Abstract
The NMDA-type glutamate receptor is a heteromeric complex composed of the NR1 and at least one of the NR2 subunits. Switching from the NR2B to the NR2A subunit is thought to underlie functional alteration of the NMDA receptor during synaptic maturation, and it is generally believed that it results in preferential localization of NR2A subunits on the synaptic site and that of NR2B subunits on the extracellular site in the mature brain. It has also been proposed that activation of the NR2A and NR2B subunits results in long-term potentiation (LTP) and long-term depression (LTD), respectively. Furthermore, recent reports suggest that synaptic and extrasynaptic receptors may have distinct roles in synaptic plasticity as well as in gene expression associated with neuronal death. Here, we have investigated whether NR2B subunit-containing receptors are present and functional at mature synapses in the lateral nucleus of the amygdala (LA) and the CA1 region of the hippocampus, comparing their properties between the two brain regions. We have found, in contrast to the above hypotheses, that the NR2B subunit significantly contributes to synaptic transmission as well as LTP induction. Furthermore, its contribution is greater in the LA than in the CA1 region, and biophysical properties of NMDA receptors and the NR2B/NR2A ratio are different between the two brain regions. These results indicate that NR2B subunit-containing NMDA receptors accumulate on the synaptic site and are responsible for the unique properties of synaptic function and plasticity in the amygdala.
The NMDA receptor plays central roles in neural development, synaptic plasticity and pathological changes in the CNS (Choi, 1988; McDonald & Johnston, 1990; Collingridge & Bliss, 1995). The induction of LTP is blocked by NMDA receptor antagonists (Collingridge & Bliss, 1995), and spatial learning is impaired by the pharmacological blockade of NMDA receptors in vivo (Morris et al. 1986) and by the genetic deletion of NMDA receptor subunits (Sakimura et al. 1995; Tsien et al. 1996). Recent studies suggest that the NMDA receptor also plays a critical role in synaptic plasticity in the amygdala (Bauer et al. 2002; Nakazawa et al. 2006), which is associated with the acquisition and expression of fear memory (Davis, 1992; LeDoux, 2000). The LA is the region where the sensory inputs associated with the formation of fear memory (for instance, tone and footshock) first converge during fear conditioning (Romanski et al. 1993). Infusion of NMDA-receptor antagonists into the LA blocks the acquisition and expression of fear memory (Miserendino et al. 1990; Lee & Kim, 1998; Lee et al. 2001), suggesting that NMDA receptor-dependent synaptic plasticity in the LA underlies the cellular changes that mediate associative fear learning. Thus, the NMDA receptor functions as a key molecule for the induction of LTP in the hippocampus and amygdala.
The NMDA receptor is a heteromeric complex containing NR1 (GluRζ) subunits, which are obligatory for channel activation, and at least one of NR2A–2D (GluRɛ1-ɛ4) subunits (Kutsuwada et al. 1992; Monyer et al. 1992; Ishii et al. 1993; Cull-Candy et al. 2001), which determine pharmacological and biophysical properties of the NMDA receptor such as the Mg2+ sensitivity, single channel conductance, open probability, and current decay time constant as well as the interaction with intracellular signalling and scaffolding molecules (Monyer et al. 1994; Mori & Mishina, 1995). The expression of NR2B subunits is developmentally regulated: neurons express NR2B subunit-containing NMDA receptors alone at birth, and start to express NR2A subunits gradually when nascent synapses are formed extensively after birth (McDonald & Johnston, 1990; Watanabe et al. 1992; Monyer et al. 1994). This developmental switch of the subunit expression is interpreted as the cause of the disproportional distribution of NMDA receptor subunits in mature neurons: NR2A subunit-containing NMDA receptors are localized on the synaptic site and NR2B subunit-containing receptors on the extrasynaptic site (Tovar & Westbrook, 1999). However, the exact nature and impact of this process are not well characterized (Stocca & Vicini, 1998; Mohrmann et al. 2000).
Recently, a number of reports suggest that NR2A and NR2B subunit-containing receptors may have different, and sometimes opposing, roles in the regulation of synaptic plasticity, receptor localization and signal transduction. For instance, NR2A and NR2B subunit-containing receptors may be involved specifically in LTP and LTD, respectively (Liu et al. 2004; Massey et al. 2004). A recent study has shown that administration of the NR2B-selective antagonist ifenprodil (Williams, 1993) to the LA blocks the acquisition of fear memory (Rodrigues et al. 2001), suggesting that the synaptic NR2B subunit may play a role in synaptic plasticity in the LA. However, it is not known whether NR2B subunits exist at synapses in the LA and whether they contribute to the induction of synaptic plasticity. In this study, we have characterized the electrophysiological properties of NMDA receptors and examined functional contributions of NR2B subunit-containing NMDA receptors to synaptic transmission and plasticity at the thalamo-LA synapse, comparing them with those at Schaffer collateral/commissural–CA1 synapses.
