Abstract
Purinergic signalling plays a major role in the function of every organ including the brain. A growing body of evidence also suggests that purinergic signalling is important in the development of the retina, cochlea and neocortex. In these three contexts release of ATP through the spontaneous gating of connexin hemichannels in cells, respectively, of the retinal pigment epithelium, Köllicker's organ, and the radial glia triggers waves of intracellular Ca2+ release. In the case of the developing retina and cortex, the released ATP acts to control proliferation of neuronal precursor cells, while in the cochlea it coordinates the spontaneous activity of adjacent hair cells to refine the tonotopic maps in the cochlear nucleus. Recently ATP-derived ADP signalling has been implicated at the very earliest stages of development, notably in triggering the gene expression necessary for formation of the eye. It is now timely to test the extent to which connexin hemichannel-mediated ATP release and accompanying Ca2+ waves contribute to all stages of development.
Introduction
Understanding the development of the nervous system remains one of the great challenges in contemporary biology. In a structure of this complexity, cell-to-cell interactions must play an essential role in creating the intricately ordered final structure. There are many ways that cells can interact, encompassing membrane bound ligands and receptors (e.g. Notch) as well as diffusible messengers capable of interacting with membrane bound receptors (e.g. Wnt). Recent evidence suggests that ATP and related purines can be added to the list of diffusible signalling messengers that contribute to intercellular interactions during development.
This review will highlight three striking examples of purinergic signalling in ‘late’ development – events occurring within organs already substantially developed – and the recent finding that purinergic signalling also plays a role during the very earliest stages of development. My aims are to bring out common principles underlying the three examples of late stage development, to describe the recent finding that ATP signalling triggers eye development, and to propose that the principles uncovered during late development can guide our thinking on signalling mechanisms during early development.
The relevant signalling components in these examples of development involve: P2X receptors, ionotropic ATP receptors; P2Y receptors, G-protein coupled ATP receptors; IP3, inositol 1,4,5-trisphosphate; and the connexins, a gene family of subunits that make up connexons or hemichannels (each composed of 6 connexins) and gap junctions (2 connexons docked together to make the intercellular channel).
This review does not seek to present a comprehensive overview of the role of purinergic signalling in development. For that I direct the reader to a recent review by Zimmermann (2006).
‘Late’ stages of development
Retina
The developing retina is a multilayered structure (Fig. 1A and B). The outermost layer, the retinal pigment epithelium (RPE) is essential for the normal development of the underlying layers (Raymond & Jackson, 1995), collectively termed the neural retina. The portion of the neural retina closest to the RPE is called the ventricular zone, and it is here that progenitor cells divide to generate the neurons and glia that comprise the fully developed retina. The rate of cell division in the ventricular zone depends upon the presence of, and close association with, the RPE, suggesting signalling between these two tissue layers.
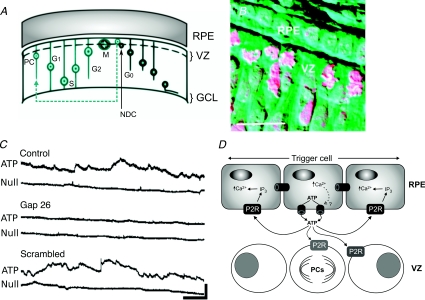
Figure 1. Ca2+ signalling in the developing retina.
A, diagram of the retinal pigment epithelium (RPE) and neural retina showing the ventricular zone (VZ) where progenitor cells (PC) divide. The non-dividing daughter cell (NDC) migrates towards the ganglion cell layer (GCL), the progenitor cell migrates through the layers of the retina as it goes through the cell cycle (G1, S, G2, M). B, photomicrograph demonstrating the proximity of the RPE to the VZ. Mitotic cells are clearly visible in the VZ. C, spontaneous ATP release from the RPE measured in real-time with an ATP-selective biosensor. The Null sensor does not respond to ATP and acts as a control. This release is blocked by the connexin mimetic peptide Gap26, which blocks connexin 43 hemichannels selectively. A scrambled version of Gap26 has no effect on ATP release. (Scale bar 100 pA, 50 s.) D, the model of connexin hemichannel-mediated ATP release mediating Ca2+ waves in the RPE and controlling mitosis of progenitor cells in the VZ. A and B modified from Pearson et al. (2002) with permission; ©2002 by the Society for Neuroscience. C and D modified from Pearson et al. (2005), with permission from Elsevier, ©2005.
