± 0.2* | 5.2 ± 0.2* | 5.1 ± 0.2* |
| cv | 5.5 ± 0.4 | 5.0 ± 0.4* | 5.1 ± 0.3* | 5.1 ± 0.3* |
| fv | 4.6 ± 0.2 | 4.9 ± 0.2* | 5.0 ± 0.2* | 4.9 ± 0.2* |
| Temperature (oC) | ||||
| fv | 37.6 ± 0.1 | 37.8 ± 0.1* | 37.9 ± 0.1* | 38.2 ± 0.1* |
Values are means ± s.e.m. for 12 subjects, except central venous (cv) values (n = 8). a. arterial. cv. right atrium or pulmonary artery. fv. femoral venous.  .
. 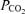 and pH values were corrected for changes in blood temperature.
and pH values were corrected for changes in blood temperature.
Different from rest, P < 0.05.
Table 3.
Blood variables at rest and during incremental knee-extensor exercise to exhaustion
| Incremental knee-extensor exercise (% peak power) | ||||||
|---|---|---|---|---|---|---|
| Rest | 26 ± 1% | 51 ± 1% | 77 ± 2% | 93 ± 2% | 100 ± 0% | |
| Haemoglobin (g·l−1) | ||||||
| a | 155 ± 2 | 157 ± 2 | 157 ± 2 | 157 ± 2 | 161 ± 3* | 163 ± 3* |
| cv | 154 ± 6 | 161 ± 3 | 158 ± 4 | 162 ± 4 | 161 ± 4 | 171 ± 4* |
| fv | 160 ± 3 | 160 ± 3 | 157 ± 4 | 164 ± 3 | 166 ± 3* | 171 ± 2* |
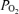 (mmHg) (mmHg) | ||||||
| a | 97 ± 2 | 96 ± 2 | 103 ± 2 | 109 ± 2* | 118 ± 3* | 122 ± 3* |
| cv | 37 ± 1 | 33 ± 2* | 30 ± 1* | 30 ± 2* | 31 ± 2* | 28 ± 1* |
| fv | 30 ± 2 | 24 ± 1* | 22 ± 1* | 21 ± 1* | 23 ± 1* | 23 ± 1* |
| O2 saturation (%) | ||||||
| a | 98.3 ± 0.1 | 98.3 ± 0.1 | 98.5 ± 0.1 | 98.7 ± 0.1 | 98.9 ± 0.2* | 98.9 ± 0.2* |
| cv | 70.8 ± 1.3 | 63.1 ± 2.6* | 54.4 ± 2.0* | 48.4 ± 2.4* | 44.3 ± 1.7* | 38.3 ± 2.2* |
| fv | 55.5 ± 3.9 | 41.6 ± 1.6* | 33.4 ± 1.6* | 28.1 ± 1.5* | 27.1 ± 1.4* | 26.3 ± 1.2* |
| O2 content (ml·l−1) | ||||||
| a | 206 ± 3 | 209 ± 3 | 210 ± 3 | 211 ± 3* | 217 ± 3* | 219 ± 3* |
| cv | 147 ± 6 | 137 ± 8* | 116 ± 5* | 106 ± 8* | 97 ± 6* | 91 ± 7* |
| fv | 122 ± 8 | 89 ± 4* | 70 ± 2* | 60 ± 3* | 59 ± 3* | 58 ± 3* |
 (mmHg) (mmHg) | ||||||
| a | 40 ± 0 | 41 ± 0 | 40 ± 1 | 40 ± 1 | 39 ± 1 | 37 ± 1 |
| cv | 46 ± 1 | 48 ± 1 | 52 ± 2* | 56 ± 2* | 59 ± 1* | 64 ± 1* |
| fv | 49 ± 1 | 53 ± 1 | 60 ± 1* | 70 ± 2* | 80 ± 3* | 81 ± 3* |
| pH | ||||||
| a | 7.41 ± 0.00 | 7.41 ± 0.01 | 7.41 ± 0.01 | 7.39 ± 0.01 | 7.37 ± 0.01* | 7.37 ± 0.01* |
| cv | 7.39 ± 0.01 | 7.38 ± 0.01 | 7.35 ± 0.01* | 7.32 ± 0.01* | 7.29 ± 0.01* | 7.23 ± 0.02* |
| fv | 7.38 ± 0.00 | 7.36 ± 0.01 | 7.32 ± 0.01* | 7.26 ± 0.01* | 7.19 ± 0.01* | 7.17 ± 0.02* |
| Lactate (mmol·l−1) | ||||||
| a | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.2 ± 0.1 | 2.0 ± 0.2* | 3.6 ± 0.2* | 4.9 ± 0.5* |
| cv | 0.9 ± 0.1 | 1.0 ± 0.1 | 1.3 ± 0.2 | 1.9 ± 0.2 | 3.2 ± 0.3* | 6.1 ± 0.9* |
| fv | 1.0 ± 0.1 | 1.3 ± 0.1 | 1.9 ± 0.2 | 3.5 ± 0.3* | 6.1 ± 0.4* | 7.7 ± 0.5* |
| Glucose (mmol·l−1) | ||||||
| a | 5.8 ± 0.1 | 5.7 ± 0.1 | 5.7 ± 0.1 | 5.5 ± 0.1 | 5.5 ± 0.1 | 5.5 ± 0.1 |
| cv | 5.7 ± 0.1 | 5.6 ± 0.2 | 5.5 ± 0.2 | 5.6 ± 0.2 | 5.5 ± 0.2 | 5.3 ± 0.2 |
| fv | 5.0 ± 0.2 | 5.3 ± 0.1 | 5.4 ± 0.2 | 5.4 ± 0.1 | 5.5 ± 0.1* | 5.4 ± 0.1* |
| Temperature (oC) | ||||||
| fv | 36.7 ± 0.1 | 36.7 ± 0.1 | 36.9 ± 0.1* | 37.2 ± 0.1* | 37.4 ± 0.1* | 37.5 ± 0.1* |
Values are means ± s.e.m. for 12 subjects, except central venous (cv) values (n = 6). a. arterial. cv. right atrium or pulmonary artery. fv. femoral venous.  .
.  and pH values were corrected for changes in blood temperature.
and pH values were corrected for changes in blood temperature.
Different from rest, P < 0.05.
Table 2.