Methods
Animals
All experiments were performed in accordance with the guidelines of Animal Care and Experimentation Committee of the University of Tokyo and Hokkaido University.
Slice preparation and electrophysiology
Male C57BL/6J mice (6–10 weeks old; CLEA Japan Inc., Japan) were deeply anaesthetized with halothane and then decapitated. The brain was quickly removed and transferred to an ice-cold medium saturated with 95% O2 and 5% CO2. The medium contained (in mm): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgSO4, 1.0 NaH2PO4, 26.2 NaHCO3 and 11 glucose. Coronal amygdaloid slices (400 μm thickness) containing the LA and transverse hippocampal slices (400 μm thickness) were prepared in the medium with a tissue slicer (Vibratome, The Vibratome Company, St Louis, MO, USA) and placed in a humidified interface-type holding chamber for at least 1 h before recordings. A single slice was then transferred to the recording chamber and submerged with a nylon net stretched out on a U-shaped piece of flattened platinum wire beneath a continuously perfusing medium (flow rate: ∼2 ml min−1) that had been saturated with 95% O2 and 5% CO2. The composition of the medium used for recordings was the same as that used for slice preparations, unless otherwise stated, except that all the perfusing solutions contained 100 μm picrotoxin to block GABAA receptor-mediated inhibitory synaptic responses. In some experiments (Figs 4 and 6), slices were preincubated with the medium containing ifenprodil, an NR2B-selective antagonist (10 μm), for at least 1 h to ensure sufficient and maximal exposure of slices to the antagonist. For amygdaloid slices, the dorsal region of the cortex was cut off with a scalpel to prevent the propagation of epileptiform activity. For hippocampal slices, the CA3 region was surgically cut off from the CA1 region. Whole-cell patch-clamp recordings were made from neurons in the dorsal subdivision of the LA and from pyramidal cells in the hippocampal CA1 region, using the ‘blind’ technique or under visual guidance with a ×60 water-immersion objective attached to an upright microscope. Cells were voltage-clamped at −90 mV to record excitatory postsynaptic currents (EPSCs), unless otherwise indicated. The whole-cell pipette solution contained (in mm): 122.5 caesium gluconate, 17.5 CsCl, 10 Hepes, 0.2 EGTA, 8 NaCl, 2 Mg-ATP and 0.3 Na3-GTP (pH 7.2; 290–310 mosmol l−1). The values of membrane potentials were corrected for the liquid junction potential at the electrode tip. For evoking synaptic responses, electrical stimuli were delivered at 0.1 Hz through a bipolar tungsten stimulating electrode placed in the ventral striatum just medial to the LA to stimulate fibres originating in the auditory thalamus for amygdaloid slices (Weisskopf et al. 1999) and in the stratum radiatum for hippocampal slices. When NMDA receptor-mediated EPSCs were recorded, 10 μm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) was present to block AMPA receptor-mediated EPSCs. LTP was induced by stimulating afferent fibres at 1 Hz for 100 s while depolarizing the cell to −10 mV. An Axopatch-1D amplifier and Digidata1322A (Molecular Devices, Union City, CA, USA) were used to record and store the data with pCLAMP9.2 software (Molecular Devices), respectively. The signal was filtered at 5 kHz and digitized at 10–20 kHz. Recording electrodes had resistances of 3–7 MΩ. The series resistance was 10–30 MΩ and monitored on-line throughout the experiment. Experiments were rejected if the series resistance changed by more than 20%. Series resistance compensation was not used. For the analysis of EPSCs, we measured their peak amplitudes. The magnitude of LTP was calculated as a percentage of the averaged EPSC amplitude value from 40 to 50 min after the conditioning relative to the baseline EPSC amplitude value. All experiments were performed at 25–27°C. Drugs used were CNQX, d-(–)-2-amino-5-phosphonovaleric acid (d-APV), ifenprodil (Tocris Bioscience, Bristol, UK) and picrotoxin (Wako Pure Chemical Industries, Osaka, Japan).
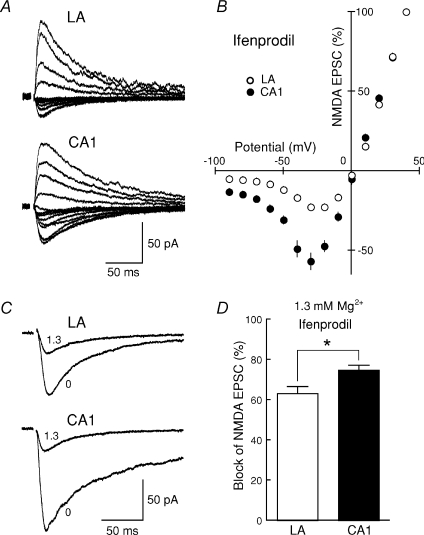
Figure 4. The difference in I–V curves and Mg2+ sensitivity of NMDA EPSCs between the LA and the CA1 region is not attributed to the difference in the contribution of NR2B subunit-containing NMDA receptors.