ATP signalling associated with Ca2+ waves occurs in both the RPE (Pearson et al. 2004, 2005) and the developing neural retina (Sugioka et al. 1996; Pearson et al. 2002). In the neural retina, activation of ATP receptors by exogenous agonists evokes an increase of intracellular Ca2+ in, and controls the rate of division of, progenitor cells (Sugioka et al. 1999; Pearson et al. 2002; Nunes et al. 2007). Spontaneous Ca2+ waves occur in the RPE and can trigger Ca2+ waves in nearby portions of the neural retina (Pearson et al. 2004). The propagation of Ca2+ waves through the RPE depends on the release of ATP (Pearson et al. 2005), possibly from a single RPE cell. As the ATP diffuses from this focal point of release, it activates P2Y receptors (G-protein coupled) on adjacent cells leading to the release of Ca2+ from internal stores and hence the appearance of a propagating Ca2+ wave (Fig. 1D). The ATP is released through the spontaneous opening of connexin 43 hemichannels. The mechanism of this spontaneous gating has not been explored but it may involve transient increases in intracellular Ca2+ (Pearson et al. 2005). Critically, specific peptide blockers of connexin 43 hemichannels not only prevent ATP release (Fig. 1C), they also slow cell division in the ventricular zone of the neural retina. This demonstrates that the RPE controls division in the neural retina through the release of ATP via connexin hemichannels, which subsequently activates P2Y receptors on the precursor cells in the ventricular zone. The function of the Ca2+ waves in the RPE remains uncertain – they are not required for the release of ATP; rather they depend upon an initial ATP release event which is sufficient for their propagation without, for example, further Ca2+-dependent ATP release (Pearson et al. 2005). In this regard, the function of Ca2+ waves in the RPE remains unclear. Perhaps they are required for the release of other factors from the RPE, or changes in gene expression, which may control other aspects of development (Pearson et al. 2005).
Cochlea
Like the retina, the cochlea is a multilayered and highly ordered structure. The inner hair cells transduce specific frequencies and contact their follower cells, the spiral ganglion neurons, which project to the cochlear nucleus in the pons. The location of the hair cells within the cochlea determines their optimal sound frequency for activation. Precise wiring of the hair cells, via the spiral ganglion neurons, creates a tonotopic map in the cochlear nucleus that is fundamental to the accurate perception of sound. Spontaneous activity in the auditory nerve fibres during development, and before the onset of hearing, is essential for the establishment of these tonotopic maps.
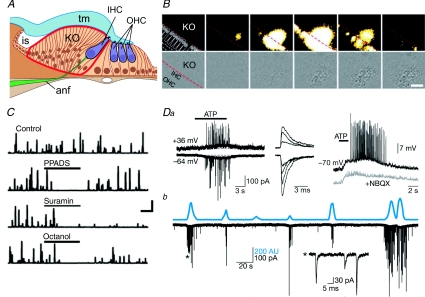
A transient structure termed Köllicker's organ (KO) is present during development of the cochlea (Fig. 2A). This is an epithelial ridge that lies adjacent to the layers containing the hair cells. In a recent study Tritsch et al. (2007) demonstrated that the cells within KO exhibit spontaneous electrical potentials. These potentials are mediated by the spontaneous release of ATP acting via P2Y and P2X receptors. Interestingly these cells also exhibit spontaneous Ca2+ waves that depend upon ATP receptor activation (Fig. 2B). Furthermore the release of ATP occurs via connexin hemichannels (Fig. 2C). The identity of these channels has not been identified, but Cx26 – mutations of which are a major cause of deafness – is an obvious candidate. The spontaneous release of ATP from KO cells depolarizes hair cells (Fig. 2D), which have ATP receptors. This causes them to release glutamate and evoke bursts of action potentials in the spiral ganglion follower neurons that comprise the afferent auditory nerve fibres. Crucially, these authors demonstrated that the activity of neighbouring hair cells is coordinated by the release of ATP from the KO cells and has little correlation with the activity of more distant hair cells. They suggest that this is a critical mechanism for establishing the tonotopic map in the cochlear nucleus prior to the onset of hearing.
