Abstract
The contractile and actomyosin ATPase properties of single fibres were examined in human diaphragm muscle obtained from patients with and without chronic obstructive pulmonary disease (COPD). Costal diaphragm biopsies were taken from five patients without evidence of COPD and from 11 age-matched individuals with varying degrees of the disease. Our aim was to establish whether changes in contractile properties of COPD diaphragm could be fully explained by the previously documented shift towards a greater proportion of type I myosin heavy chain isoform in COPD. The relative proportion of type I diaphragm fibres from non-COPD and COPD patients was measured by gel electrophoresis, and was negatively correlated with FEV1 over the full range of values investigated. There was also significant atrophy of the type I fibre population in COPD diaphragms. Isometric tension was similar among the fibre types and between the COPD and non-COPD patients. The intrinsic energetic properties of diaphragm fibres were examined by monitoring the time-resolved actomyosin ATPase activity in COPD and non-COPD fibres that produced similar isometric forces. The isometric ATPase rate in COPD fibres was reduced to 50% of the rate in non-COPD fibres; hence, the cost of isometric contraction in type I and type IIA COPD fibres was reduced to between one-third and one-half of the tension cost calculated for non-COPD fibres. The rate of force development in type I COPD fibres was reduced to 50% of the rate seen in non-COPD type-I fibres. No difference in the rate of ATP consumption between COPD and non-COPD fibres was evident during isovelocity shortening. These data extend previous findings showing that aspects of breathing mechanics during progressive COPD are associated with remodelling of the diaphragm fibre-type distribution; on top of the increase in type I fibres there are fibre-specific reductions in force development rate (type I fibres) and ATPase rate that are consistent with the impairment of cross-bridge cycling kinetics.
The diaphragm is the most important inspiratory muscle; unlike many skeletal muscles the diaphragm is constantly active. Interestingly, in patients with chronic obstructive pulmonary disease (COPD), the diaphragm is relatively resistant to fatigue so that although low frequency fatigue (a relative loss of force at low frequencies of stimulation) can be elicited in normal humans (Johnson et al. 1993; Polkey et al. 1997a), it has not been possible to do so in patients with COPD (Polkey et al. 1995, 1997b). Various theories have been advanced. Diaphragm shortening (as a consequence of increased lung volumes) may protect against fatigue (Fitch & McComas 1985; Sacco et al. 1994) and a fibre type shift occurs towards fatigue-resistant type I fibres (Levine et al. 1997; Mercadier et al. 1998).
Very little, however, is known about the properties of individual muscle fibres of the diaphragm of patients with COPD. Levine et al. (2003) studied two patients with severe COPD and two normal controls and concluded that patient fibres exhibited a lower specific force than control subjects. Ottenheijm et al. (2005) studied diaphragm muscle fibres from eight patients with moderate COPD and five healthy controls. They also concluded that the specific force was reduced in COPD, and noted slower cross-bridge cycling. Neither of these studies, however, was able to document the energetics associated with the contraction of the fibre itself. Since one of the mechanisms for fatigue resistance could be more efficient substrate turnover, we hypothesized that muscles from COPD patients would hydrolyse ATP at a slower rate than control subjects.
This hypothesis was tested in the current study by measuring both the mechanical and ATPase properties of isolated muscle fibres from the diaphragm of patients with COPD and non-COPD control subjects during fibre activation.
Methods
Subjects
Full thickness muscle biopsies (approximately 15 mm × 5 mm) were taken from the anterior costal diaphragm from five individuals without COPD (judged by a forced expiratory volume in 1 s (FEV1) > 80% predicted, and FEV1 to forced vital capacity (FVC) ratio > 0.7), and from 11 age-matched individuals with varying degrees of disease (FEV1 26.9–80% predicted; FEV1/FVC < 0.7). The biopsies were not full thickness by design, but it proved impossible to separate the underlying peritoneal mesothelium from the muscle; full-thickness defects were therefore repaired by direct continuous suture using non-absorbable braided suture. All individuals had thoracotomies for pulmonary nodules, under general anaesthesia, and gave prior written informed consent to participate. Patients who had previously undergone thoracic surgery were excluded from the study, as were individuals with known heart failure, neurological disease, diabetes mellitus, epilepsy, and implanted cardiac defibrillators or pacemakers. The protocol was approved by the local research ethics committee. Spirometry and lung volumes determined by whole body plethysmography were measured as previously described (Moore et al. 2006). Global inspiratory and expiratory muscle strength was assessed using techniques described in the American Thoracic Society/European Respiratory Society joint statement on expiratory muscle testing (ATS/ERS, 2002).
Baseline individual anthropometric and pulmonary function data are shown in Table 1. Based on the Global Initiative for Obstructive Lung Disease (GOLD) criteria (Pauwels et al. 2001), three patients had mild COPD (stage I), six patients had moderate COPD (stage II), and two patients had very severe COPD (stage IV). Both COPD and non-COPD groups were well matched for age, height and weight whilst, as expected, the COPD patient group had a significantly lower mean percentage predicted FEV1 and FEV1/FVC ratio compared with the non-COPD group. Average values for the available measurements on sniff inspiratory pressure (SnIP), maximum inspiratory pressure (PImax), and maximum expiratory pressure (PEmax) were not significantly different between COPD patients and non-COPD patients. However, as it was not possible to obtain measurements of in vivo inspiratory and expiratory muscle strength on all patients (Table 1) because of the relatively low number of measurements, particularly for the non-COPD patients, we presently caution against an outright conclusion that there were no differences in in vivo muscle strength between the patient groups. There was no difference in the degree of hyperinflation between the two groups, as defined by a raised mean percentage predicted total lung capacity (TLC; P = 0.203), functional residual capacity (FRC; P = 0.072), and residual volume (RV; P = 0.131). However, the four patients with the greatest obstruction (FEV1: 26.9–52.3% predicted; FEV1/FVC: 26.4–42.4%) showed significant hyperinflation compared to controls (TLC, P = 0.01; RV, P = 0.006; FRC, P = 0.001) and RV/TLC (P = 0.05).
Table 1.