A, after slices were preincubated in the medium containing 10 μm ifenprodil for at least 1 h, NMDA EPSCs were recorded from neurons in the LA and the CA1 region at +40 to −90 mV in the presence of ifenprodil. B, the I–V relationship of NMDA EPSCs in the LA (○, n = 6) and the CA1 region (•, n = 7) in the presence of ifenprodil. The current amplitudes were normalized to the value obtained at +40 mV in each cell, and the values were then averaged for all cells. C, after slices were preincubated in the medium containing 10 μm ifenprodil for at least 1 h, NMDA EPSCs were recorded at a membrane potential of −40 mV in 0 (nominally Mg2+-free solution) and 1.3 mm Mg2+ solutions in the presence of ifenprodil. D, the Mg2+ sensitivity (1.3 mm) of NMDA EPSCs was calculated for each cell, and the values were averaged for all cells. The Mg2+ sensitivity in the presence of ifenprodil was still significantly lower in the LA than in the CA1 region.
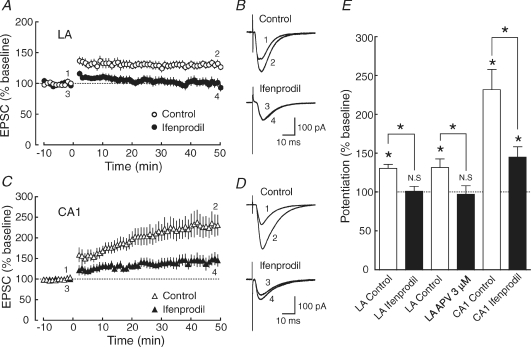
Figure 6. The induction of LTP in the LA requires the activation of NR2B subunit-containing NMDA receptors to a greater extent than in the CA1 region.
A, the averaged time course of LTP in the LA (○: control, n = 19; •: ifenprodil, n = 14; P = 0.0124). The EPSC peak amplitude was normalized in each experiment to the averaged EPSC peak amplitude during the baseline period (−10–0 min). LTP was induced by applying 100 pulses at 1 Hz while depolarizing the cell to −10 mV at time 0. B, sample traces of EPSCs (average of 10 consecutive responses) in the LA recorded at the times indicated by the numbers in A. C, the averaged time course of LTP in the CA1 region (▵: control, n = 17; ▴: ifenprodil, n = 13; P = 0.0123). D, sample traces of EPSCs (average of 10 consecutive responses) in the CA1 region recorded at the times indicated by the numbers in C. E, summary of the effects of ifenprodil on LTP induction in the LA and the CA1 region. The reduction of LTP in the presence of ifenprodil in the LA (left: control, open bars, P < 0.0001; ifenprodil, filled bars, P = 0.807) was larger than in the CA1 region (right: control, open bars, P = 0.00012; ifenprodil, filled bars, P = 0.00533). Student's t test (paired) was used for the statistical analysis.
Immunogold electron microscopy
The post-embedding immunogold analysis was performed as previously described (Fukaya et al. 2003). Under deep pentobarbital anaesthesia (100 mg (kg body weight−1)), C57BL/6J mice at 8 weeks of age were perfused transcardially with 4% paraformaldehyde–0.1% glutaraldehyde in 0.1 m sodium phosphate buffer (pH 7.2). The LA and hippocampal slices were cryoprotected with 30% sucrose in 0.1 m phosphate buffer (PB), and frozen rapidly with liquid propane in a Leica EM CPC unit. Frozen sections were immersed in 0.5% uranyl acetate in methanol at −90°C in a Leica AFS freeze-substitution unit, infiltrated at −45°C with Lowicryl HM-20 resin (Lowi, Waldkraiburg, Germany), and polymerized with UV light. After etching with saturated sodium ethanolate solution for 3 s, ultra-thin sections on nickel grids were treated successively with 1% human serum albumin (Wako Pure Chemical Industries)–0.1% Triton X-100 in Tris-buffered saline (HTBST pH 7.5) for 1 h, a rabbit anti-NR2A or NR2B antibody (15 μg ml−1 for each) (Watanabe et al. 1998) in HTBST overnight, and colloidal gold (10 nm)-conjugated anti-rabbit IgG (1: 100, British Biocell International, Cardiff, UK) in HTBST for 2 h. Finally, grids were stained with uranyl acetate for 15 min, and examined with an H-7100 electron microscope (Hitachi, Tokyo, Japan). For semiquantitative analyses, the number of gold particles for NR2A and NR2B subunits was counted at asymmetrical axo-spinous synapses in the LA and hippocampal CA1 region, and the NR2B/NR2A ratio was calculated in each region. To compare synaptic and extrasynaptic distribution, the density of gold particles on the synaptic and extrasynaptic membranes of spines was measured using IPlab software (Nippon Roper, Tokyo, Japan). Immunogold particles were judged as cell membrane-associated labelling where there was less than 35 nm from the cell membrane to the centre of the gold particles (Matsubara et al. 1996).