Figure 2. ATP signalling regulates activity of hair cells in the developing cochlea.
A, diagram of the developing cochlea demonstrating the proximity of Köllicker's organ (KO) to the inner hair cells (IHC). OHC, outer hair cells; tm, tympanic membrane; anf, auditory nerve fibre. B, spontaneous Ca2+ wave occurring in KO spreads to the inner hair cells (top row). This Ca2+ wave is accompanied by a spontaneous optical change (SOC), which acts as a proxy measure for the changes in intracellular Ca2+ (bottom row). C, the SOCs can be quantified and plotted against time. Their frequency of occurrence is diminished by PPADS and suramin (P2 receptor antagonists) and octanol (gap junction channel blocker). Da, application of ATP evokes an inward current in IHCs accompanied by fast EPSCs. The underlying depolarization is unaffected by NBQX (AMPA receptor antagonist), which blocks the fast EPSCs. b, most SOCs are accompanied by inward currents in the IHCs, demonstrating the Ca2+ wave and accompanying release of ATP from KO spontaneously stimulates the IHCs. A–D adapted from Tritsch et al. (2007) by permission from Macmillan Publishers Ltd, ©2007.
Neural progenitor cells
In the developing cortex, radial glial cells are now recognized as neural progenitor cells (Malatesta et al. 2000; Noctor et al. 2001; reviewed by Rakic, 2003a,b, 2006) and give rise to most if not all excitatory cortical neurons (Noctor et al. 2002; Anthony et al. 2004). Their cell bodies are found in the ventricular zone of the developing cortex (next to the lateral ventricle), and they have a radial process that projects dorsally to reach the subpial surface where it ramifies into an endfoot in the marginal zone. After asymmetrical division, their daughter cells migrate dorsally along the radial process and differentiate into the neurons found in the cortical layers. In this sense the radial glia are also supporting cells that help to direct the development of the complex cortical structure (Rakic, 1971, 1972, 1988).
ATP signalling among the radial glial cells controls their entry into the S-phase of the cell cycle. Weissman et al. (2004) demonstrated the presence of spontaneous propagating Ca2+ waves among the radial glia. These waves were blocked by ATP receptor antagonists, particularly those specific for the P2Y1 receptor. The ATP is released from the radial glial cells via the spontaneous gating of connexin hemichannels. The particular hemichannel involved has not been identified, but radial glial cells possess both connexins 43 and 26 (Elias et al. 2007). The increasing incidence of ATP sensitivity of cells, as well as the increasing frequency of, and distance of propagation of, the spontaneous Ca2+ waves occurs about midway through neurogenesis. The Ca2+ waves may be initiated in a cell cycle-dependent manner, and the rate of division may depend on P2Y receptor-mediated signalling. Additional studies have lent further support to these findings (Lin et al. 2007).
Common features
From these three examples several prominent mechanistic features emerge (cf. Fig. 1D). Firstly there is an intimate relation between the release of ATP and the presence of intracellular Ca2+ waves: the propagation of the Ca2+ waves depends upon the release and extracellular diffusion of ATP.
Secondly, ATP appears to be released via the apparently spontaneous gating of connexin hemichannels, and is independent of extracellular Ca2+. The Ca2+ waves themselves, as they depend upon release of Ca2+ from intracellular stores, do not require a Ca2+ influx. As pannexins are also permeable to ATP (Bao et al. 2004), release of ATP via the homologous innexin channels could play a role in invertebrate development too. Interestingly, there is strong coordination between the transcription of gap junction proteins and P2 receptors (Iacobas et al. 2007).