Baseline anthropometric and pulmonary function data
| Subject No. | Age (year) | Sex (M/F) | BMI (kg m−1) | FEV1 (l) | FEV1 (% pred.) | FVC (% pred.) | FEV1/FVC (%) | SnIP (cmH2O) | PImax (cmH2O) | PEmax (cmH2O) | TLC (% pred.) | RV (% pred.) | FRC (% pred.) | RV/TLC (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 60 | F | 38.9 | 2.3 | 108.5 | 133.7 | 68.1 | 101.3 | 67.1 | 83.4 | 124.3 | 121.7 | 110.3 | 38.6 |
| P2 | 68 | M | 24.0 | 2.8 | 96.0 | 110.3 | 67.3 | 97.5 | 71.2 | 99.3 | 101.4 | 105.6 | 122.5 | 39.3 |
| P3 | 34 | M | 28.2 | 2.3 | 69.0 | 94.0 | 69.8 | 101.7 | 132.3 | 129.0 | n/a | n/a | n/a | n/a |
| P4 | 46 | F | 17.7 | 0.9 | 26.9 | 72.3 | 32.2 | 57.0 | 20.6 | 53.1 | 127.5 | 256.4 | 208.6 | 65.6 |
| P5 | 53 | M | n/a | 2.6 | 81.0 | 117.6 | 55.3 | n/a | n/a | n/a | 106.0 | 83.7 | 108.7 | 26.5 |
| P6 | 53 | F | 21.3 | 0.8 | 27.0 | 87.5 | 26.4 | n/a | n/a | n/a | 167.9 | 317.3 | 245.9 | 66.0 |
| P7 | 64 | F | 23.2 | 1.6 | 79.3 | 94.9 | 69.8 | 97.8 | 52.2 | 69.6 | 99.1 | 119.7 | 111.2 | 49.1 |
| P8 | 53 | M | 26.6 | 2.0 | 51.0 | 137.1 | 29.6 | n/a | n/a | n/a | 136.7 | 175.9 | 189.6 | 39.8 |
| P9 | 80 | M | 26.3 | 1.3 | 52.3 | 91.1 | 42.4 | n/a | n/a | n/a | 125.6 | 183.4 | 165.3 | 61.4 |
| P10 | 75 | M | 23.3 | 2.1 | 63.0 | 76.0 | 62.0 | 91.8 | 61.9 | 77.9 | 82.0 | 91.0 | 101.1 | 41.0 |
| P11 | 64 | M | 32.2 | 2.2 | 74.4 | 91.6 | 63.4 | 125.7 | 93.6 | 124.2 | 109.6 | 127.6 | 97.3 | 46.9 |
| Mean | 59.1 | n/a | 26.2 | 1.9 | 66.2 | 100.6 | 52.5 | 96.1 | 71.3 | 90.9 | 117.1 | 158.2 | 146.0 | 47.4 |
| ± s.e.m. | (4.0) | (n/a) | (1.9) | (0.2) | (7.8) | (6.5) | (5.0) | (7.7) | (13.1) | (10.6) | (7.4) | (23.0) | (15.9) | (4.0) |
| C1 | 35 | M | 28.1 | 3.8 | 93.0 | 92.0 | 84.0 | 165.9 | 141.2 | 188.7 | n/a | n/a | n/a | n/a |
| C2 | 39 | M | 27.7 | 4.1 | 107.3 | 109.2 | 82.1 | n/a | n/a | n/a | 101.2 | 100.9 | 97.0 | n/a |
| C3 | 83 | M | 25.2 | 3.1 | 132.2 | 114.1 | 84.8 | 88.9 | 49.3 | 46.2 | 95.5 | 90.9 | 87.1 | 42.0 |
| C4 | 69 | F | 22.5 | 1.9 | 96.3 | 110.8 | 71.9 | 79.4 | n/a | n/a | 91.8 | 90.2 | 89.7 | 41.2 |
| C5 | 58 | F | 25.3 | 2.6 | 107.5 | 126.8 | 71.9 | 93.5 | 53.3 | 79.6 | 111.0 | 97.7 | 96.9 | 33.0 |
| Mean | 56.8 | n/a | 25.8 | 3.1 | 107.3 | 110.6 | 78.9 | 106.9 | 81.2 | 104.8 | 99.9 | 94.7 | 92.7 | 38.7 |
| ± s.e.m. | (9.0) | (n/a) | (1.0) | (0.4) | (6.9) | (5.6) | (2.9) | (19.9) | (30.0) | (43.0) | (4.2) | (2.5) | (2.5) | (2.9) |
Fibre preparation
The muscle biopsies were chemically permeabilized with Triton X-100, and fibres were prepared as detailed previously (Moore et al. 2006). In addition, compliance in the T-clip attachment regions was minimized by cross-linking with glutaraldehyde as previously described (He et al. 1997).
Experimental apparatus
The set-up has previously been described in detail (He et al. 1997, 1999). It comprised six 30 μl troughs, cut into the rotating stainless steel stage mounted on a Zeiss upright microscope. The fibre was mounted horizontally between two hooks (0.1 mm diameter stainless steel wire) whilst fully immersed in relaxing solution. One of the hooks was connected to a tension transducer (AE801 sensor element, Memscap AS, Skoppum, Norway), whilst the other was attached to a servomotor, adapted from a loudspeaker coil. Once mounted, the stage could be rotated in < 5 s in order to change the solution bathing the fibre. Experimental temperature (20°C) was maintained within each trough by a refrigeration system, whilst surface tension prevented the solution from leaking out of the ends of the troughs. One of the troughs in the rotating stage had a quartz window, allowing fibres to be illuminated by light pulses from a frequency-doubled ruby laser. Fibre activation was initiated by a single laser pulse, which resulted in the rapid photo-release of ATP from NPE-caged ATP (P3-1(2-nitrophenyl) ethyl ester of ATP).
Phosphate-binding protein and ATPase measurements
The ATP hydrolysis rate was determined in 66 fibres by measuring the change in fluorescence of a phosphate binding protein labelled with a coumarin fluorophore H-(2[l-malemidyl]ethyl)-7-diethylamino-coumarin-3carboximide phosphate binding protein(MDCC-PBP) diffused into the fibre, as previously described (He et al. 2000). Excitation of the Pi probe was achieved at 440 nm by a 4 mW laser diode module (PPM16-LD1504-G2; Laser 2000, Kettering, UK) aligned to the epi-fluorescence illumination port of the microscope. Background fluorescence was minimized by immersing the muscle fibre in silicone oil during these measurements. Teflon blocks (2 mm × 1 mm) on the walls of the trough at either end held drops of water in place, so that the oil in the central section of the trough did not leak out of the ends.
The fluorescence signal was corrected for aci-nitro decay, as previously described (He et al. 1998). The concentration of Pi released in the fibre was related to the amplitude of the fluorescence signal by:
| (1) |
where [Pi] is the concentration of Pi (in mm) bound to MDCC-PBP, Ft is the amplitude of the fluorescence signal at time t, Fmin is the fluorescence signal prior to photolytic release of ATP, [MDCC-PBP] is the concentration (in mm) of phosphate binding protein, and Fmax is the fluorescence signal when the MDCC-PBP was saturated with Pi. Ft, Fmin and Fmax were in arbitrary units, which in practice were the photomultiplier tube current converted to voltage after amplification. The ATPase rate was derived from the gradient of the fluorescence signal calibrated in terms of Pi concentration.
Experimental protocol
Procedures were performed as previously described (He et al. 1997, 1998, 1999). Briefly, once mounted in the quartz trough, the fibre was incubated for 30 min in a relaxing solution containing 1% (v/v) Triton X-100 to remove any remaining membranous material that had not been removed during the initial permeabilization. When returned to relaxing solution, the resting sarcomere length was set to 2.4 μm by adjusting fibre length while monitoring the first order laser diffraction signal, using a helium–neon laser (632.8 nm, LGK 7627; Lambda Photometrics, Harpenden, UK). A resting sarcomere length of 2.4 μm is 5–15% longer than estimates of in vivo sarcomere length for the non-COPD and COPD diaphragm (Orozco-Levi et al. 1999). However, resting tension was not notably elevated in single permeabilized fibres at a sarcomere length of 2.4 μm; fibres were not ‘stretched’ at this length, in keeping with our previous work (Moore et al. 2006), which showed that the passive force characteristics of single diaphragm fibres were similar for COPD and non-COPD patients in the range of sarcomere lengths from 2.0 to 2.5 μm. We opted for a resting length of 2.4 μm partly to facilitate comparisons of diaphragm mechanics and energetics with observations made on other mammalian skeletal muscle fibres (He et al. 1997, 1998, 1999). Moreover, during shortening-contractions a standard starting length of 2.4 μm meant that sarcomeres would shorten into a general length range that overlapped the apparently optimal lengths for force production in COPD and non-COPD fibres. We received small diaphragm biopsies too infrequently to quantify directly the specific force–length relationships for each of the fibre-types in both COPD and non-COPD patients.
Fibre length was determined by measuring the distance between the two points of end fixation using the microscope eye-piece graticule. The average fibre cross-sectional area (CSA) at the resting sarcomere length was determined optically (assuming an elliptical cross-section) from the mean of three width and depth measurements.