Blood variables at rest and during incremental cycling to exhaustion
| Incremental cycling (% peak power) | ||||||
|---|---|---|---|---|---|---|
| Rest | 25% | 50% | 75% | 90% | 100% | |
| Haemoglobin (g·l−1) | ||||||
| a | 153 ± 2 | 155 ± 2 | 158 ± 3* | 159 ± 3* | 161 ± 3* | 166 ± 3* |
| cv | 157 ± 4 | 160 ± 4 | 161 ± 3 | 161 ± 4 | 163 ± 4 | 168 ± 5* |
| fv | 158 ± 3 | 154 ± 4 | 155 ± 5 | 157 ± 5 | 161 ± 4 | 167 ± 4* |
 (mmHg) (mmHg) | ||||||
| a | 112 ± 2 | 102 ± 1* | 95 ± 2* | 92 ± 3* | 94 ± 3* | 99 ± 3* |
| cv | 40 ± 1 | 32 ± 2* | 25 ± 1* | 22 ± 1* | 22 ± 2* | 22 ± 2* |
| fv | 31 ± 1 | 23 ± 1* | 20 ± 1* | 18 ± 1* | 18 ± 1* | 17 ± 1* |
| O2 saturation (%) | ||||||
| a | 98.8 ± 0.1 | 98.1 ± 0.1 | 97.3 ± 0.2* | 96.5 ± 0.4* | 95.9 ± 0.4* | 95.3 ± 0.4* |
| cv | 70.5 ± 0.6 | 45.5 ± 3.5* | 35.0 ± 2.8* | 26.5 ± 2.7* | 21.5 ± 3.0* | 17.3 ± 2.3* |
| fv | 49.5 ± 2.8 | 34.8 ± 1.3* | 26.3 ± 1.0* | 20.2 ± 1.0* | 16.8 ± 1.2* | 12.6 ± 1.2* |
| O2 content (ml·l−1) | ||||||
| a | 206 ± 3 | 207 ± 3 | 209 ± 3 | 209 ± 4 | 210 ± 4 | 214 ± 4* |
| cv | 149 ± 4 | 99 ± 9* | 77 ± 7* | 59 ± 7* | 49 ± 8* | 40 ± 6* |
| fv | 105 ± 5 | 69 ± 3* | 52 ± 3* | 39 ± 3* | 33 ± 3* | 26 ± 3* |
 (mmHg) (mmHg) | ||||||
| a | 40 ± 1 | 41 ± 1 | 42 ± 0* | 43 ± 1 | 42 ± 1* | 36 ± 1* |
| cv | 49 ± 1 | 56 ± 1 | 60 ± 1* | 71 ± 1* | 81 ± 4* | 85 ± 3* |
| fv | 56 ± 1 | 57 ± 1 | 64 ± 2 | 72 ± 1* | 85 ± 2* | 91 ± 3* |
| pH | ||||||
| a | 7.38 ± 0.01 | 7.38 ± 0.01 | 7.36 ± 0.01 | 7.34 ± 0.01* | 7.28 ± 0.02* | 7.19 ± 0.02* |
| cv | 7.34 ± 0.01 | 7.32 ± 0.01 | 7.31 ± 0.01 | 7.25 ± 0.01* | 7.19 ± 0.02* | 7.07 ± 0.02* |
| fv | 7.31 ± 0.01 | 7.31 ± 0.01 | 7.28 ± 0.01 | 7.22 ± 0.01* | 7.13 ± 0.03* | 7.02 ± 0.03* |
| Lactate (mmol·l−1) | ||||||
| a | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.6 ± 0.1* | 3.1 ± 0.2* | 7.0 ± 0.5* | 14.0 ± 0.7* |
| cv | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.7 ± 0.3* | 3.8 ± 0.6* | 7.9 ± 0.9* | 14.1 ± 1.1* |
| fv | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.7 ± 0.1* | 3.3 ± 0.2* | 7.8 ± 0.8* | 15.2 ± 0.7* |
| Glucose (mmol·l−1) | ||||||
| a | 5.2 ± 0.2 | 4.9 ± 0.2 | 5.0 ± 0.2 | 5.0 ± 0.2 | 5.1 ± 0.2 | 5.1 ± 0.2 |
| cv | 4.9 ± 0.2 | 4.6 ± 0.2 | 4.7 ± 0.2 | 4.7 ± 0.3 | 4.7 ± 0.2 | 4.9 ± 0.2 |
| fv | 4.6 ± 0.2 | 4.5 ± 0.2 | 4.8 ± 0.2 | 4.8 ± 0.2 | 4.8 ± 0.3 | 5.0 ± 0.3* |
| Temperature (oC) | ||||||
| fv | 37.5 ± 0.1 | 37.6 ± 0.1* | 37.8 ± 0.1* | 38.1 ± 0.1* | 38.5 ± 0.1* | 38.8 ± 0.1* |
Values are means ± s.e.m. for 12 subjects, central venous (cv) values (n = 8). a. arterial. cv. right atrium or pulmonary artery. fv. femoral venous.  .
. 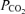 and pH values were corrected for changes in blood temperature.
and pH values were corrected for changes in blood temperature.
Different from rest, P < 0.05.
During incremental cycling, systemic 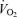 increased linearly until exhaustion (r2 = 0.993, P < 0.05; slope 9.4 ml min−1 W−1) because non-locomotor tissue
increased linearly until exhaustion (r2 = 0.993, P < 0.05; slope 9.4 ml min−1 W−1) because non-locomotor tissue  increased from ∼0.4 l min−1 at rest and light exercise to 0.8 ± 0.2 l min−1 at exhaustion (Figs 4 and 5). During incremental knee-extensor exercise, systemic
increased from ∼0.4 l min−1 at rest and light exercise to 0.8 ± 0.2 l min−1 at exhaustion (Figs 4 and 5). During incremental knee-extensor exercise, systemic  increased in a hyperbolic fashion above 73 ± 1% peak power in association with an increase in non-locomotor tissue
increased in a hyperbolic fashion above 73 ± 1% peak power in association with an increase in non-locomotor tissue  from ∼0.4 l min−1 at rest and moderate exercise to 1.1 ± 0.2 l min−1 at exhaustion (P < 0.05). The attenuation in LBF and leg
from ∼0.4 l min−1 at rest and moderate exercise to 1.1 ± 0.2 l min−1 at exhaustion (P < 0.05). The attenuation in LBF and leg  during incremental and supramaximal cycling, compared with the linear increase during incremental knee-extensor exercise, resulted in a lower leg
during incremental and supramaximal cycling, compared with the linear increase during incremental knee-extensor exercise, resulted in a lower leg 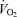 for a given work load at exhaustion during both types of cycling compared to the small muscle mass exercise model (7.0 ± 0.5 and 8.6 ± 0.4 versus 12.2 ± 0.7 ml min−1 W−1 during supramaximal cycling, incremental cycling and incremental knee extension, respectively, P < 0.05). For the systemic circulation, the rate of rise in
for a given work load at exhaustion during both types of cycling compared to the small muscle mass exercise model (7.0 ± 0.5 and 8.6 ± 0.4 versus 12.2 ± 0.7 ml min−1 W−1 during supramaximal cycling, incremental cycling and incremental knee extension, respectively, P < 0.05). For the systemic circulation, the rate of rise in  per unit of power was also higher during incremental knee-extensor exercise compared to incremental cycling (130 versus 41 ml min−1 W−1; Fig. 4) and therefore blood flow to non-locomotor tissues was higher, particularly at exhaustion (i.e. 11.5 ± 1.7 versus 6.0 ± 1.0 l min−1; P < 0.05; Fig. 5).