Thirdly, the signalling occurs between different tissue layers in the retina and cochlea. The same is not immediately evident for the neural progenitor cells. However the radial glia, as well as being the progenitors, perform the role of supporting and directing migration of the daughter cells (Elias et al. 2007) and could thus be considered functionally equivalent to the RPE of the retina or the KO cells of the cochlea. However, an important distinction is that the radial glial cells themselves undergo proliferation in contrast to the RPE cells and KO cells. Finally, P2Y receptors play a critical role in each example and trigger release of Ca2+ from internal stores via an IP3-dependent mechanism.
The paradigm of synaptic release of neurotransmitters such as ATP involves their packaging in vesicles and release via Ca2+-dependent exocytosis (Edwards & Gibb, 1993). These three examples of ATP release during development involve a channel-mediated release mechanism. This raises the question as to whether there may be an advantage to utilization of this mechanism, as opposed to exocytosis, for developmental signalling. One possibility is that signalling may need to be accomplished over quite large distances, e.g. from one tissue layer to another, and channel-mediated release may give a greater amount of ATP for diffusion over these distances compared to exocytosis, which typically at synapses operates in the context of specialized structures over the much shorter distance of the synaptic cleft. Assuming that ATP carries only 1% of the total charge going through a connexin hemichannel, such as those composed of Cx43 subunits, then in 1 s around 3 × 105 ATP molecules will leave via a single channel (cf. Saez et al. 2005). This is equivalent to the fusion of 100 vesicles (if they contain 3 × 103 molecules of ATP). Thus a complement of 102 connexin hemichannels can effectively allow release of ATP equivalent to 105 vesicles per second. This channel-mediated mechanism may therefore be a more effective and less energetically costly mechanism for the release of larger quantities of ATP compared to exocytosis.
The mechanism of apparent spontaneous gating of the hemichannels remains largely opaque, although Pearson et al. (2005) demonstrated a dependence on intracellular Ca2+ transients. It will be fascinating to explore whether this hemichannel gating is truly spontaneous, or whether underlying mechanistic reasons for gating at particular times with particular characteristics can be uncovered.
‘Early’ stages
Until recently there was little direct evidence for the involvement of ATP-mediated signalling in early development. However, a growing body of indirect evidence – the early developmental expression of key molecules involved in purinergic signalling – made this hypothesis likely. In the frog embryo at least three P2Y receptors are expressed early in development, around the time of gastrulation (Bogdanov et al. 1997; Devader et al. 2007; Masséet al. 2007). Furthermore various enzymes with ectoATPase activity (Devader et al. 2006), including those from the E-NTPDase family (Masséet al. 2006), are also expressed around the time of gastrulation. During early mammalian development there is also extensive expression of both receptors and ectonucleotidases (Cheung et al. 2003; Langer et al. 2007).
Eye development
The eye develops from anterior ectoderm at either side of the neural plate. This is orchestrated by a network of eye field transcription factors (EFTFs) that has been at least partially conserved through evolution. In vertebrates, the EFTF genes include Pax6, Six3, Rx1 and Lhx2 (Zuber et al. 2003). Homologous genes control the development of the eye in Drosophila. Although the EFTFs are well known, the mechanism by which they are activated to specify the eye has remained unclear. Very recently Masséet al. (2007) have provided compelling evidence for the involvement of purine-mediated signalling at these very early developmental stages, in the development of both the nervous system and the eye in Xenopus.