Statistical analysis
The data are expressed as the mean ± s.e.m. Student's t test (unpaired, unless otherwise stated) was used to determine whether there was a significant difference (*P < 0.05) in the mean between the two sets of data. N.S., not significant.
Results
Contribution of the NR2B subunit to synaptic transmission
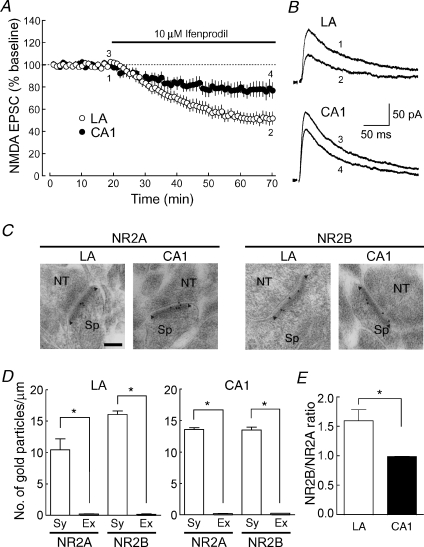
We first examined whether NR2B subunits contributed to NMDA receptor-mediated normal synaptic transmission at thalamo-LA synapses with whole-cell patch-clamp recordings from principal cells in the LA, comparing it with that at Schaffer collateral/commissural–CA1 synapses (CA1 synapses) (Fig. 1A and B). Ifenprodil, which has often been used as an NR2B subunit-selective antagonist, significantly inhibited NMDA EPSCs in both types of synapses; however, the inhibition at the thalamo-LA synapse (open circles: by 48.1 ± 5.2%, n = 10, at 60–70 min) was significantly larger than that at CA1 synapses (filled circles: by 22.3 ± 6.5%, n = 7, P = 0.00708). It has been reported that the time course of synaptic responses of the NMDA receptor consisting of NR1 and NR2A subunits is faster than that of the NMDA receptor consisting of NR1 and NR2B subunits (Köhr & Seeburg, 1996). The decay time constant of NMDA EPSCs in the LA became much faster when the NR2B subunit was selectively blocked by ifenprodil (70.2 ± 5.0 ms), compared with that in the control external solution (87.4 ± 4.1 ms): the difference was highly significant (n = 10, P = 0.00611). On the other hand, the decay time constant in the CA1 region was changed only slightly in the presence of ifenprodil (69.8 ± 5.1 ms), compared with that in the control external solution (77.0 ± 3.7 ms): the difference was marginally significant (n = 7, P = 0.0433). However, the decay time constant of NMDA EPSCs in the control external solution was not significantly different (P = 0.0930) between the CA1 region and LA. These results suggest that the NR2B subunit is a member of the NMDA receptor complex on the synaptic site and that its contribution is greater in the LA.
Figure 1. NMDA receptors at synapses in the LA contain the larger number of NR2B subunits than in the CA1 region.
A, the NR2B subunit-selective antagonist ifenprodil (10 μm; filled bar) reduced NMDA EPSCs to a greater extent in the LA than in the CA1 region (LA: ○, n = 10; CA1, •, n = 7; P = 0.00708 at 60–70 min). Cells were voltage-clamped at +30 mV. Each data point represents the averaged EPSC peak amplitude for 1 min that was normalized to the baseline EPSC peak amplitude. B, sample traces of NMDA EPSCs (average of 10 consecutive responses) in the LA and the CA1 region recorded at the times indicated by the numbers in A. C, the postembedding immunogold staining for NR2A and NR2B subunits at asymmetrical axo-spinous synapses in the LA and the CA1 region. NT, nerve terminal; Sp, spine. Arrowheads indicate the edge of the PSD. Calibration bar, 100 nm. D, the density of immunogold particles for NR2A and NR2B subunits on the synaptic (Sy) and extrasynaptic (Ex) membranes of spines in the LA (left) and the CA1 region (right). E, the NR2B/NR2A ratio of immunogold labelling in the LA and the CA1 region.