Prior to activation, the fibre was exposed to a pre-rigor solution (as relaxing solution except for a low ATP concentration) for 2 min, followed by 7 min in a calcium-free rigor solution (−ATP, −Ca2+). Contaminant phosphate was removed from both the calcium-free rigor and activating solutions by addition of a phosphate ‘mop’ (1 U ml−1 purine nucleoside phosphorylase and 0.5 mm 7-methylguanosine). Subsequently, the fibre was transferred to a trough filled with a calcium-containing rigor solution (5 min), before finally being incubated for 7 min in a loading solution (32 μm free Ca2+, 20 mm EGTA, 2 mm free Mg2+, 20 mm glutathione, 60 mm TES (2-(N-tris(hydroxymethyl)methyl-2-amino)ethanesulfonic acid), 5 mm NPE-caged ATP, 1.2 mm Pi-free MDCC-PBP). All incubating solutions were pH 7.1 with ionic strength of 150 mm (adjusted with potassium propionate). Whilst incubating solution constituents essentially mirrored those previously described (He et al. 1997), no ATP-regenerating system was present in our activating solution.
Following all the incubation steps, the fibre was transferred to the quartz trough, filled with silicone oil. The epifluorescence head of the microscope was lowered so that the objective made contact with the silicone fluid and the shutter for the fluorescence excitation light was opened. Fibres were activated by photolytic release of ATP induced by a light pulse (347 nm) emitted by a frequency doubled ruby laser (Lasermetrics, Saddle Brook, NJ, USA). Laser photolysis resulted in the release of 1.5 mm ATP from 5 mm NPE caged ATP. At a predetermined time after photolysis, the fibre was shortened at a predetermined velocity. The extent of movement was fixed at 180 μm, corresponding to 7–10% of fibre length, whilst the applied velocity of shortening varied from 0 to 1 muscle lengths per second (ML s−1). At the end of the shortening phase, the epifluorescence microscope head was lifted, and the fibre was returned to relaxing solution. Only a single contraction per fibre was performed so as to minimize contraction- or light-induced damage.
Determination of MHC composition
Following each experiment, single muscle fibres were placed in a cryo-vial containing 20 μl sample buffer (80 mm Tris-HCl, pH 6.8, 2.3% (w/v) sodium dodecyl sulphate (SDS), 5% β-mercaptoethanol (v/v), 10 mm dithiothreitol (DTT), 13.6% (w/v) sucrose, 0.01% (v/v) bromophenol blue, 0.1 mm phenylmethanesulphonyl fluoride (PMSF), 2 μm leupeptin, 1 μm pepstatin, 12.5% (v/v) glycerol) and stored at −20°C for up to 2 months prior to the biochemical analysis. The MHC isoform expression of each single muscle fibre, as well as homogenized pieces of diaphragm muscle excised from the midcostal region were determined electrophoretically, as previously described (Moore et al. 2006). The relative proportions of MHC isoforms in homogenized diaphragm were determined by densitometric analysis from scanned gel images using ImageJ analysis software (ImageJ 1.32j, National Institutes of Health, Bethesda, MD, USA). Gaussian curves were fitted to the data using Microsoft Excel with the Solver add-in.
Determination of fibre-type and fibre cross-sectional area in stained sections
Muscle fibre typing and distribution were evaluated by myofibrillar ATPase (mATPase) staining at pH 4.6, following the standard method of Dubowitz (1985). At pH 4.6, type I fibres stained dark, type IIA fibres stained light, and type IIX fibres stained intermediate. Fibre cross-sectional area (CSA) was determined in > 200 myofibres per mATPase stained section. Images of cells were magnified and outlined using ImageJ in order to evaluate CSA and fibre cross-sectional roundness. The roundness index (recorded as a value between 0 and 1) is the ratio of the cell area relative to the area of a circle that fully enclosed that cell; circular cells had a value approaching 1, while non-circular cells had smaller values.
Data collection and analysis
Fluorescence, force, sarcomere length and motor output signals were sampled at 8 kHz, digitized and saved with a TestPoint program (Measurement Computing Corporation, Norton, MA, USA).
Data were expressed as the mean ± 1 s.e.m., unless otherwise indicated. Student's t test was used for between-group comparison of lung function values, and two-way analysis of variance was used for comparisons among fibre groups. All least-square minimizations were carried out in Microsoft Excel using the Solver add-in tool.
Maximum isometric tension (Po) was taken as the force level immediately preceding the applied length change. The length change was applied before a true plateau level of force because we wanted to be sure that the shortening-induced increase in Pi release was completed before the onset of MDCC-PBP saturation. During shortening, tension declined, first steeply and then more slowly until the end of the shortening period: the average tension (P) during the slow decline phase was considered as the tension during the shortening phase (Ottenheijm et al. 2005). P multiplied by shortening velocity (V) was used to calculate mechanical power (W) measured in watts per litre (W l−1) of muscle (with muscle volume calculated from CSA × fibre length).
The ATP hydrolysis rate was calculated over intervals of 0.15 mm Pi, corresponding to one hydrolytic cycle in all myosin heads, assuming a myosin active site concentration of 0.15 mm (He et al. 2000). The ATPase rate was determined during both the phase immediately prior to fibre shortening and during isovelocity shortening.
The force–velocity relationships of COPD and non-COPD fibres expressing different MHC isoforms were interpolated with Hill's hyperbolic equation (Hill 1938):
 |
(2) |
Where Po is the isometric tension, P is the tension at shortening velocity V and the fit parameters a and b were obtained by least-square minimization. In isometric conditions, P/Po = 1. The maximal shortening velocity Vmax is then given by b/a at P = 0. The same numerical values of the parameters were used in the power–velocity equation:
| (3) |
The optimal velocity for peak mechanical power (Vopt) was determined from the power–velocity relationship.
Thermodynamic efficiency was determined from the power–velocity and ATPase–velocity relationships; efficiency (E) was calculated as:
| (4) |
where the value used for ΔGATP was 50 J mmol−1 (He et al. 2000). The relationship between efficiency and shortening velocity was used to determine peak thermodynamic efficiency, and values for shortening velocity and ATPase rate at peak efficiency.
Results
Relationship between the proportion of type I fibres and pulmonary function measurements
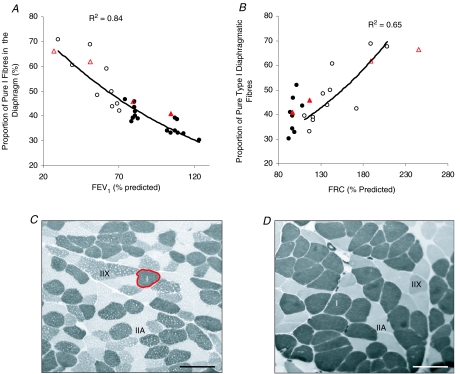
Figure 1 shows the relationship between the proportion of pure Type I fibres and measurements of pulmonary function: FEV1 (Fig. 1A) and FRC (Fig. 1B) in COPD and non-COPD diaphragm. It should be noted that the relative proportion of type I fibres in the diaphragm was determined for most (but not all) subjects detailed in Table 1, and that electrophoretic data are reported for a few additional subjects for whom anthropometric data are not documented in Table 1. A clear negative correlation between the percentage of type I fibres and FEV1 was found across the full range of values studied (FEV1 27–123% predicted; R2 = 0.84). A similar negative correlation also existed between the percentage of type I fibres in the diaphragm at values of FRC > 100% (R2 = 0.65), but no correlation was evident at FRC < 100% predicted.
Figure 1. Diaphragm fibre-type remodelling correlates with pulmonary functions in COPD patients.