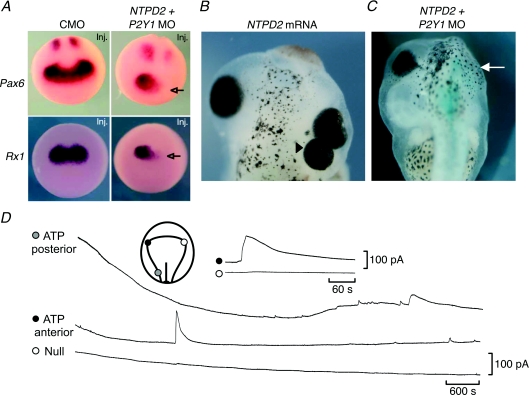
Massé and colleagues (2007) found that over-expression of E-NTPDase2 (ecto nucleoside triphosphate diphosphohydrolase) in the anterior part of the embryo could induce ectopic eye formation (Fig. 3B). This effect of E-NTPDase2 over-expression was exacerbated by simultaneous over-expression of the P2Y1 receptor – selective for ADP, the product of E-NTPDase 2, in Xenopus. Manipulation of the expression of E-NTPDase2 altered the expression of the endogenous EFTFs such as Pax6, Rx1, Otx2 and Six3. In particular simultaneous knockdown of the expression of E-NTPDase2 and the P2Y1 receptor could almost completely abolish Pax6 and Rx1 expression (Fig. 3A). This key observation demonstrates that endogenous purinergic signalling by ADP, generated by E-NTPDase2 and acting on the P2Y1 receptor, lies upstream of the EFTFs. This manipulation also greatly reduced the size of the eyes and, in some cases, prevented them from developing (Fig. 3C). By making biosensor recordings from the location of the eye field, Masséet al. (2007) demonstrated a brief period of ATP release (Fig. 3D). This presumably supplies the substrate for E-NTPDase2 and precedes the activation of the P2Y1 receptor.
Figure 3. Development of the eye depends on purine-mediated signalling.
A, morpholino antisense knockdown of E-NTPDase2 and P2Y1R expression (NTPD2 + P2Y1 MO) targeted to one side of the embryo reduces the expression of the EFTFs Pax6 and Rx1 on the targeted side (arrow), compared to expression in embryos injected with a control morpholino construct (CMO). This demonstrates that ADP-mediated signalling lies upstream of EFTF expression. B, over-expression of E-NTPDase2 can trigger formation of extra eyes (triangle). C, morpholino knock down of E-NTPDase2 and P2Y1 receptors can prevent the eye from developing (arrow). D, ATP signalling in the very early embryo. A single period of ATP release is seen in the location of the future eye field. In the posterior neural plate frequent ATP release events are seen, suggesting a role for ATP signalling in the development of the nervous system. Compare these ATP signalling events with those of Fig. 1C. Adapted from Masséet al. (2007).
Several aspects of this novel mechanism are rather intriguing. A transient period of ATP release lasting only a few minutes appears sufficient to evoke an essentially permanent effect (the development of the eye). P2Y1 receptors will internalize after relatively brief exposure to their agonist (30 min, Tulapurkar et al. 2006), perhaps suggesting why the period of ATP release is so short. The EFTFs form a ‘reverberatory’ gene network. Rx1 activates Pax6, which then activates Six3, Lhx2 and tll, each of which reinforces the expression of Pax6 (Zuber et al. 2003). Pax6 can also up-regulate its own expression. The existence of this positive feedback within the EFTF network explains why only a brief signal may be required to trigger a very long lasting effect. This work raises many unanswered questions for future investigation, such as the identity of the ATP-releasing cells, the mechanism by which ATP is released, and how activation of the P2Y1 receptor turns on EFTF expression.
A hypothesis for early development
Application of the consensus model of ATP signalling evident from later stages of development would predict that ATP release in the early embryo should also occur through spontaneous hemichannel gating and underlie the generation of intracellular Ca2+ waves. Interestingly as in the retina and cochlea, this signalling during early development occurs within the context of a multilayered structure where signals from mesoderm may influence the developmental fate of the overlying ectoderm.
Ca2+ waves occur during early development
Several studies have reported the occurrence of Ca2+ waves around the time of gastrulation in both zebra fish and Xenopus embryos (Webb & Miller, 2003, 2006). A gradual rise in intracellular Ca2+ coupled with transient waves occurs between stages 9 and 12 in the dorsal ectoderm of Xenopus (Leclerc et al. 2006). Interestingly, from stage 10 onwards the Ca2+ transients were restricted to the dorsal animal cap (region of prospective forebrain, midbrain and eyes). This overlaps with the approximate location and time of the ATP release observed by Masséet al. (2007).