per unit of power was also higher during incremental knee-extensor exercise compared to incremental cycling (130 versus 41 ml min−1 W−1; Fig. 4) and therefore blood flow to non-locomotor tissues was higher, particularly at exhaustion (i.e. 11.5 ± 1.7 versus 6.0 ± 1.0 l min−1; P < 0.05; Fig. 5).
Figure 5. Regional blood flow, vascular conductance and 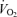 versus power during cycling and knee-extensor exercise.
versus power during cycling and knee-extensor exercise.
Systemic, leg and non-legged blood flow, vascular conductance and 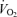 during incremental knee-extensor exercise and incremental and supramaximal cycling to exhaustion plotted against power output. Data are means ± s.e.m. for 8 subjects, except for
during incremental knee-extensor exercise and incremental and supramaximal cycling to exhaustion plotted against power output. Data are means ± s.e.m. for 8 subjects, except for  during incremental cycling and knee-extensor exercise (n = 12) and blood flow and vascular conductance during knee-extensor exercise (n = 6). *Non-legged value different from rest, P < 0.05.
during incremental cycling and knee-extensor exercise (n = 12) and blood flow and vascular conductance during knee-extensor exercise (n = 6). *Non-legged value different from rest, P < 0.05.
Leg and systemic haemodynamics
LBF and leg O2 delivery as a function of leg  increased linearly during knee-extensor exercise within the full range of work rate (5.7 and 1.3 l min−1 (l
increased linearly during knee-extensor exercise within the full range of work rate (5.7 and 1.3 l min−1 (l  )−1, respectively; r2 = 0.990–0.998; Fig. 6). During incremental cycling, the rate of rise in LBF and leg O2 delivery at low work rates was similar to that during knee-extensor exercise, but it was attenuated (P < 0.05) at intensities above 50% of leg
)−1, respectively; r2 = 0.990–0.998; Fig. 6). During incremental cycling, the rate of rise in LBF and leg O2 delivery at low work rates was similar to that during knee-extensor exercise, but it was attenuated (P < 0.05) at intensities above 50% of leg  peak (LBF: 3.5 versus 6.0 l min−1 (l
peak (LBF: 3.5 versus 6.0 l min−1 (l  )−1 and leg O2 delivery: 0.8 versus 1.2 l min−1 (l
)−1 and leg O2 delivery: 0.8 versus 1.2 l min−1 (l  )−1 at ≥50%versus ≤50% of leg
)−1 at ≥50%versus ≤50% of leg  , respectively; r2 = 0.927–0.999). Arterial, central venous and femoral venous lactate increased to 10–15 mmol l−1 during supramaximal and incremental cycling and to ∼7 mmol l−1 during incremental knee-extensor exercise (Tables 1–3). However, the increase in net leg lactate release was greater during knee-extensor exercise than during cycling despite the higher O2 delivery for a given work rate in the former (20.6 ± 2.2 versus 13.2 ± 3.2 mmol min−1, respectively; Fig. 6B).
, respectively; r2 = 0.927–0.999). Arterial, central venous and femoral venous lactate increased to 10–15 mmol l−1 during supramaximal and incremental cycling and to ∼7 mmol l−1 during incremental knee-extensor exercise (Tables 1–3). However, the increase in net leg lactate release was greater during knee-extensor exercise than during cycling despite the higher O2 delivery for a given work rate in the former (20.6 ± 2.2 versus 13.2 ± 3.2 mmol min−1, respectively; Fig. 6B).
Figure 6. Leg blood flow, O2 delivery and vascular conductance versus leg  during cycling and knee-extensor exercise.
during cycling and knee-extensor exercise.
One-legged blood flow, O2 delivery and vascular conductance during incremental knee-extensor exercise and incremental and supramaximal cycling to exhaustion plotted against one-legged 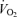 . Note that the increase in leg blood flow was attenuated above 50% peak power (slope 6.1 versus 3.6 l min−1(
. Note that the increase in leg blood flow was attenuated above 50% peak power (slope 6.1 versus 3.6 l min−1( )−1, respectively, P < 0.05). Data are means ± s.e.m. for 12 subjects, except during supramaximal cycling (n = 8).
)−1, respectively, P < 0.05). Data are means ± s.e.m. for 12 subjects, except during supramaximal cycling (n = 8).
During both incremental cycling and knee-extensor exercise, leg vascular conductance was attenuated above 50% of its respective leg  peaks; however, LBF was only attenuated from the linear perfusion-to-power relationship during cycling (Fig. 6C). The
peaks; however, LBF was only attenuated from the linear perfusion-to-power relationship during cycling (Fig. 6C). The  increased linearly as a function of systemic
increased linearly as a function of systemic  during both knee extensions (6.5 l (l
during both knee extensions (6.5 l (l  )−1) and cycling (4.6 l (l
)−1) and cycling (4.6 l (l  )−1) to ∼440 W (Fig. 7). The plateau in
)−1) to ∼440 W (Fig. 7). The plateau in  during maximal and supramaximal cycling was associated with a decline in SV since HR increased until exhaustion. The rate of rise in SV and HR per litre
during maximal and supramaximal cycling was associated with a decline in SV since HR increased until exhaustion. The rate of rise in SV and HR per litre 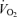 was higher during incremental knee-extensor exercise compared to incremental cycling (Fig. 7B and C). In all exercise modes, SV declined before exhaustion.
was higher during incremental knee-extensor exercise compared to incremental cycling (Fig. 7B and C). In all exercise modes, SV declined before exhaustion.
Figure 7. Systemic haemodynamics versus systemic  during cycling and knee-extensor exercise.
during cycling and knee-extensor exercise.
Cardiac output, stroke volume and heart rate during incremental knee-extensor exercise, incremental and supramaximal cycling to exhaustion plotted against systemic  . Note the linear increase in cardiac output up to ∼93%
. Note the linear increase in cardiac output up to ∼93% (slope 4.7 l min−1(
(slope 4.7 l min−1( )−1). Data are means ± s.e.m. for 8 subjects, except heart rate (n = 12) and cardiac output and stroke volume during knee-extensions (n = 6).