A and B, relationship between the proportion of slow, type I fibres versus FEV1 (A) and FRC (B). Each pulmonary function measurement is expressed as a percentage of the predicted normal value. The filled circles were derived from densitometry of MHC isoforms on SDS-PAGE gels using muscle homogenates of non-COPD patients, whilst the open circles represent data from muscle homogenates of COPD patients (Moore et al. 2006). The relative proportion of type I fibres was determined for most (but not all) subjects detailed in Table 1; hence, electrophoretic data are reported for some additional subjects for whom anthropometric data are not documented in Table 1. The triangles in A and B show data derived from mATPase stained sections (open triangles show data from COPD patient diaphragm, whilst filled triangles show results obtained from non-COPD patients). The data derived from SDS-PAGE in each panel were fitted with a Microsoft Excel exponential trend line (in B, this was fitted only to the COPD data, because no correlation between FRC and the proportion of type I fibres was apparent in the control data), along with the correlation coefficient. C and D, staining of mATPase in sections of costal diaphragm revealed the increased proportion of type I fibres in COPD patients. C shows a section from a non-COPD individual in the highest spirometric quartile (area of the type I fibre highlighted in red is 3703 μm2), and D shows a COPD patient in the lowest spirometric quartile. Scale bar = 100 μm.
Importantly, our electrophoretic method of myosin heavy chain separation was verified using ATPase staining techniques (Fig. 1C and D). In biopsies (n = 4) where both techniques were employed, the proportion of type I fibres determined electrophoretically (circles) was found to be similar to the area fraction of MHC-1 fibres determined histochemically (triangles were within ±5–18% of circles, Fig. 1A). Figure 1C shows a transverse section through a diaphragm biopsy of a non-COPD patient from the uppermost spirometric quartile, whilst Fig. 1D shows a cross-section through diaphragm muscle from a severe COPD patient (lowest spirometric quartile). Both sections were histochemically stained for mATPase. The typical mosaic distribution of MHC isoforms is shown in Fig. 1C, whilst Fig. 1D shows a greater proportion of type I fibres in the diaphragm.
Fibre cross-sectional area
The median diaphragm fibre CSAs were 2845, 2835 and 2290 μm2 for type I, IIA and IIX COPD fibres, respectively, compared with 3955, 3645 and 2740 μm2 for type I, IIA and IIX fibres in the non-COPD group. The cumulative distributions (assessed by the Kolmogorov–Smirnov test) of type I and type IIA fibres were different; there was a greater proportion of smaller type I fibres and greater proportions of both small and large type IIA fibres in the COPD muscle (P < 0.001 and P < 0.005 for type I and type IIA fibres, respectively). Despite this difference, it was just the type I mean fibre area that was affected by COPD indicating that there was significant type I fibre atrophy compared to the non-COPD patients (P < 0.01). The within-group differences in fibre area are noted in Table 2. There was no correlation between the degree of airflow obstruction (% predicted FEV1) and the mean size of type I, IIA or IIX fibres.
Table 2.
Cross-sectional areas derived by measuring fibres (minimum 200 per biopsy) from ATPase stained sections through the muscle biopsies. Muscle was sampled from patients P7–P11 and C2–C5 in Table 1
| Type I (μm2) | Type IIA (μm2) | Type IIX (μm2) | |
|---|---|---|---|
| Non-COPD | 4143 ± 65 | 3834 ± 74 | 3072 ± 81§ |
| COPD | 3455 ± 114* | 4030 ± 250† | 3268 ± 139 |
P < 0.01 different from non-COPD type I
P < 0.01 different from other COPD fibres
P < 0.01 different from other non-COPD fibres.
Type I and type IIX diaphragm fibres from COPD and non-COPD patients had similar roundness indices (0.77 ± 0.01 and 0.786 ± 0.004 for type I; 0.76 ± 0.02 and 0.75 ± 0.01 for type IIX COPD and non-COPD fibres, respectively). COPD type IIA fibres were slightly less circular than those from non-COPD patients (0.74 ± 0.01 versus 0.79 ± 0.01, respectively; P < 0.05).
Performance of single fibres from the diaphragm
Contractions were performed on a total of 102 fibres from COPD patients (n = 68) and non-COPD patients (n = 34). On the basis of the electrophoretic separation of MHC isoforms, the fibres were individually classified into one of three groups: type I, which expressed only MHC-1; type IIA, which contained only MHC-2A; and type IIX, which contained only MHC-2X. Fibres which coexpressed MHC-1 and MHC-2A (n = 4) or MHC-2A and MHC-2X (n = 5) were excluded from further analysis. Some fibres were used for mechanical analysis only and others for mechanics and ATPase measurements therefore the number of single fibres analysed per parameter differs.
Figure 2 shows typical records of force development, shortening and Pi liberation in type I and type IIA human diaphragm muscle fibres at 20°C. The rate of tension rise (t1/2) was greater in fast type II (Fig. 2A) than in slow type I (Fig. 2B) fibres. Moreover, the gradient of the fluorescence signal, and hence the rate of Pi release (Fig. 2C and D), was greater in fast than in slow fibres. During initial isometric contraction, the ATP consumption rate declined after an initial transient fast phase and thereafter continued to decrease. The decline was more pronounced in fast than in slow fibres. When the fibre was allowed to shorten, tension decreased whilst the rate of Pi release increased, as indicated by the higher gradient of the fluorescence signal. At the end of the shortening phase, tension redeveloped and the rate of Pi release decreased to below the preshortening value, indicating gradual saturation of the phosphate binding protein.
Figure 2. Typical records of the contractions of a fast fibre segment (left) and a slow fibre segment (right).
A and B, tension records. The laser flash at time 0.2 s resulted in the photolytic release of ATP from NPE-caged ATP followed by tension rise. When tension had nearly reached a plateau (and ATPase activity had reached a quasi-steady state, see C and D) the fibre segment was allowed to shorten at a constant velocity (E and F), and tension decreased to a lower level. After the shortening period tension redeveloped, and a new plateau was reached. C and D, fluorescence signal, which, after calibration, provides a measure of Pi released by the hydrolysis of ATP. A fast initial phase was followed by a quasi-steady state period. When the fibre was allowed to shorten, the gradient of the signal increased. However, following the end of the shortening period, the rate of Pi release fell below that observed during the preshortening period. Saturation of the signal is seen (C) when total Pi released approached 1.2 mm.E and F, motor output, i.e. the signal indicating the change in the length of the fibre segment.
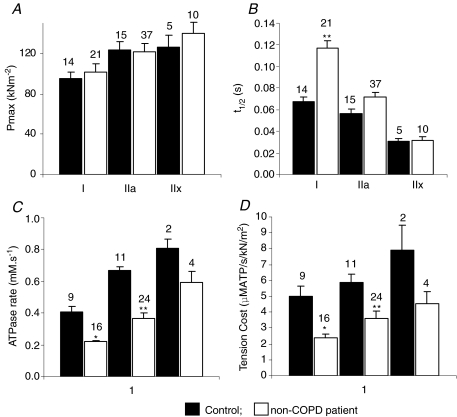
The half-time for force development (t1/2; Fig. 3B), isometric ATPase rate (Fig. 3C) and economy (ratio of ATP consumption rate during shortening and peak force; Fig. 3D) showed strong fibre type dependence in both COPD and control groups, whilst the absolute values of isometric tension were similar between fibres expressing different MHC isoforms (Fig. 3A). Both isometric ATPase rate and economy were significantly reduced in type I and type IIA COPD fibres compared to non-COPD subjects (P < 0.05 and P < 0.01, respectively); however, the rate of force development was significantly reduced only in type I COPD fibres compared to non-COPD (P < 0.01). ATPase data were collected from too few non-COPD type IIX fibres to enable variance analysis to be performed, but the data indicate that for these fibres too, COPD decreases the ATPase rate and improves the economy of isometric contraction.
Figure 3. Functional characterization of the fibre groups.