Ca2+ signalling has been investigated in explanted tissue, where it shows patterns similar to that seen in situ (Wallingford et al. 2001; Webb et al. 2005). The waves of Ca2+ depend upon release from internal stores. There is a lack of consensus over whether the Ca2+ waves are necessary for the activation of gene expression, but the convergent extension movements that cells of the involuting marginal zone undergo during gastrulation appear to depend on these waves. So far the dependence of these waves on ATP signalling has not been investigated. Nevertheless, application of the consensus model of ATP-dependent Ca2+ waves would predict that both the waves and convergent extension should be blocked by application of the appropriate ATP receptor antagonists.
Occurrence of gap junction proteins in early development
Several connexins are expressed during, and have been implicated in, early development in Xenopus (de Boer & van der Heyden, 2005; de Boer et al. 2006). In an experiment performed well before knowledge of connexins and gap junction structure, Warner et al. (1984) injected an antibody raised against a gap junction protein (now known to be connexin 32) into the dorsal blastomere of an eight-cell embryo. This targeted anterior dorsal structures including nervous system. The embryos developed with a range of head defects including reduction of nervous system and eye (and in some cases complete loss) on the injected side. This phenotype is remarkably similar to the effect following knock down of expression of E-NTPDase2 and the P2Y1 receptor reported by Masséet al. (2007).
Around 10 years after this experiment, Paul et al. (1995) generated a Cx32–43 chimera that was unable to assemble with the endogenous Cx38 present in early Xenopus embryos – a dominant negative mutant. Injection of the mRNA for this construct into the dorsal blastomere of the eight-cell embryos gave a similar phenotype to the Warner et al. results, including loss of the eye on the injected side. This phenotype could be rescued by coinjection of Cx37 mRNA, which could form gap junctions in the presence of the chimeric construct, demonstrating that the phenotype was specifically due to loss of functional gap junction channels.
Both these studies suggest that the developmental defects arise from the disruption of functional gap junctions between cells in the early embryo. While this may indeed be true, it is also possible to reinterpret these findings as an effect on connexin hemichannel function interfering with the release of ATP and the effective propagation of intracellular Ca2+ waves rather than gap junction coupling per se.
Concluding remarks
Intracellular Ca2+ signalling is prevalent during early development, together with the early expression of connexins. This raises the prospect that connexin hemichannels could play an important signalling role at these developmental stages. Indeed some evidence strongly implicates the connexins in development of anterior dorsal structures such as the eye (Paul et al. 1995) – however, it remains to be established whether the connexins participate as hemichannels or fully fledged gap junctions. ADP signalling is critical in turning on genes for development of the eye around the time of gastrulation. Circumstantial evidence suggests that the consensual model of hemichannel-mediated ATP release, giving rise to Ca2+ waves in developing tissues, may apply to very early stages of development. Extension of purinergic signalling to the control of gene expression at the very earliest stages of development marks an exciting new frontier in understanding the cell–cell interactions required to convert the genetic blueprint into a fully developed organism. Further explorations of this hypothesis are urgently needed.