)−1). Data are means ± s.e.m. for 8 subjects, except heart rate (n = 12) and cardiac output and stroke volume during knee-extensions (n = 6).
Discussion
This study explored the regulatory limits of  and muscle perfusion by comparing systemic and leg haemodynamic responses to two-legged maximal and supramaximal cycling versus one-legged maximal knee-extensor exercise in the same individuals. There were three key findings in this study. Firstly, systemic and leg perfusion and O2 delivery levelled off during supramaximal cycling at values similar to those observed during maximal cycling in spite of a greater metabolic demand, thereby supporting the hypothesis that there is an upper limit in systemic and muscle perfusion which is unrelated to total energy turnover. Secondly, during incremental one- and two-legged exercise, leg perfusion and O2 delivery were the same up to a power of ∼100 W and were intimately related to leg
and muscle perfusion by comparing systemic and leg haemodynamic responses to two-legged maximal and supramaximal cycling versus one-legged maximal knee-extensor exercise in the same individuals. There were three key findings in this study. Firstly, systemic and leg perfusion and O2 delivery levelled off during supramaximal cycling at values similar to those observed during maximal cycling in spite of a greater metabolic demand, thereby supporting the hypothesis that there is an upper limit in systemic and muscle perfusion which is unrelated to total energy turnover. Secondly, during incremental one- and two-legged exercise, leg perfusion and O2 delivery were the same up to a power of ∼100 W and were intimately related to leg  , irrespective of whether power was generated only by the quadriceps muscles during knee-extensor exercise or spread among the different leg muscles during cycling. However, the rate of rise in
, irrespective of whether power was generated only by the quadriceps muscles during knee-extensor exercise or spread among the different leg muscles during cycling. However, the rate of rise in 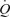 was greater during knee-extensor exercise and did not reach a plateau, unlike during maximal cycling, suggesting that blood flow to non-exercising tissues only increased when the central circulation was performing far below its capacity. Thirdly, the blunted muscle perfusion during maximal and supramaximal cycling was associated with a plateau in limb muscle vascular conductance and
was greater during knee-extensor exercise and did not reach a plateau, unlike during maximal cycling, suggesting that blood flow to non-exercising tissues only increased when the central circulation was performing far below its capacity. Thirdly, the blunted muscle perfusion during maximal and supramaximal cycling was associated with a plateau in limb muscle vascular conductance and  . Collectively, these observations suggest that muscle vasoconstriction and the attainment of limits of cardiac function underlie the inability of the circulatory system to meet the increasing metabolic demands of both the locomotor and non-locomotor muscles and tissues during intense whole-body exercise.
. Collectively, these observations suggest that muscle vasoconstriction and the attainment of limits of cardiac function underlie the inability of the circulatory system to meet the increasing metabolic demands of both the locomotor and non-locomotor muscles and tissues during intense whole-body exercise.
Perfusion and O2 supply
Leg and systemic blood flow and O2 delivery increased rapidly during the first minute of supramaximal cycling, but levelled off at values similar to those established during maximal cycling. The magnitude of the attenuation in the circulatory response in the heavily exercising leg muscles is evidenced by the ∼35% lower LBF and leg O2 delivery for a given power during supramaximal cycling compared to maximal knee-extensor exercise. The plateau in leg and systemic perfusion during supramaximal whole-body exercise is in accordance with reports in miniature swine and humans running on a treadmill or cycling, all of which have shown a levelling off in muscle blood flow and  at maximal and supramaximal intensities (Mitchell et al. 1958; Mortensen et al. 2005; Stringer et al. 2005; Brink-Elfegoun et al. 2007). These findings during supramaximal cycling, however, contrasted with the constant perfusion-to-power ratio and close matching between perfusion and
at maximal and supramaximal intensities (Mitchell et al. 1958; Mortensen et al. 2005; Stringer et al. 2005; Brink-Elfegoun et al. 2007). These findings during supramaximal cycling, however, contrasted with the constant perfusion-to-power ratio and close matching between perfusion and  observed during incremental knee-extensor exercise to exhaustion (Andersen & Saltin, 1985; Richardson et al. 1995) and during incremental cycling to ∼100 W.
observed during incremental knee-extensor exercise to exhaustion (Andersen & Saltin, 1985; Richardson et al. 1995) and during incremental cycling to ∼100 W.
The range of exercise intensities employed in this study makes it possible to identify the critical intensity at which perfusion and O2 supply become dissociated from the developed power. In this light, we found that LBF and leg O2 delivery plateaued from 80% to 110% of peak power in association with a parallel levelling off in  (Knight et al. 1992; Rosenmeier et al. 2004; González-Alonso et al. 2006; Calbet et al. 2007) (Fig. 3). When the haemodynamic responses are expressed as a function of
(Knight et al. 1992; Rosenmeier et al. 2004; González-Alonso et al. 2006; Calbet et al. 2007) (Fig. 3). When the haemodynamic responses are expressed as a function of  , it is also evident that the restriction in perfusion occurred before
, it is also evident that the restriction in perfusion occurred before 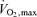 was reached (∼90% of
was reached (∼90% of  ) and that LBF and
) and that LBF and 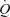 responses during the first minute of supramaximal cycling were proportional to the increase in
responses during the first minute of supramaximal cycling were proportional to the increase in 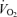 (Figs 6 and 7). Taken together, these findings support that muscle perfusion, and thus O2 delivery, becomes conspicuously restricted during both maximal or supramaximal exercise prior to the attainment of
(Figs 6 and 7). Taken together, these findings support that muscle perfusion, and thus O2 delivery, becomes conspicuously restricted during both maximal or supramaximal exercise prior to the attainment of  . Furthermore, the circulatory adjustments to severe exercise are driven by factors sensing aerobic energy demand rather than total energy demand.
. Furthermore, the circulatory adjustments to severe exercise are driven by factors sensing aerobic energy demand rather than total energy demand.