A–D, mechanical and energetic parameters of isometric contraction. The bar graphs show the average values of isometric tension (A; n = 102), half-time for force development (expressed as the time taken for force to reach half its maximal value following photolytic release of ATP; t1/2) (B; n = 102), the steady rate of ATP release, measured from the rate of fluorescence increase of the phosphate binding protein (C; n = 66) and cost of force production (the rate of ATP hydrolysis divided by isometric force) (D; n = 66) for the three fibre groups (type I, IIA and IIX) at 20°C. Filled bars show data from non-COPD subjects (n = 34), whilst open bars show data obtained from COPD patients (n = 68). n values are shown above each bar. *P < 0.05 different from non-COPD group. **P < 0.01 different from non-COPD group.
Force–velocity properties
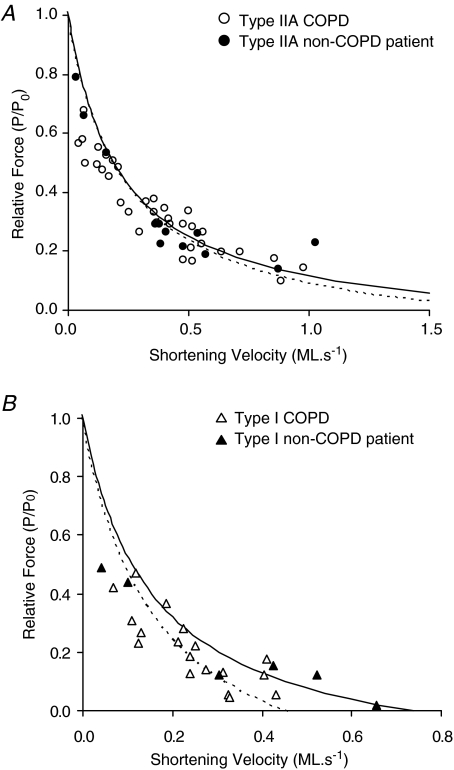
Relative forces (P/Po) during isovelocity shortening were determined for a range of shortening velocities in type IIA (Fig. 4A) and type I (Fig. 4B) COPD and non-COPD fibres. The data for each fibre type were fitted with hyperbolic Hill equations, the parameters of which are reported in Table 3. Variance analysis showed that the two fibre types exhibited distinct values of maximum shortening velocity (Vmax) (P < 0.01 and P < 0.05, non-COPD and COPD fibres, respectively); however, no difference in the F–V curves plotted for COPD and non-COPD patient fibres was observed. The maximum power (Wmax) produced by both COPD and non-COPD fibres was also dependent on fibre type (P < 0.01), but whilst type I COPD and non-COPD fibres produced similar Wmax values, the maximum power exhibited by control type IIA fibres was significantly greater than that produced by COPD type IIA fibres (P < 0.05) (Table 3).
Figure 4. Force–velocity curves.
A, F–V curves for type IIA control (n = 11; R2 = 0.61) and COPD (n = 34; R2 = 0.70) fibres. B, F–V curves for type I control (n = 6; R2 = 0.81) and COPD (n = 18; R2 = 0.88) fibres. Each data point represents a different fibre. Data points were interpolated using eqn (1) (Methods), with best fits obtained using Microsoft Excel's Solver, the parameters of which are reported in Table 3. Curves for non-COPD fibres are plotted with continuous lines, whilst COPD fibre curves are illustrated by dashed lines.
Table 3.
Comparison of diaphragm and limb fibre performance at 20°C
| Source of muscle | Po (kN m−2) | Isometric ATPase (mm.s−1) | Tension cost (μm s−1 kN−1 m−2) | b (ML s−1) | Vmax (ML s−1) | a/Po | Wmax (W l−1) | Vopt (ML s−1) | Peak Thermo-dynamic efficiency | V at peak efficienty (ML s−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Type I fibres | ||||||||||
| Vastus* | 150.0 ± 7.6 | 0.24 ± 0.05 | 2.15 ± 0.16 | 0.08 ± 0.01 | 1.15 ± 0.36 | 0.07 ± 0.04 | 7.0 | 0.25 | 0.34 | 0.1 |
| Non-COPD diaphragm | 95.6 ± 5.9 | 0.41 ± 0.04 | 5.00 ± 0.64 | 0.156 | 0.74 | 0.21 | 5.6 | 0.22 | 0.19 | 0.12 |
| COPD diaphragm | 101.1 ± 8.9 | 0.22 ± 0.01 | 2.40 ± 0.23 | 0.159 | 0.46 | 0.35 | 5.5 | 0.16 | 0.2 | 0.10 |
| Type IIA fibres | ||||||||||
| Vastus* | 170.0 ± 13.6 | 0.65 ± 0.08 | 3.80 ± 0.46 | 0.30 ± 0.03 | 2.37 ± 0.34 | 0.14 ± 0.03 | 28.8 | 0.55 | 0.41 | 0.3 |
| Non-COPD diaphragm | 123.6 ± 8.0 | 0.67 ± 0.02 | 5.89 ± 0.49 | 0.218 | 2.85 | 0.08 | 15.1 | 0.60 | 0.18 | 0.18 |
| COPD diaphragm | 121.9 ± 8.0 | 0.37 ± 0.03 | 3.6 ± 0.5 | 0.235 | 1.99 | 0.12 | 13.9 | 0.48 | 0.2 | 0.18 |
From He et al. (2000).
ATP consumption during shortening
During isovelocity shortening the ATP consumption rate increased above the isometric level (Ao) and reached a new value (As) (Fig. 2C and D). The ATP consumption rate during shortening (As) was roughly proportional to shortening velocity, such that at fast velocities of shortening, ATP was used more rapidly than at slow shortening velocities (Fig. 5). Whilst type IIA fibres consumed energy more rapidly than type I fibres in both COPD and non-COPD fibres, no difference in the rate of ATP consumption between non-COPD and COPD fibres was shown.
Figure 5. Energy utilization during isovelocity shortening.
The ATPase rates of COPD (open symbols) and non-COPD (filled symbols) type I (n = 7 and 5, respectively) and type IIA (n = 6 and 9, respectively) fibres at various velocities of shortening are shown. Triangles show data from type I fibres, whilst data from type IIA fibres are shown by circles.
Discussion
The use of human diaphragm biopsies imposes experimental limitations. Firstly, we assume that small muscle biopsies are representative of the entire diaphragm and that there are no differences between the costal and crural diaphragm, although there is evidence suggesting that in COPD there is increased injury in the costal compared to the crural diaphragm region (Scott et al. 2006). Although only healthy diaphragm muscle biopsies were used as non-COPD controls, the muscle samples were taken from relatively elderly subjects (58.0 ± 6.5 year). Several effects of ageing on skeletal muscle have been described, including an age-related decline in force generation (Lowe et al. 2002; Trappe et al. 2003). All subjects (COPD and controls) were undergoing thoracotomy for pulmonary nodules so that the presence of malignancy, other health related conditions or prescribed medication may have influenced our results.
In the present study, we judged the non-COPD patients to be a reasonable surrogate for a true control group partly because there was only minor overlap (at around 70% FEV1; Fig. 1A) of the relationships between fibre type and FEV1 for COPD and non-COPD groups, and partly because many of the non-COPD subjects approached or exceeded 100% of expected FEV1. Factors like subject fitness and differences in nodule characteristics are likely to account for the variation in diaphragm fibre type distribution in non-COPD patients. The non-COPD diaphragm fibre results are also broadly comparable with other studies on human skeletal muscles where large differences between type I and type IIA fibres are seen for the rate of force development (t1/2), isometric ATPase activity (Ao), shortening velocity and contraction costs (Larsson & Moss 1993; Bottinelli et al. 1996; Stienen et al. 1996; He et al. 2000). In Table 3, we have included results from the only previous study of limb muscle fibres (He et al. 2000) that employed the same methods used here. Some parameters are similar for diaphragm and vastus fibres and some are not (Table 3). Differences in subject age and fitness level are likely to be important factors in this comparison. In addition, these parameters are temperature dependent (Ferenczi et al. 1984; Ranatunga 1984; De Ruiter & de Haan 2000), so such comparisons at in vivo temperature will be important in the future for evaluating the physiological relevance of any differences in size, and mechanical and thermodynamic properties of normal diaphragm and limb muscle fibres.