To confirm that the NR2B subunit was localized on the synaptic site with a technically different approach, we next performed the postembedding immunogold electron-microscopic analysis at excitatory, asymmetrical axo-spinous synapses in the LA and the CA1 region (Fig. 1C–E). In the two brain regions, both NR2A and NR2B subunits were enriched on the synaptic membrane, which was defined as the membrane associated with the postsynaptic density (PSD), an electron-dense submembrane cytoskeleton. In contrast, these subunits were far less expressed at the extrasynaptic membrane in the spine, which was defined as the membrane outside the edge of the PSD (arrowheads in Fig. 1C). The density of synaptic versus extrasynaptic NR2A subunits in the spine (particles μm−1) was 10.45 ± 1.74 versus 0.24 ± 0.02, respectively, in the LA (n = 30 synapses, P = 0.00417) and 13.59 ± 0.29 versus 0.21 ± 0.03, respectively, in the CA1 region (n = 30, P < 0.00001); and that of NR2B subunits was 16.08 ± 0.56 versus 0.18 ± 0.09, respectively, in the LA (n = 34, P < 0.00001) and 13.51 ± 0.45 versus 0.25 ± 0.01, respectively, in the CA1 region (n = 22, P < 0.00001). The relative abundance of synaptic NR2B subunits to synaptic NR2A subunits was significantly greater (n = 300 synapses for each subunit from 3 mice, P = 0.0178) in the LA (1.60 ± 0.19) than in the CA1 region (1.00 ± 0.04) (Fig. 1E). Taken together, these data indicate that NMDA receptors are highly accumulated on synaptic sites in both brain regions, but the content of NR2B subunits relative to NR2A subunits is higher at the thalamo-LA synapse than at the CA1 synapse.
Comparison of electrophysiological properties of synaptic NMDA receptors between the LA and the CA1 region
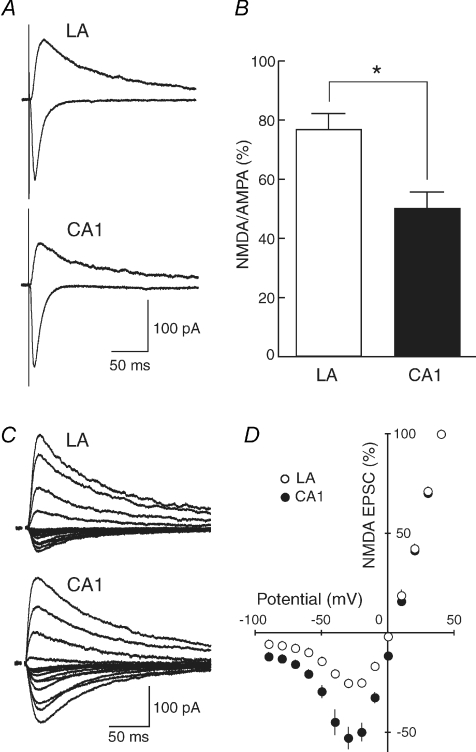
Because the relative contribution of NR2B subunits to basal synaptic transmission in the LA was greater than that in the CA1 region, we next compared the properties of NMDA EPSCs between the two brain regions to test whether the difference in the relative abundance of NR2B subunits affected properties of NMDA EPSCs. We first measured the ratio of NMDA EPSC to AMPA EPSC amplitudes. AMPA EPSCs were recorded at a membrane potential of −90 mV in the voltage-clamp mode and NMDA EPSCs were then recorded at the same stimulus strength in the presence of 10 μm CNQX, a non-NMDA receptor antagonist, at +40 mV to relieve the voltage-dependent Mg2+ block of NMDA receptor channels (Fig. 2A). The ratio of NMDA to AMPA EPSC amplitudes was significantly larger (P = 0.00461) in the LA (76.7 ± 5.3%, n = 13) than in the CA1 region (50.1 ± 5.3%, n = 7) (Fig. 2B).
Figure 2. Distinct basic properties of synaptic transmission between the LA and the CA1 region.
A, AMPA receptor-mediated EPSCs (downward traces) and NMDA receptor-mediated EPSC (upward traces) were recorded at membrane potentials of −90 and +40 mV, respectively. When NMDA EPSCs were recorded, 10 μm CNQX was present to block AMPA EPSCs. B, the ratio of amplitudes of NMDA EPSCs to those of AMPA EPSCs was calculated for each cell, and the values were then averaged for all cells. The ratio was significantly larger in the LA than in the CA1 region (LA, n = 13; CA1, n = 7; P = 0.00461). C, NMDA EPSCs were recorded at membrane potentials between +40 and −90 mV. Stimulus artifacts were truncated for clarity. D, the current–voltage relationship of NMDA synaptic currents in the LA (○, n = 11) and the CA1 region (•, n = 12). The current amplitudes were normalized to the value obtained at +40 mV in each cell, and the values were then averaged for all cells. NMDA EPSCs in the LA displayed significantly smaller synaptic currents in the I–V curve at membrane potentials between 0 and −90 mV than in the CA1 region.