Central and local limitations to muscle perfusion and O2 supply
The plateau in blood flow and thus O2 delivery to locomotor skeletal muscle during severe whole-body exercise might be the result of the interaction of local and central reflexes signalling metabolic, thermal, mechanical and vascular events in many regions of the body including skeletal muscle, heart and brain (Rowell, 1974, 2004; González-Alonso et al. 2004; Secher et al. 2008). Blood flow and vascular conductance dynamics in the leg and systemic circulations during increasing cycling intensity were similar (see Fig. 5), indicating that the levelling off in LBF and  were mechanistically associated. Notably, MAP, CVP and PAMP did not decline with increasing exercise intensity, signifying that perfusion pressure and its regulation were not compromised when blood flow to the leg failed to increase. It therefore seems that insufficient
were mechanistically associated. Notably, MAP, CVP and PAMP did not decline with increasing exercise intensity, signifying that perfusion pressure and its regulation were not compromised when blood flow to the leg failed to increase. It therefore seems that insufficient  and local muscle vasoconstriction, but not inadequate perfusion pressure, are the major factors limiting skeletal muscle blood flow during severe exercise.
and local muscle vasoconstriction, but not inadequate perfusion pressure, are the major factors limiting skeletal muscle blood flow during severe exercise.
A salient observation in this study is the intimate relationship between the alterations in  and the changes in leg vascular conductance across all exercise modes and intensities (Fig. 8A; r = 0.954; P < 0.01). This implies that muscle vasoconstriction is related to the plateau in
and the changes in leg vascular conductance across all exercise modes and intensities (Fig. 8A; r = 0.954; P < 0.01). This implies that muscle vasoconstriction is related to the plateau in  . Yet, to support a causal relationship between alterations in cardiac function and muscle vasoconstriction, we need to establish that the plateau in
. Yet, to support a causal relationship between alterations in cardiac function and muscle vasoconstriction, we need to establish that the plateau in  is not caused by a reduced peripheral demand for blood flow and O2 supply. During incremental cycling, we observed that upper body blood flow and vascular conductance remained stable at all cycling intensities while local
is not caused by a reduced peripheral demand for blood flow and O2 supply. During incremental cycling, we observed that upper body blood flow and vascular conductance remained stable at all cycling intensities while local  increased (Harms et al. 1997; Calbet et al. 2007). In contrast, when exercise was performed only with the quadriceps muscles and
increased (Harms et al. 1997; Calbet et al. 2007). In contrast, when exercise was performed only with the quadriceps muscles and  increased linearly, blood flow to non-exercising tissues (including the contralateral leg) increased with increasing local
increased linearly, blood flow to non-exercising tissues (including the contralateral leg) increased with increasing local  reaching values twice as high as those seen during cycling (Fig. 5). Consequently, peripheral demand for blood flow and O2 supply is enhanced during maximal and supramaximal cycling, but that demand cannot be met because
reaching values twice as high as those seen during cycling (Fig. 5). Consequently, peripheral demand for blood flow and O2 supply is enhanced during maximal and supramaximal cycling, but that demand cannot be met because 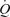 is compromised owing to a decline in SV and peripheral vasoconstriction is limiting blood flow in exercising and non-exercising muscles and tissues. Studies showing that
is compromised owing to a decline in SV and peripheral vasoconstriction is limiting blood flow in exercising and non-exercising muscles and tissues. Studies showing that  at rest is inversely related to resting muscle sympathetic nerve activity (MSNA) and to baroreflex control of MSNA at rest suggest that mechanistic links exist communicating the functioning of cardiac and skeletal muscle (Charkoudian et al. 2005, 2006).
at rest is inversely related to resting muscle sympathetic nerve activity (MSNA) and to baroreflex control of MSNA at rest suggest that mechanistic links exist communicating the functioning of cardiac and skeletal muscle (Charkoudian et al. 2005, 2006).
Figure 8. Leg vascular conductance versus cardiac output and femoral venous saturation during cycling and knee-extensor exercise.
Leg vascular conductance during incremental knee-extensor exercise, incremental and supramaximal cycling to exhaustion plotted against cardiac output (A) and femoral venous saturation (B). Data are means ± s.e.m. for 8 (A) or 12 (B) subjects.
The mechanisms causing vasoconstriction in the intensely contracting leg muscles could include an augmented local vasoconstrictor activity, a diminished vasodilator activity or both (Strandell & Shepherd, 1967; Rowell, 1997; Buckwalter & Clifford, 2001). Regarding the first possibility, MSNA and circulating noradrenaline increase exponentially above moderate exercise intensities, with the magnitude of the increase being greater during large compared to small muscle mass exercise (Seals et al. 1988; Savard et al. 1989; Richter et al. 1992; Saito et al. 1993; Richardson et al. 1995; Rosenmeier et al. 2004). A close temporal relationship therefore exists between the increase in sympathetic vasoconstrictor activity and the attenuation in LBF and 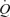 during incremental cycling and possibly supramaximal exercise (Pawelczyk et al. 1992). Perfusion could also be restrained during maximal knee-extensor exercise as leg vascular conductance levels off at high intensities and sympathetic vasoconstrictor activity increases (Richardson et al. 1999). In that regard, infusions of ATP and adenosine have minimal effects on LBF during both maximal knee-extensor and cycling exercise (Calbet et al. 2006; Barden et al. 2007; Lundby et al. 2008), but induce profound leg vasodilatation at rest and during submaximal exercise (Rådegran & Calbet, 2001; González-Alonso et al. 2002; Rosenmeier et al. 2004; Barden et al. 2007; González-Alonso et al. 2008). It therefore seems that the sympathetic system is heavily involved in both the local vasoconstriction causing the plateau in leg muscle blood flow during whole-body exercise and the increased vasoconstrictor tone restricting blood flow during maximal small muscle mass exercise.
during incremental cycling and possibly supramaximal exercise (Pawelczyk et al. 1992). Perfusion could also be restrained during maximal knee-extensor exercise as leg vascular conductance levels off at high intensities and sympathetic vasoconstrictor activity increases (Richardson et al. 1999). In that regard, infusions of ATP and adenosine have minimal effects on LBF during both maximal knee-extensor and cycling exercise (Calbet et al. 2006; Barden et al. 2007; Lundby et al. 2008), but induce profound leg vasodilatation at rest and during submaximal exercise (Rådegran & Calbet, 2001; González-Alonso et al. 2002; Rosenmeier et al. 2004; Barden et al. 2007; González-Alonso et al. 2008). It therefore seems that the sympathetic system is heavily involved in both the local vasoconstriction causing the plateau in leg muscle blood flow during whole-body exercise and the increased vasoconstrictor tone restricting blood flow during maximal small muscle mass exercise.
Sympathetic control of vascular conductance and blood flow can be attenuated, or even abolished, in contracting muscles, a phenomenon termed functional sympatholysis (Remensnyder et al. 1962). Metabolic vasodilatation may override sympathetic vasoconstrictor activity to optimize blood flow and O2 delivery to active muscle despite sympathetic activation (Remensnyder et al. 1962; Rosenmeier et al. 2004). The present observation that LBF failed to increase during supramaximal exercise, despite an extreme metabolic stimulus to vasodilate, indicates that metabolic vasodilatation does not offset sympathetic vasoconstrictor activity during intense whole-body exercise.