Not all contractile parameters were described for each fibre because of the complexity of the experimental procedures. Preoperative in vivo characterization of the diaphragm by measurement of twitch transdiaphragmatic pressure (Polkey et al. 1996) could not be obtained for most patients because of the clinical pressures associated with bringing patients to thoracotomy.
Performance characteristics of diaphragm fibres
Isometric force production
Our sample covered the full spectrum of COPD severities from mild to very severe, judged by GOLD criteria (Pauwels et al. 2001), and demonstrated the previously reported fibre type shift towards a preponderance of fatigue-resistant type I fibres with advancing disease severity. Average isometric tension was found to be similar among the different diaphragm fibre types tested. Moreover, there was no effect of COPD on average peak force production.
The similar forces for COPD and non-COPD fibres contrasts with previously published studies (Levine et al. 2003; Ottenheijm et al. 2005) which report reduced isometric force in the COPD diaphragm. One possible mechanistic explanation for this difference relates to the fact that fibres in the present study were contracted at low (Pi), owing to the binding characteristics of the PBP-MDCC. Increasing (Pi), which would have been a feature of isometric contractions in previous studies, will reduce force. At present we cannot rule out the possibility that increasing (Pi) may reduce cross-bridge force more in diaphragm fibres from COPD patients. The small difference in isomeric force observed here for different fibre types may also be caused by low Pi concentration in our protocol (Fig. 3A).
Another possible reason for the differences between studies relates to the criteria used for excluding data from further analysis. In the present study, fibres were excluded if they broke during activation or if maximum force production was low. We also excluded COPD fibres which had unusually long (>3 μm) sarcomere lengths, despite being unstretched during dissection. In our experience these are features of fibre damage. The dissection of diaphragm biopsies is generally more difficult than it is for limb muscle biopsies because of the abundance of extracellular matrix. Diaphragm fibres are thus more prone to damage during dissection; this is perhaps part of the reason for the lower isometric forces in diaphragm fibres compared with vastus muscle (Table 3), and may be a factor when comparing diaphragm fibre mechanics in different studies. In the present study, we analysed 40% of the total non-COPD fibres and 29% of total COPD fibres that were activated. We applied the same exclusion criteria to both the COPD and non-COPD groups. Even so, at present we cannot rule out the possibility that features such as ‘low-force’ and abnormal sarcomere length hold physiological relevance for the disease state.
The advantage of analysing fibres that produced similar isometric forces is that we gain insights into the effect of COPD on the intrinsic energetic aspects of diaphragm force production and sarcomere shortening. We provide new data showing that the time-resolved isometric ATPase rate and the ATP cost of maintaining isometric force were reduced in type I and IIA COPD fibres compared to the non-COPD fibre-types, whilst the rate of force development was significantly impaired only in type I COPD fibres. The ATP cost of maintaining isometric contraction was greater in type IIA fibres than in type I fibres, a trend that was also evident for limb muscle fibres (Table 3).
Active shortening
During active shortening of fibres from both COPD and non-COPD patients, the ATPase rate increased in proportion to shortening velocity and in proportion to the ATPase rate preceding the shortening phase. However, there was no significant difference in the rate of energy utilization in COPD and non-COPD fibres during shortening.
To our knowledge, these data provide the first estimate of fibre-specific force–velocity relationships for human diaphragm muscle (Fig. 4). There were mostly only minor differences in the force–velocity parameters between muscle groups and in response to COPD. At the present time, we are unsure of the physiological relevance of the apparently lower Vmax and force–velocity curvature for type I diaphragm fibres compared to type I limb muscles (Table 3, He et al. 2000). The differences in fitness level and age of the subject in each set of experiments may be important for explaining this difference. We also note that the Vmax value for type I diaphragm fibres was reduced in COPD patients (Table 3). We caution that this result is based on a small number of observations for the non-COPD fibres (Fig. 4B), but we do note that it is consistent with the atrophy of COPD type I fibres in diaphragm sections (Table 2). Future study of force–velocity characteristics using mechanics approaches with repeat contractions will help provide estimates of Vmax variances, and to either confirm or revise the fibre-specific force–velocity parameters listed in Table 3.
Power and thermodynamic efficiency
The values for Vopt in COPD fibres were lower than in non-COPD fibres (Table 3). Thus, maximum power, which was not affected in COPD fibres, seems to have occurred at a lower shortening velocity in the diseased state (Vopt in COPD was 72% and 80% of the non-COPD type I and type IIA fibres, respectively). The Wmax for type II diaphragm fibres was lower than in vastus, whereas power output of type I fibres in vastus and the diaphragm are similar. The features of power production in the intact mixed-fibre diaphragm might therefore be expected to be more similar to limb muscles with predominantly type I fibres. Indeed, in mouse models, Wmax for intact diaphragm muscle at 25°C was 31 W kg−1 (Stevens & Faulkner 2000); this value is closer to the Wmax for the mouse soleus (Wmax = 42 W kg−1) than for mouse extensor digitorum longus (Wmax = 137 W kg−1) (Barclay 1996).
The shortening velocity that produced peak thermodynamic efficiency (Table 3) was always lower than the value for Vopt (the velocity that gave maximum power). This is consistent with previous observations made with skinned fibres from human vastus (He et al. 2000) and with intact fast-twitch fibre bundles from fish (Curtin & Woledge 1993).
Peak thermodynamic efficiencies were approximately 20% for both type I and type IIA diaphragm fibres. For a given fibre type, there were very similar power output values and ATPase rates at the shortening velocity that produced peak efficiency. Peak thermodynamic efficiencies are higher in vastus muscle fibres, perhaps reflecting the younger age and increased fitness of subjects in the earlier study. However, it may be that diaphragm fibres display intrinsically low energetic and mechanical efficiencies. In vivo mechanical efficiencies for human respiratory muscles are generally low (< 20%), but also tend to be highly variable and possibly reduced in patients with obstructive lung diseases (McGregor & Becklake 1961). Similarly, the efficiency of the oxygen cost of breathing is about 20% (Scano et al. 2006) and may be lower than 10% in healthy elderly and COPD subjects (Barrends et al. 1998). The low thermodynamic efficiencies were apparently unchanged in COPD patients. It may be that molecular- and cellular-level adaptations in the COPD diaphragm protect energetic efficiency, and improve the cost of isometric force production in specific fibre types, but in vivo mechanical efficiencies are most sensitive to the overall remodelling of fibre types and to increasing lung hyperinflation during progressive COPD (McGregor & Becklake 1961). Our data indicate that low thermodynamic efficiency in diaphragm fibres arose because of relatively high ATPase activity and relatively low power at peak efficiency.