One of the characteristic properties of NMDA receptors is the blockade of the receptor channel by extracellular Mg2+ at negative membrane potentials (Mayer et al. 1984; Nowak et al. 1984). The current–voltage (I–V) curves were constructed by recording NMDA EPSCs from principal neurons in the LA and pyramidal cells in the CA1 region at the membrane potentials between +40 and −90 mV (Fig. 2C and D) to examine whether the difference in the relative abundance of NR2B subunits affected the I–V relationship, although the sensitivity to Mg2+ is similar between the NR2A and NR2B receptor (Mori & Mishina, 1995). The current amplitudes were normalized to the value obtained at +40 mV in each cell, and the values were then averaged for all cells examined (Fig. 2D). The I–V curve in the LA (open circles, n = 11) was significantly different (P < 0.05) from that in the CA1 region (filled circles, n = 12) at the membrane potentials between 0 and 90 mV.
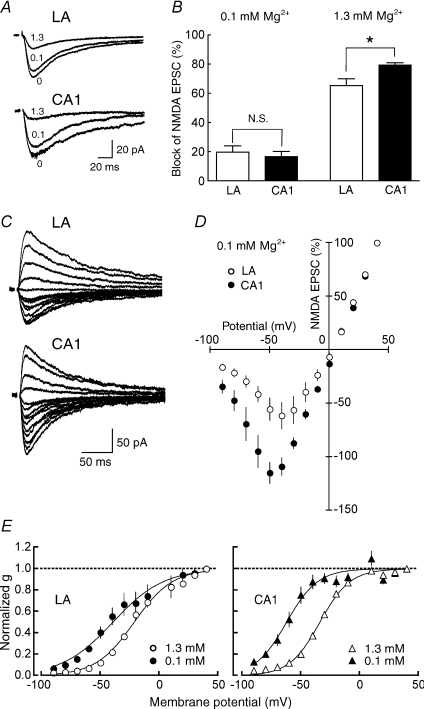
To test whether the difference in the I–V curve was associated with the difference in Mg2+ sensitivity of NMDA receptors between the two brain regions, we compared the inhibition of NMDA EPSCs caused by various concentrations of extracellular Mg2+. After obtaining a stable baseline of NMDA EPSCs at −40 mV in a nominally Mg2+-free solution, the external solution was switched successively to 0.1 mm and 1.3 mm extracellular Mg2+ solutions (Fig. 3A). The blocking effects of extracellular Mg2+ on NMDA EPSCs were 19.9 ± 4.2% (n = 7) in the LA and 16.9 ± 3.5% (n = 5, P = 0.614) in the CA1 region in the presence of 0.1 mm Mg2+ (Fig. 3B, left); and 65.8 ± 4.7% (n = 7) in the LA and 79.3 ± 2.2% (n = 5, P = 0.0457) in the CA1 region in the presence of 1.3 mm Mg2+ (Fig. 3B, right). Thus, the sensitivity to 1.3 mm Mg2+ was significantly lower in the LA than in the CA1 region, but that to 0.1 mm Mg2+ was comparable between the two brain regions at −40 mV. We then examined whether the difference in the I–V curves between the LA and the CA1 region was attributable entirely to the difference in the sensitivity to extracellular Mg2+. Because the sensitivity of NMDA receptors at 0.1 mm Mg2+ was comparable between the LA and the CA1 region (Fig. 3B, left), the difference in the I–V curve should disappear in 0.1 mm Mg2+, if it is totally attributed to the difference in Mg2+ sensitivity; however, the I–V curve of NMDA EPSCs was still significantly different between the LA and the CA1 neurons at 0 to −90 mV (Fig. 3C and D). Therefore, the difference in the I–V curves is at least partially, but not completely, explained by the difference in the sensitivity of NMDA receptors to extracellular Mg2+.
Figure 3. The lower sensitivity of NMDA receptors to extracellular Mg2+ in the LA.