A blunted vasodilatory activity due to depressed O2-related vasodilatation, lower concentration of vasoactive substances and/or interference with vasodilatory signalling transduction pathways could be another explanation for the blunted leg muscle blood flow during maximal and supramaximal exercise. In the present study, we also found a tight correlation between the increases in leg vascular conductance and the reductions in femoral venous O2 saturation across all exercise modes and intensities (r = 0.945; Fig. 8B). Thus, the higher femoral venous oxyhaemoglobin (O2Hb) reflecting a lower  might be responsible for the lower blood flows during supramaximal compared to maximal cycling. A role of O2Hb in the control of muscle blood flow is supported in these subjects by the previously reported observation that LBF during submaximal knee-extensor exercise increased from ∼5 l min−1 in control conditions to ∼8 l min−1 with exposure to anaemia + plasma volume expansion + hypoxia and decreased to ∼4.5 l min−1 during polycythaemia + hyperoxia, while O2 delivery and demand were kept constant and
might be responsible for the lower blood flows during supramaximal compared to maximal cycling. A role of O2Hb in the control of muscle blood flow is supported in these subjects by the previously reported observation that LBF during submaximal knee-extensor exercise increased from ∼5 l min−1 in control conditions to ∼8 l min−1 with exposure to anaemia + plasma volume expansion + hypoxia and decreased to ∼4.5 l min−1 during polycythaemia + hyperoxia, while O2 delivery and demand were kept constant and  accommodated to the changes in muscle perfusion (González-Alonso et al. 2006). In this construct, the high concentration of lactate in the blood during maximal and supramaximal exercise may impair erythrocyte ATP release (Rozier et al. 2007) and/or its signalling transduction pathway (Mortensen et al. 2007). Regardless of the mechanisms, the restrictions in leg muscle blood flow during maximal and supramaximal whole-body exercise are closely related to an enhanced local vasoconstriction and the plateau in
accommodated to the changes in muscle perfusion (González-Alonso et al. 2006). In this construct, the high concentration of lactate in the blood during maximal and supramaximal exercise may impair erythrocyte ATP release (Rozier et al. 2007) and/or its signalling transduction pathway (Mortensen et al. 2007). Regardless of the mechanisms, the restrictions in leg muscle blood flow during maximal and supramaximal whole-body exercise are closely related to an enhanced local vasoconstriction and the plateau in  and demonstrate the existence of an upper limit in cardiovascular function in exercising humans.
and demonstrate the existence of an upper limit in cardiovascular function in exercising humans.
Circulatory limitations to muscle metabolism during intense exercise
The present data shed more light into the circulatory limitations to maximal and supramaximal exercise. Reductions in perfusion and O2 delivery to exercising muscles will limit the duration and the intensity of exercise as demonstrated by studies occluding flow to the exercising limbs (Kemp et al. 1994; Krustrup et al. 2003; Lanza et al. 2006) or reducing O2 supply in exercising humans and isolated muscle preparations via heat stress, hypoxia or ischaemia (Richardson et al. 1995; Hogan et al. 1996; Calbet et al. 2003; González-Alonso & Calbet, 2003). The progressive attenuation in O2 delivery to the locomotor limbs above 60% of 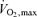 was associated with the classic exponential increase in leg lactate release, indicative of augmented anaerobic ATP production which can be sustained only for a short period due to the low capacity of the anaerobic energy pathways (Fig. 6). These findings speak against the ‘central governor’ model of fatigue postulating that an anticipatory feedforward mechanism terminates maximal exercise by depressing central motor drive before
was associated with the classic exponential increase in leg lactate release, indicative of augmented anaerobic ATP production which can be sustained only for a short period due to the low capacity of the anaerobic energy pathways (Fig. 6). These findings speak against the ‘central governor’ model of fatigue postulating that an anticipatory feedforward mechanism terminates maximal exercise by depressing central motor drive before  and skeletal muscle blood flow are compromised (Noakes, 1997, 1998; Noakes & Marino, 2007). Rather, our findings support that
and skeletal muscle blood flow are compromised (Noakes, 1997, 1998; Noakes & Marino, 2007). Rather, our findings support that  and locomotor skeletal muscle blood flow are indeed compromised prior to the attainment of
and locomotor skeletal muscle blood flow are indeed compromised prior to the attainment of  in parallel with a decline in SV (González-Alonso, 2008). In this line, the rapid plateau in
in parallel with a decline in SV (González-Alonso, 2008). In this line, the rapid plateau in  and LBF after only ∼1 min of supramaximal exercise and the somewhat lower systemic
and LBF after only ∼1 min of supramaximal exercise and the somewhat lower systemic  (92% of
(92% of  ) (Mitchell et al. 1958; Åstrand & Saltin, 1961; Hawkins et al. 2007) suggest that suppressed muscle O2 delivery and declining SV are important factors encompassing the chain of events leading to fatigue not only during constant and incremental maximal exercise, but also during supramaximal exercise lasting ≤2 min.
) (Mitchell et al. 1958; Åstrand & Saltin, 1961; Hawkins et al. 2007) suggest that suppressed muscle O2 delivery and declining SV are important factors encompassing the chain of events leading to fatigue not only during constant and incremental maximal exercise, but also during supramaximal exercise lasting ≤2 min.
In summary, these findings suggest that, despite the higher metabolic energy demand, systemic and locomotor skeletal muscle perfusion, vascular conductance, O2 delivery and aerobic metabolism become progressively more restricted during high-intensity cycling. The blunted muscle perfusion during exercise engaging a large compared to small muscle mass is associated with enhanced vasoconstrictor activity and a plateau in 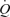 . Collectively, these results indicate that cardiac function and local vasoconstriction limit the ability of the circulatory system the meet the increasing demands of the locomotor and non-locomotor muscle and tissues during whole-body exercise.
. Collectively, these results indicate that cardiac function and local vasoconstriction limit the ability of the circulatory system the meet the increasing demands of the locomotor and non-locomotor muscle and tissues during whole-body exercise.
Acknowledgments
We give special thanks to the volunteer subjects for their enthusiasm. We also thank Peter Nissen, Troels Munch and Jacob Mørkeberg for the excellent technical assistance. This study was supported by the Gatorade Sports Science Institute and the Novo Nordisk Foundation. S.P., N.H.S. and J.G.-A. were supported by The Copenhagen Hospital System.