Relationship between measurements of pulmonary function and distribution of type I fibres
A fast to slow fibre type transformation has been well established in the diaphragm of COPD patients over recent years (Levine et al. 1997, 2003; Mercadier et al. 1998); interestingly an opposite process occurs in the quadriceps, which are under-active in patients with COPD, and is associated with reduced endurance (Swallow et al. 2007). In the current study, the percentage of type I fibres in the diaphragm increased as respiratory function decreased (FEV1 and FRC), in line with previous findings (Levine et al. 2003). One difference from the present study was that Levine et al. (2003) found that the proportion of type I fibres increased appreciably only when FEV1 < 60% predicted. In the present study, the proportion of type I fibres increased over a wider range of FEV1 values (123 to 27% predicted). Our observation of type I COPD fibre atrophy is in accordance with the findings of Levine et al. (2002). However, fibre atrophy coupled with the observation that there was not a correlation between FEV1 (% predicted) and type I CSA suggests that the type I fibre atrophy is a feature of both moderate and severe cases of COPD. The chief effect of progressive COPD on diaphragm muscle structure appears to have been on the remodelling of fibre types.
We have shown here that a fast to slow fibre-type transformation was evident in patients with mild to moderate COPD, and that fibre-type distribution also varied in non-COPD patients over the range of normal expected expiratory lung volumes. The relationship between type I fibre proportion and FEV1 for non-COPD patients (filled black symbols in Fig. 1) is likely to reflect differences in the fitness level of the subjects, but we do not rule out possible detrimental effects of diaphragm inflammation and/or nodule size, distribution, and classification (e.g. benign or malignant), particularly within the cluster of observations between 70 and 100% of FEV1. With more measurements in the future it might be possible to evaluate the extent to which these factors explain the variance in our observations.
Significance of the findings
The present study shows differences in the time course of contractile response of type I and type II fibres of the diaphragm. Isometric ATPase rate was significantly reduced in both type I and type IIA COPD fibres compared to non-COPD fibres, resulting in lower ATP cost of isometric contraction in COPD fibres. The rate of force development was significantly reduced only in type I COPD fibres. Together these changes suggest that cross-bridge cycling kinetics are impaired in COPD fibres. The difference in isometric ATPase rate between COPD and non-COPD fibres may be accounted for by differences in cross-bridge number, although the fact that COPD and non-COPD fibres produced similar isometric tensions means that for this to be true, each cross-bridge in COPD muscle must exert more force compared to cross-bridges in the non-COPD muscle. A redistribution of cross-bridge states, toward a greater proportion of force-bearing AM.ADP.Pi and AM.ADP states (e.g., see Siththanandan et al. 2006), may be an important adaptation to compensate for proposed loss of MHC content in the diseased diaphragm. These data support the findings of Ottenheijm et al. (2005) and Moore et al. (2006) who found a reduced MHC content per half-sarcomere in patients with COPD compared to controls. Similarly, the tendency for Vopt to be reduced in type I and type IIA COPD fibres is consistent with fibre-type specific reductions in MHC content in the COPD diaphragm.
We, and others, have previously shown that COPD diaphragm is associated with a longer, more extensible titin compared to non-COPD muscle (Ottenheijm et al. 2006). This structural change in the titin molecule may weaken the structural stability of the muscle filaments, resulting in fibre damage under increased respiratory loads. This change could also have added to the difficulty with diaphragm fibre dissections, and may have contributed to the single-fibre energetic features during isometric contraction by introducing greater shortening against the series elastic component of fibre compliance. We had too few measurements of sarcomere change in the present study to evaluate internal shortening aspects of isometric contraction.
Furthermore, in accordance with our observations of fibre damage, previous studies (Orozco-Levi et al. 1999; 2001) have also revealed sarcomere disruption in all human diaphragm samples, but found it to be more prevalent in patients with COPD. Additionally, MacGowan et al. (2001) revealed a correlation between increasing severity of airflow obstruction and increased area fraction of abnormal muscle. Whilst injury may be caused at a greater rate in COPD diaphragm as a result of increased mechanical stresses or increased susceptibility to damage, recent work by Ottenheijm et al. (2005) showed enhanced degradation of cytoskeletal proteins in COPD diaphragm. A combination of these factors may account for the increased prevalence of fibre damage in the COPD diaphragm.
Overall, the most important finding of the current study is that COPD affects fibres in a separate manner to the well-documented switch toward an increased population of type I fibres. We have shown that under isometric conditions, COPD fibres use energy at a slower rate than fibres from non-COPD subjects, despite producing similar specific forces. These changes suggest that COPD muscle fibres have fewer active cross-bridges, each exerting greater force than those in control muscle. This phenomenon could offer an additional insight into the fatigue resistance observed in human in vivo studies. The signalling mechanisms driving this phenotypic change are unknown but deserve investigation since they could offer a treatment approach to muscle weakness in COPD.
Acknowledgments
The study was funded by the MRC, NHLI and DMETA. Our thanks Dr Chris Barclay (Griffith University, Australia) and Prof Nancy Curtin (Imperial College London) for critically reviewing the manuscript, to Dr Martin Webb and Mrs Jackie Hunter for production and supply of the phosphate binding protein, and also Dr Martin Webb, Dr John Corrie and Mr Gordon Reid for the MDCC and NPE-caged ATP. We are also grateful to Ms Lorraine Lawrence (NHLI, Imperial College) for her assistance with the mATPase staining.
References
- ATS/ERS. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- Barclay CJ. Mechanical efficiency and fatigue of fast and slow muscles of the mouse. J Physiol. 1996;497:718–794. doi: 10.1113/jphysiol.1996.sp021809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrends EM, Schols AM, Nusmeier CM, Van Der Griten CP, Wouters EF. Breathing efficiency during inspiratory threshold loading in patients with chronic obstructive pulmonary disease. Clin Physiol. 1998;18:235–244. doi: 10.1046/j.1365-2281.1998.00097.x. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Canepari M, Pellegrino MA, Reggiani C. Force–velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol. 1996;495:573–586. doi: 10.1113/jphysiol.1996.sp021617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin NA, Woledge RC. Efficiency of energy conversion during sinusoidal movement of white muscle fibres from the dogfish. Scyliorhinus canicula. J Exp Biol. 1993;183:137–147. doi: 10.1242/jeb.158.1.343. [DOI] [PubMed] [Google Scholar]
- De Ruiter CJ, De Haan A. Temperature effect on the force/velocity relationship of the fresh and fatigued human adductor pollicis muscle. Pflugers Archiv. 2000;440:163–170. doi: 10.1007/s004240000284. [DOI] [PubMed] [Google Scholar]
- Dubowitz V. Muscle Biopsy: A Practical Approach. 2. London: Bailliere Tindall; 1985. pp. 19–40. [Google Scholar]