A, NMDA EPSCs were recorded at a membrane potential of −40 mV in the extracellular Mg2+ concentrations indicated by the numbers (in mm): 0 (nominally Mg2+-free solution), 0.1 and 1.3. B, the fraction of NMDA EPSCs blocked by extracellular Mg2+ was calculated for each cell, and the values were averaged for all cells. The blocking effects of extracellular Mg2+ on NMDA EPSCs were shown at the concentration of 0.1 mm (left) and 1.3 mm (right), respectively. The Mg2+ sensitivity was significantly lower in the LA than in the CA1 region in 1.3 mm Mg2+ (LA, n = 7; CA1, n = 5; P = 0.0457) but not in 0.1 mm Mg2+ (LA, n = 7; CA1, n = 5; P = 0.614). C, NMDA EPSCs were recorded at +40 to −90 mV in 0.1 mm Mg2+. D, the current–voltage relationship of NMDA synaptic currents in the LA (○, n = 8) and the CA1 region (•, n = 8) in 0.1 mm Mg2+. The current amplitudes were normalized to the value obtained at +40 mV in each cell, and the values were then averaged for all cells. The I–V curve of NMDA EPSC amplitudes was still significantly (P < 0.05) different between the LA and CA1 neurons at 0 to −90 mV. E, the conductance–voltage plots of NMDA EPSCs in 0.1 (filled symbols) and 1.3 mm Mg2+ (open symbols) in the LA (left) and the CA1 region (right). The conductance was normalized to the value at +40 mV. The data were fitted with Boltzmann functions (LA: [Mg2+]o = 1.3 mm, g/gmax = 1/(1 + exp(−21.2 − V)/16.4), [Mg2+]o = 0.1 mm, g/gmax = 1/(1 + exp(−39.4 − V)/21.6); CA1: [Mg2+]o = 1.3 mm, g/gmax = 1/(1 + exp(−33.8 − V)/12.9), [Mg2+]o = 0.1 mm, g/gmax = 1/(1 + exp(−62.2 − V)/13.3)).
To examine the Mg2+ sensitivity of NMDA receptors more systematically, we converted the I–V curves to conductance–voltage plots and fitted them with Boltzmann functions (Fig. 3E). The membrane potential at which the NMDA receptor channel exhibited 50% of the maximal conductance (V50) in 1.3 mm Mg2+ was −21.2 ± 0.9 mV (n = 11) and −33.8 ± 0.8 mV (n = 12) in the LA and the CA1 region, respectively, and there was a significant difference between the two brain regions (P = 0.00075). There was also a significant difference (P = 0.0159) in the V50 in 0.1 mm Mg2+ between the LA (−39.4 ± 2.4 mV, n = 8) and the CA1 region (−62.2 ± 1.4 mV, n = 8). Furthermore, the difference between V50 in 1.3 mm Mg2+ and V50 in 0.1 mm Mg2+ was larger in the CA1 region (26.5 mV) than that in the LA (18.7 mV). These results suggest that the sensitivity of the NMDA receptor to Mg2+ is generally higher in the CA1 region than that in the LA.
We next examined whether the difference in the I–V curve and the Mg2+ sensitivity of NMDA receptors between the LA and the CA1 region shown above was attributable to the difference in the relative abundance of the NR2B subunit between the two brain regions. After slices were preincubated with ifenprodil (10 μm) for at least 1 h, NMDA EPSCs were recorded at the membrane potentials between +40 and −90 mV in the continuous presence of ifenprodil (10 μm), and the current–voltage relationship of NMDA synaptic currents in the LA and the CA1 region was examined (Fig. 4A and B). The profile of the I–V curve was still significantly different between the two regions (Fig. 4B) as in the absence of ifenprodil (Fig. 2D). We also investigated the sensitivity of NMDA receptors to extracellular Mg2+ in the presence of ifenprodil (10 μm). When NMDA EPSCs were recorded in nominally Mg2+-free and 1.3 mm Mg2+ solutions at −40 mV (Fig. 4C), the Mg2+ sensitivity was still significantly lower (P = 0.0161) (Fig. 4D) in the LA (63.4 ± 3.3%, n = 6) than in the CA1 region (75.0 ± 2.3%, n = 6) as in the absence of ifenprodil (Fig. 3B, right). Collectively, the differences in the I–V curve and the Mg2+ sensitivity of NMDA receptors between the LA and the CA1 region were not attributable to the difference in the relative abundance of the NR2B subunit between the two regions.
Involvement of NR2B subunit-containing synaptic NMDA receptors in LTP induction
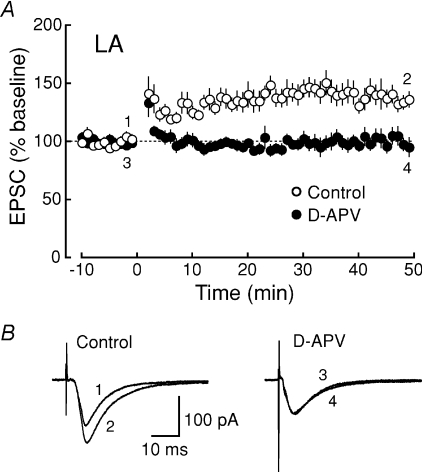
In many types of central synapses, the activation of NMDA receptors is required for synaptic plasticity (Bliss & Collingridge, 1993). We first confirmed that the activation of NMDA receptors was required for LTP induction in the LA (Fig. 5) (Nakazawa et al. 2006). LTP was induced by applying 100 pulses at 1 Hz while depolarizing the cell to −10 mV in the absence (open circles, 137.6 ± 7.2% of baseline, n = 8) and presence (filled circles, 100.7 ± 6.3% of baseline, n = 5) of the NMDA receptor antagonist d-APV (50 μm) (Fig. 5A and B). LTP in the LA induced by this pairing protocol was completely blocked by d-APV (P = 0.00461), indicating that this form of LTP was dependent on the NMDA receptor. To assess a possible role of the NR2B subunit in LTP induction, we next investigated the effect of the blockade of NR2B subunit-containing NMDA receptors on LTP induction. After slices were preincubated with the NR2B subunit-selective antagonist ifenprodil (10 μm) for at least 1 h, LTP was induced by the pairing in the continuous presence of ifenprodil. LTP was reduced in magnitude in both the LA (control, open circles, 130.8 ± 5.4% of baseline, n = 19; ifenprodil, filled circles, 101.5 ± 6.2% of baseline, n = 14; P = 0.00124) (Fig. 6A and B) and the CA1 region (control, open triangles, 232.0 ± 26.3% of baseline, n = 17; ifenprodil, filled triangles, 145.4 ± 13.4% of baseline, n = 13; P = 0.0123) (Fig. 6C and D). In the LA, LTP disappeared almost completely in the presence of ifenprodil (P = 0.807) (Fig. 6E, left), whereas LTP was significantly (P = 0.00533) but only partially inhibited in the CA1 region (Fig. 6E, right), suggesting that LTP induction in the LA required the activation of NR2B subunit-containing NMDA receptors to a greater extent than in the CA1 region.