References
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åstrand PO, Saltin B. Oxygen uptake during the first minutes of heavy muscular exercise. J Appl Physiol. 1961;16:971–976. doi: 10.1152/jappl.1961.16.6.971. [DOI] [PubMed] [Google Scholar]
- Baratt-Boyes BG, Wood E. The oxygen saturation of blood in the venae cavae, right-heart chambers, and pulmonary vessels of healthy subjects. J Laboratory Clin Med. 1957;50:93–106. [PubMed] [Google Scholar]
- Barden J, Lawrenson L, Poole JG, Kim J, Wray DW, Bailey DM, Richardson RS. Limitations to vasodilatory capacity and Vo2 max in trained human skeletal muscle. Am J Physiol Heart Circ Physiol. 2007;292:H2491–H2497. doi: 10.1152/ajpheart.01396.2006. [DOI] [PubMed] [Google Scholar]
- Brink-Elfegoun T, Kaijser L, Gustafsson T, Ekblom B. Maximal oxygen uptake is not limited by a central nervous system governor. J Appl Physiol. 2007;102:781–786. doi: 10.1152/japplphysiol.00566.2006. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Clifford PS. The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exerc Sport Sci Rev. 2001;29:159–163. doi: 10.1097/00003677-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Boushel R, Rådegran G, Søndergaard H, Wagner PD, Saltin B. Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol Regul Integr Comp Physiol. 2003;284:R291–R303. doi: 10.1152/ajpregu.00155.2002. [DOI] [PubMed] [Google Scholar]
- Calbet JA, González-Alonso J, Helge JW, Sondergaard H, Munch-Andersen T, Boushel R, Saltin B. Cardiac output and leg and arm blood flow during incremental exercise to exhaustion on the cycle ergometer. J Appl Physiol. 2007;103:969–978. doi: 10.1152/japplphysiol.01281.2006. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Jensen-Urstad M, van Hall G, Holmberg HC, Rosdahl H, Saltin B. Maximal muscular vascular conductances during whole body upright exercise in humans. J Physiol. 2004;558:319–331. doi: 10.1113/jphysiol.2003.059287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet JA, Lundby C, Sander M, Robach P, Saltin B, Boushel R. Effects of ATP-induced leg vasodilation on Vo2 peak and leg O2 extraction during maximal exercise in humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R447–R453. doi: 10.1152/ajpregu.00746.2005. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005;568:315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol. 2006;572:821–827. doi: 10.1113/jphysiol.2005.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp MD, Laughlin MH. Regulation of skeletal muscle perfusion during exercise. Acta Physiol Scand. 1998;162:411–419. doi: 10.1046/j.1365-201X.1998.0324e.x. [DOI] [PubMed] [Google Scholar]
- González-Alonso J. Point: Stroke volume does decline during exercise at maximal effort in healthy individuals. J Appl Physiol. 2008;104:275–276. doi: 10.1152/japplphysiol.00595.2007. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Calbet JA. Reductions in systemic and skeletal muscle blood flow and oxygen delivery limit maximal aerobic capacity in humans. Circulation. 2003;107:824–830. doi: 10.1161/01.cir.0000049746.29175.3f. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Dalsgaard MK, Osada T, Volianitis S, Dawson EA, Yoshiga CC, Secher NH. Brain and central haemodynamics and oxygenation during maximal exercise in humans. J Physiol. 2004;557:331–342. doi: 10.1113/jphysiol.2004.060574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: role of erythrocyte count and oxygenation state of haemoglobin. J Physiol. 2006;572:295–305. doi: 10.1113/jphysiol.2005.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R, Secher NH, Dawson EA, Dutour SP. Haemodynamic responses to exercise, ATP infusion and mechanical compression in humans: insight into the role of muscle mechanisms on cardiovascular control. J Physiol. 2008;586:2405–2417. doi: 10.1113/jphysiol.2008.152058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Quistorff B, Krustrup P, Bangsbo J, Saltin B. Heat production in human skeletal muscle at the onset of intense dynamic exercise. J Physiol. 2000;524:603–615. doi: 10.1111/j.1469-7793.2000.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- Hawkins MN, Raven PB, Snell PG, Stray-Gundersen J, Levine BD. Maximal oxygen uptake as a parametric measure of cardiorespiratory capacity. Med Sci Sports Exerc. 2007;39:103–107. doi: 10.1249/01.mss.0000241641.75101.64. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Kurdak SS, Arthur PG. Effect of gradual reduction in O2 delivery on intracellular homeostasis in contracting skeletal muscle. J Appl Physiol. 1996;80:1313–1321. doi: 10.1152/jappl.1996.80.4.1313. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Nauss LA, Warner MA, Warner DO. Sympathetic modulation of blood flow and O2 uptake in rhythmically contracting human forearm muscles. Am J Physiol Heart Circ Physiol. 1992;263:H1078–H1083. doi: 10.1152/ajpheart.1992.263.4.H1078. [DOI] [PubMed] [Google Scholar]
- Kemp GJ, Thompson CH, Barnes PR, Radda GK. Comparisons of ATP turnover in human muscle during ischemic and aerobic exercise using 31P magnetic resonance spectroscopy. Magn Reson Med. 1994;31:248–258. doi: 10.1002/mrm.1910310303. [DOI] [PubMed] [Google Scholar]
- Knight DR, Poole DC, Schaffartzik W, Guy HJ, Prediletto R, Hogan MC, Wagner PD. Relationship between body and leg Vo2 during maximal cycle ergometry. J Appl Physiol. 1992;73:1114–1121. doi: 10.1152/jappl.1992.73.3.1114. [DOI] [PubMed] [Google Scholar]