References
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Bogdanov YD, Dale L, King BF, Whittock N, Burnstock G. Early expression of a novel nucleotide receptor in the neural plate of Xenopus embryos. J Biol Chem. 1997;272:12583–12590. doi: 10.1074/jbc.272.19.12583. [DOI] [PubMed] [Google Scholar]
- Cheung KK, Ryten M, Burnstock G. Abundant and dynamic expression of G protein-coupled P2Y receptors in mammalian development. Dev Dyn. 2003;228:254–266. doi: 10.1002/dvdy.10378. [DOI] [PubMed] [Google Scholar]
- de Boer TP, Kok B, Roel G, van Veen TA, Destree OH, Rook MB, Vos MA, de Bakker JM, van der Heyden MA. Cloning, embryonic expression, and functional characterization of two novel connexins from Xenopus laevis. Biochem Biophys Res Commun. 2006;349:855–862. doi: 10.1016/j.bbrc.2006.08.121. [DOI] [PubMed] [Google Scholar]
- de Boer TP, van der Heyden MA. Xenopus connexins: how frogs bridge the gap. Differentiation. 2005;73:330–340. doi: 10.1111/j.1432-0436.2005.00026.x. [DOI] [PubMed] [Google Scholar]
- Devader C, Drew CM, Geach TJ, Tabler J, Townsend-Nicholson A, Dale L. A novel nucleotide receptor in Xenopus activates the cAMP second messenger pathway. FEBS Lett. 2007;581:5332–5336. doi: 10.1016/j.febslet.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Devader C, Webb RJ, Thomas GM, Dale L. Xenopus apyrase (xapy), a secreted nucleotidase that is expressed during early development. Gene. 2006;367:135–141. doi: 10.1016/j.gene.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ. ATP – a fast neurotransmitter. FEBS Lett. 1993;325:86–89. doi: 10.1016/0014-5793(93)81419-z. [DOI] [PubMed] [Google Scholar]
- Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- Iacobas DA, Suadicani SO, Iacobas S, Chrisman C, Cohen MA, Spray DC, Scemes E. Gap junction and purinergic p2 receptor proteins as a functional unit: insights from transcriptomics. J Membr Biol. 2007;217:83–91. doi: 10.1007/s00232-007-9039-7. [DOI] [PubMed] [Google Scholar]
- Langer D, Ikehara Y, Takebayashi H, Hawkes R, Zimmermann H. The ectonucleotidases alkaline phosphatase and nucleoside triphosphate diphosphohydrolase 2 are associated with subsets of progenitor cell populations in the mouse embryonic, postnatal and adult neurogenic zones. Neuroscience. 2007;150:863–879. doi: 10.1016/j.neuroscience.2007.07.064. [DOI] [PubMed] [Google Scholar]
- Leclerc C, Neant I, Webb SE, Miller AL, Moreau M. Calcium transients and calcium signalling during early neurogenesis in the amphibian embryo Xenopus laevis. Biochim Biophys Acta. 2006;1763:1184–1191. doi: 10.1016/j.bbamcr.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Lin JH, Takano T, Arcuino G, Wang X, Hu F, Darzynkiewicz Z, Nunes M, Goldman SA, Nedergaard M. Purinergic signaling regulates neural progenitor cell expansion and neurogenesis. Dev Biol. 2007;302:356–366. doi: 10.1016/j.ydbio.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- Massé K, Bhamra S, Eason R, Dale N, Jones EA. Purine-mediated signalling triggers eye development. Nature. 2007;449:1058–1062. doi: 10.1038/nature06189. [DOI] [PubMed] [Google Scholar]
- Massé K, Eason R, Bhamra S, Dale N, Jones EA. Comparative genomic and expression analysis of the conserved NTPDase gene family in Xenopus. Genomics. 2006;87:366–381. doi: 10.1016/j.ygeno.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Wong WS, Clinton BK, Kriegstein AR. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci. 2002;22:3161–3173. doi: 10.1523/JNEUROSCI.22-08-03161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes PH, Calaza KD, Albuquerque LM, Fragel-Madeira L, Sholl-Franco A, Ventura AL. Signal transduction pathways associated with ATP-induced proliferation of cell progenitors in the intact embryonic retina. Int J Dev Neurosci. 2007;25:499–508. doi: 10.1016/j.ijdevneu.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Paul DL, Yu K, Bruzzone R, Gimlich RL, Goodenough DA. Expression of a dominant negative inhibitor of intercellular communication in the early Xenopus embryo causes delamination and extrusion of cells. Development. 1995;121:371–381. doi: 10.1242/dev.121.2.371. [DOI] [PubMed] [Google Scholar]