- Ferenczi MA, Goldman YE, Simmons RM. The dependence of force and shortening velocity on substrate concentration in skinned muscle fibres from Rana temporaria. J Physiol. 1984;350:519–543. doi: 10.1113/jphysiol.1984.sp015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch S, McComas A. Influence of human muscle length on fatigue. J Physiol. 1985;362:205–213. doi: 10.1113/jphysiol.1985.sp015671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He ZH, Bottinelli R, Pellegrino MA, Ferenczi MA, Reggiani C. ATP consumption and efficiency of human single muscle fibers with different myosin isoform composition. Biophys J. 2000;79:945–961. doi: 10.1016/S0006-3495(00)76349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He ZH, Chillingworth RK, Brune M, Corrie JE, Trentham DR, Webb MR, Ferenczi MA. ATPase kinetics on activation of rabbit and frog permeabilized isometric muscle fibres: a real time phosphate assay. J Physiol. 1997;501:125–148. doi: 10.1111/j.1469-7793.1997.125bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He ZH, Chillingworth RK, Brune M, Corrie JE, Webb MR, Ferenczi MA. The efficiency of contraction in rabbit skeletal muscle fibres, determined from the rate of release of inorganic phosphate. J Physiol. 1999;517:839–854. doi: 10.1111/j.1469-7793.1999.0839s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Stienen GJ, Barends JP, Ferenczi MA. Rate of phosphate release after photoliberation of adenosine 5-triphosphate in slow and fast skeletal muscle fibers. Biophys J. 1998;75:2389–2401. doi: 10.1016/s0006-3495(98)77683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B Biol Sci. 1938;126:136–195. doi: 10.1098/rspb.1949.0019. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Babcock MA, Suman OE, Dempsey JA. Exercise-induced diaphragmatic fatigue in healthy humans. J Physiol. 1993;460:385–405. doi: 10.1113/jphysiol.1993.sp019477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol. 1993;472:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Gregory C, Nguyen T, Shrager J, Kaiser L, Rubinstein N, Dudley G. Bioenergetic adaptation of individual human diaphragmatic myofibers to severe COPD. J Appl Physiol. 2002;92:1205–1213. doi: 10.1152/japplphysiol.00116.2001. [DOI] [PubMed] [Google Scholar]
- Levine S, Kaiser L, Leferovich J, Tikunov B. Cellular adaptations in the diaphragm in chronic obstructive pulmonary disease. N Engl J Med. 1997;337:1799–1806. doi: 10.1056/NEJM199712183372503. [DOI] [PubMed] [Google Scholar]
- Levine S, Nguyen T, Kaiser LR, Rubinstein NA, Maislin G, Gregory C, Rome LC, Dudley GA, Sieck GC, Shrager JB. Human diaphragm remodeling associated with chronic obstructive pulmonary disease: clinical implications. Am J Respir Crit Care Med. 2003;168:706–713. doi: 10.1164/rccm.200209-1070OC. [DOI] [PubMed] [Google Scholar]
- Lowe DA, Thomas DD, Thompson LV. Force generation, but not myosin ATPase activity, declines with age in rat muscle fibers. Am J Physiol Cell Physiol. 2002;283:C187–C192. doi: 10.1152/ajpcell.00008.2002. [DOI] [PubMed] [Google Scholar]
- Macgowan NA, Evans KG, Road JD, Reid WD. Diaphragm injury in individuals with airflow obstruction. Am J Respir Crit Care Med. 2001;163:1654–1659. doi: 10.1164/ajrccm.163.7.2001042. [DOI] [PubMed] [Google Scholar]
- McGregor M, Becklake MR. The relationship of oxygen cost of breathing to respiratory mechanical work and respiratory force. J Clin Invest. 1961;41:971–980. doi: 10.1172/JCI104336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercadier JJ, Schwartz K, Schiaffino S, Wisnewsky C, Ausoni S, Heimburger M, Marrash R, Pariente R, Aubier M. Myosin heavy chain gene expression changes in the diaphragm of patients with chronic lung hyperinflation. Am J Physiol Lung Cell Mol Physiol. 1998;274:L527–L534. doi: 10.1152/ajplung.1998.274.4.L527. [DOI] [PubMed] [Google Scholar]
- Moore AJ, Stubbings A, Swallow EB, Dusmet M, Goldstraw P, Porcher R, Moxham J, Polkey MI, Ferenczi MA. Passive properties of the diaphragm in COPD. J Appl Physiol. 2006;101:1400–1405. doi: 10.1152/japplphysiol.01614.2005. [DOI] [PubMed] [Google Scholar]
- Orozco-Levi M, Gea J, Lloreta JL, Felez M, Minguella J, Serrano S, Broquetas JM. Subcellular adaptation of the human diaphragm in chronic obstructive pulmonary disease. Eur Respir J. 1999;13:371–378. doi: 10.1183/09031936.99.13237199. [DOI] [PubMed] [Google Scholar]
- Orozco-Levi M, Lloreta J, Minguella J, Serrano S, Broquetas JM, Gea J. Injury of the human diaphragm associated with exertion and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1734–1739. doi: 10.1164/ajrccm.164.9.2011150. [DOI] [PubMed] [Google Scholar]
- Ottenheijm CA, Heunks LM, Hafmans T, Van Der Ven PF, Benoist C, Zhou H, Labeit S, Granzier HL, Dekhuijzen PN. Titin and diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:527–534. doi: 10.1164/rccm.200507-1056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenheijm CA, Heunks LM, Sieck GC, Zhan WZ, Jansen SM, Degens H, de Boo T, Dekhuijzen PN. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:200–205. doi: 10.1164/rccm.200502-262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care. 2001;46:798–825. [PubMed] [Google Scholar]
- Polkey MI, Kyroussis D, Hamnegard CH, Hughes PD, Rafferty GF, Moxham J, Green M. Paired phrenic nerve stimuli for the detection of diaphragm fatigue in humans. Eur Respir J. 1997a;10:1859–1864. doi: 10.1183/09031936.97.10081859. [DOI] [PubMed] [Google Scholar]
- Polkey MI, Kyroussis D, Hamnegard CH, Mills GH, Green M, Moxham J. Diaphragm strength in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;154:1310–1317. doi: 10.1164/ajrccm.154.5.8912741. [DOI] [PubMed] [Google Scholar]
- Polkey MI, Kyroussis D, Hamnegard CH, Mills GH, Hughes PD, Green M, Moxham J. Diaphragm performance during maximal voluntary ventilation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997b;155:642–648. doi: 10.1164/ajrccm.155.2.9032207. [DOI] [PubMed] [Google Scholar]
- Polkey MI, Kyroussis D, Keilty SE, Hamnegard CH, Mills GH, Green M, Moxham J. Exhaustive treadmill exercise does not reduce twitch transdiaphragmatic pressure in patients with COPD. Am J Respir Crit Care Med. 1995;152:959–964. doi: 10.1164/ajrccm.152.3.7663810. [DOI] [PubMed] [Google Scholar]
- Ranatunga KW. The force–velocity relation of rat fast- and slow-twitch muscles examined at different temperatures. J Physiol. 1984;351:517–529. doi: 10.1113/jphysiol.1984.sp015260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco P, McIntyre DB, Jones DA. Effects of length and stimulation frequency on fatigue of the human tibialis anterior muscle. J Appl Physiol. 1994;77:1148–1154. doi: 10.1152/jappl.1994.77.3.1148. [DOI] [PubMed] [Google Scholar]
- Scano G, Grazzini M, Stendardi L, Gigliotti F. Respiratory muscle energetics during exercise in healthy subjects and patients with COPD. Resp Med. 2006;100:1896–1906. doi: 10.1016/j.rmed.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Scott A, Wang X, Road JD, Reid WD. Increased injury and intramuscular collagen of the diaphragm in COPD: autopsy observations. Eur Respir J. 2006;27:51–59. doi: 10.1183/09031936.06.00143004. [DOI] [PubMed] [Google Scholar]
- Siththanandan VB, Donnelly JL, Ferenczi MA. Effect of strain on actomyosin kinetics in isometric muscle fibres. Biophys J. 2006;90:3653–3665. doi: 10.1529/biophysj.105.072413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens ED, Faulkner JA. The capacity of mdx mouse diaphragm muscle to do oscillatory work. J Physiol. 2000;522:457–466. doi: 10.1111/j.1469-7793.2000.t01-3-00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienen GJ, Kiers JL, Bottinelli R, Reggiani C. Myofibrillar ATPase activity in skinned human skeletal muscle fibres: fibre type and temperature dependence. J Physiol. 1996;493:299–307. doi: 10.1113/jphysiol.1996.sp021384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow EB, Gosker HR, Ward KA, Moore AJ, Dayer MJ, Hopkinson NS, Schols AM, Moxham J, Polkey MI. A novel technique for non-volitional assessment of quadriceps muscle endurance in man. J Appl Physiol. 2007;103:739–746. doi: 10.1152/japplphysiol.00025.2007. [DOI] [PubMed] [Google Scholar]
- Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol. 2003;552:47–58. doi: 10.1113/jphysiol.2003.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]