- Koskolou MD, Calbet JA, Rådegran G, Roach RC. Hypoxia and the cardiovascular response to dynamic knee-extensor exercise. Am J Physiol Heart Circ Physiol. 1997;272:H2655–H2663. doi: 10.1152/ajpheart.1997.272.6.H2655. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Ferguson RA, Kjaer M, Bangsbo J. ATP and heat production in human skeletal muscle during dynamic exercise: higher efficiency of anaerobic than aerobic ATP resynthesis. J Physiol. 2003;549:255–269. doi: 10.1113/jphysiol.2002.035089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Wigmore DM, Befroy DE, Kent-Braun JA. In vivo ATP production during free-flow and ischaemic muscle contractions in humans. J Physiol. 2006;577:353–367. doi: 10.1113/jphysiol.2006.114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby C, Boushel R, Robach P, Moller K, Saltin B, Calbet JA. During hypoxic exercise some vasoconstriction is needed to match O2 delivery with O2 demand at the microcirculatory level. J Physiol. 2008;586:123–130. doi: 10.1113/jphysiol.2007.146035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Sproule BJ, Chapman CB. The physiological meaning of the maximal oxygen intake test. J Clin Invest. 1958;37:538–547. doi: 10.1172/JCI103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Dawson EA, Yoshiga CC, Dalsgaard MK, Damsgaard R, Secher NH, González-Alonso J. Limitations to systemic and locomotor limb muscle oxygen delivery and uptake during maximal exercise in humans. J Physiol. 2005;566:273–285. doi: 10.1113/jphysiol.2005.086025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, González-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol. 2007;581:853–861. doi: 10.1113/jphysiol.2006.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noakes TD. 1996 J.B. Wolffe Memorial Lecture. Challenging beliefs: ex Africa semper aliquid novi. Med Sci Sports Exerc. 1997;29:571–590. doi: 10.1097/00005768-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Noakes TD. Maximal oxygen uptake: ‘classical’ versus ‘contemporary’ viewpoints: a rebuttal. Med Sci Sports Exerc. 1998;30:1381–1398. doi: 10.1097/00005768-199809000-00007. [DOI] [PubMed] [Google Scholar]
- Noakes TD, Marino FE. Arterial oxygenation, central motor output and exercise performance in humans. J Physiol. 2007;585:919–921. doi: 10.1113/jphysiol.2007.145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummela A, Rusko H. Time course of anaerobic and aerobic energy expenditure during short-term exhaustive running in athletes. Int J Sports Med. 1995;16:522–527. doi: 10.1055/s-2007-973048. [DOI] [PubMed] [Google Scholar]
- Pawelczyk JA, Hanel B, Pawelczyk RA, Warberg J, Secher NH. Leg vasoconstriction during dynamic exercise with reduced cardiac output. J Appl Physiol. 1992;73:1838–1846. doi: 10.1152/jappl.1992.73.5.1838. [DOI] [PubMed] [Google Scholar]
- Perrey S, Candau R, Millet GY, Borrani F, Rouillon JD. Decrease in oxygen uptake at the end of a high-intensity submaximal running in humans. Int J Sports Med. 2002;23:298–304. doi: 10.1055/s-2002-29082. [DOI] [PubMed] [Google Scholar]
- Rådegran G, Calbet JA. Role of adenosine in exercise-induced human skeletal muscle vasodilatation. Acta Physiol Scand. 2001;171:177–185. doi: 10.1046/j.1365-201x.2001.00796.x. [DOI] [PubMed] [Google Scholar]
- Remensnyder J, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J, Wagner PD. Evidence of O2 supply-dependent Vo2 max in the exercise-trained human quadriceps. J Appl Physiol. 1999;86:1048–1053. doi: 10.1152/jappl.1999.86.3.1048. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Kennedy B, Knight DR, Wagner PD. High muscle blood flows are not attenuated by recruitment of additional muscle mass. Am J Physiol Heart Circ Physiol. 1995;269:H1545–H1552. doi: 10.1152/ajpheart.1995.269.5.H1545. [DOI] [PubMed] [Google Scholar]
- Richter EA, Kiens B, Hargreaves M, Kjaer M. Effect of arm-cranking on leg blood flow and noradrenaline spillover during leg exercise in man. Acta Physiol Scand. 1992;144:9–14. doi: 10.1111/j.1748-1716.1992.tb09261.x. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, González-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54:75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Neural control of muscle blood flow: importance during dynamic exercise. Clin Exp Pharmacol Physiol. 1997;24:117–125. doi: 10.1111/j.1440-1681.1997.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Ideas about control of skeletal and cardiac muscle blood flow (1876–2003): cycles of revision and new vision. J Appl Physiol. 2004;97:384–392. doi: 10.1152/japplphysiol.01220.2003. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Saltin B, Kiens B, Christensen NJ. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? Am J Physiol Heart Circ Physiol. 1986;251:H1038–H1044. doi: 10.1152/ajpheart.1986.251.5.H1038. [DOI] [PubMed] [Google Scholar]
- Rozier MD, Zata VJ, Ellsworth ML. Lactate interferes with ATP release from red blood cells. Am J Physiol Heart Circ Physiol. 2007;292:H3038–H3042. doi: 10.1152/ajpheart.01238.2006. [DOI] [PubMed] [Google Scholar]
- Saito M, Tsukanaka A, Yanagihara D, Mano T. Muscle sympathetic nerve responses to graded leg cycling. J Appl Physiol. 1993;75:663–667. doi: 10.1152/jappl.1993.75.2.663. [DOI] [PubMed] [Google Scholar]
- Saltin B, Calbet JA. Point: in health and in a normoxic environment, Vo2max is limited primarily by cardiac output and locomotor muscle blood flow. J Appl Physiol. 2006;100:744–745. doi: 10.1152/japplphysiol.01395.2005. [DOI] [PubMed] [Google Scholar]
- Savard GK, Richter EA, Strange S, Kiens B, Christensen NJ, Saltin B. Norepinephrine spillover from skeletal muscle during exercise in humans: role of muscle mass. Am J Physiol Heart Circ Physiol. 1989;257:H1812–H1818. doi: 10.1152/ajpheart.1989.257.6.H1812. [DOI] [PubMed] [Google Scholar]
- Seals DR, Victor RG, Mark AL. Plasma norepinephrine and muscle sympathetic discharge during rhythmic exercise in humans. J Appl Physiol. 1988;65:940–944. doi: 10.1152/jappl.1988.65.2.940. [DOI] [PubMed] [Google Scholar]
- Secher NH, Seifert T, van Lieshout JJ. Cerebral blood flow and metabolism during exercise: implications for fatigue. J Appl Physiol. 2008;104:306–314. doi: 10.1152/japplphysiol.00853.2007. [DOI] [PubMed] [Google Scholar]
- Strandell T, Shepherd JT. The effect in humans of increased sympathetic activity on the blood flow to active muscles. Acta Med Scand Suppl. 1967;472:146–167. doi: 10.1111/j.0954-6820.1967.tb12622.x. [DOI] [PubMed] [Google Scholar]
- Stringer WW, Whipp BJ, Wasserman K, Porszasz J, Christenson P, French WJ. Non-linear cardiac output dynamics during ramp-incremental cycle ergometry. Eur J Appl Physiol. 2005;93:634–639. doi: 10.1007/s00421-004-1258-3. [DOI] [PubMed] [Google Scholar]
- Thomas C, Hanon C, Perrey S, Le Chevalier JM, Couturier A, Vandewalle H. Oxygen uptake response to an 800-m running race. Int J Sports Med. 2005;26:268–273. doi: 10.1055/s-2004-820998. [DOI] [PubMed] [Google Scholar]



 during high-intensity whole-body exercise in humans
during high-intensity whole-body exercise in humans