- Pearson RA, Catsicas M, Becker DL, Bayley P, Luneborg NL, Mobbs P. Ca2+ signalling and gap junction coupling within and between pigment epithelium and neural retina in the developing chick. Eur J Neurosci. 2004;19:2435–2445. doi: 10.1111/j.0953-816X.2004.03338.x. [DOI] [PubMed] [Google Scholar]
- Pearson R, Catsicas M, Becker D, Mobbs P. Purinergic and muscarinic modulation of the cell cycle and calcium signaling in the chick retinal ventricular zone. J Neurosci. 2002;22:7569–7579. doi: 10.1523/JNEUROSCI.22-17-07569.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46:731–744. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus rhesus. J Comp Neurol. 1971;141:283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P. Elusive radial glial cells: historical and evolutionary perspective. Glia. 2003a;43:19–32. doi: 10.1002/glia.10244. [DOI] [PubMed] [Google Scholar]
- Rakic P. Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex. 2003b;13:541–549. doi: 10.1093/cercor/13.6.541. [DOI] [PubMed] [Google Scholar]
- Rakic P. A century of progress in corticoneurogenesis: from silver impregnation to genetic engineering. Cereb Cortex. 2006;16(Suppl. 1):i3–17. doi: 10.1093/cercor/bhk036. [DOI] [PubMed] [Google Scholar]
- Raymond SM, Jackson IJ. The retinal pigmented epithelium is required for development and maintenance of the mouse neural retina. Curr Biol. 1995;5:1286–1295. doi: 10.1016/s0960-9822(95)00255-7. [DOI] [PubMed] [Google Scholar]
- Saez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta. 2005;1711:215–224. doi: 10.1016/j.bbamem.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugioka M, Fukuda Y, Yamashita M. Ca2+ responses to ATP via purinoceptors in the early embryonic chick retina. J Physiol. 1996;493:855–863. doi: 10.1113/jphysiol.1996.sp021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugioka M, Zhou WL, Hofmann HD, Yamashita M. Involvement of P2 purinoceptors in the regulation of DNA synthesis in the neural retina of chick embryo. Int J Dev Neurosci. 1999;17:135–144. doi: 10.1016/s0736-5748(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- Tulapurkar ME, Zundorf G, Reiser G. Internalization and desensitization of a green fluorescent protein-tagged P2Y nucleotide receptor are differently controlled by inhibition of calmodulin-dependent protein kinase II. J Neurochem. 2006;96:624–634. doi: 10.1111/j.1471-4159.2005.03594.x. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Ewald AJ, Harland RM, Fraser SE. Calcium signaling during convergent extension in Xenopus. Curr Biol. 2001;11:652–661. doi: 10.1016/s0960-9822(01)00201-9. [DOI] [PubMed] [Google Scholar]
- Warner AE, Guthrie SC, Gilula NB. Antibodies to gap-junctional protein selectively disrupt junctional communication in the early amphibian embryo. Nature. 1984;311:127–131. doi: 10.1038/311127a0. [DOI] [PubMed] [Google Scholar]
- Webb SE, Miller AL. Calcium signalling during embryonic development. Nat Rev Mol Cell Biol. 2003;4:539–551. doi: 10.1038/nrm1149. [DOI] [PubMed] [Google Scholar]
- Webb SE, Miller AL. Ca2+ signaling and early embryonic patterning during the blastula and gastrula periods of zebrafish and Xenopus development. Biochim Biophys Acta. 2006;1763:1192–1208. doi: 10.1016/j.bbamcr.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Webb SE, Moreau M, Leclerc C, Miller AL. Calcium transients and neural induction in vertebrates. Cell Calcium. 2005;37:375–385. doi: 10.1016/j.ceca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Nucleotide signaling in nervous system development. Pflugers Arch. 2006;452:573–588. doi: 10.1007/s00424-006-0067-4. [DOI] [PubMed] [Google Scholar]
- Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA. Specification of the vertebrate eye by a network of eye field transcription factors. Development. 2003;130:5155–5167. doi: 10.1242/dev.00723. [DOI] [PubMed] [Google Scholar]