Figure 5. The induction of LTP at thalamo-LA synapses is NMDA receptor dependent.
A, the averaged time course of LTP in the LA (control, ○, n = 8; d-APV, •, n = 5; P = 0.00461). The EPSC peak amplitude was normalized in each experiment to the averaged EPSC peak amplitude during the baseline period (−10 to 0 min). LTP was induced by applying 100 pulses at 1 Hz while depolarizing the cell to −10 mV at time 0. B, sample traces of EPSCs (average of 10 consecutive responses) recorded at the times indicated by the numbers in A.
We next examined the effect of lower concentrations of d-APV in order to elucidate whether the NR2B subunit selectively contributed to LTP induction in the amygdala. We first examined the dose–inhibition relationship of EPSC amplitudes and found that 3 μm d-APV exhibited a similar extent of synaptic inhibition caused by 10 μm ifenprodil (43.0 ± 4.7% of control, n = 7). When treated with 3 μm d-APV, LTP in the LA was almost completely suppressed (control: 131.9 ± 11.2% of baseline, n = 8; d-APV: 97.7 ± 10.82% of baseline, n = 9, P = 0.0442), suggesting that the suppression of LTP in the LA by 10 μm ifenprodil was a quantitative, rather than qualitative, effect of the antagonists, although we cannot exclude the possibility that the NR2B subunit plays some specific role in the induction of LTP in the amygdala in some conditions.
Discussion
In the present study, we have demonstrated that NR2B subunit-containing NMDA receptors contribute substantially to synaptic NMDA receptor-mediated responses, as well as synaptic plasticity, in the LA and the hippocampal CA1 region even in adult mice. In addition, we have found for the first time that the properties and subunit composition of the NMDA receptor are different between the LA and the CA1 region. Since the properties of NR2A and NR2B subunits are differentially regulated by tyrosine phosphorylation (Köhr & Seeburg, 1996), the difference in the ratio of NR2 subunits between the CA1 region and the LA may result in qualitatively different modification of synaptic functions between the brain regions. In fact, the activation of synaptic NR2B subunit-containing NMDA receptors is involved in the induction of LTP more critically in the LA of the adult mouse (Fig. 6). Thus, our results strongly suggest that NR2B subunits are present at mature synapses and play critical roles in the induction of synaptic plasticity in adult animals.
At birth, most of the glutamatergic synapses express only NR2B subunit-containing NMDA receptors, and the expression of NR2A subunits starts after birth and continues to increase during development (Watanabe et al. 1992; Monyer et al. 1994; Sheng et al. 1994; Zhong et al. 1995). It has been suggested that NR2A subunit-containing NMDA receptors are preferentially targeted to the synaptic site, whereas NR2B subunit-containing NMDA receptors are targeted to the extrasynaptic site (Tovar & Westbrook, 1999). However, in contrast to this hypothesis, the application of ifenprodil caused a clear reduction of synaptic NMDA receptor-mediated currents in principal neurons of the adult LA, which was much larger than that in the CA1 region (Fig. 1A and B), indicating that NR2B subunit-containing NMDA receptors are functional at the synapse. In addition, the immunogold electron-microscopic analysis revealed that NR2B subunit-containing NMDA receptors were more abundantly localized on the synaptic site than the extrasynaptic site in the spine, as is the case for NR2A subunit-containing ones (Fig. 1C and D). An apparent discrepancy between the study by Tovar & Westbrook (1999) and our present study may be due to the difference in the preparation used (cultures versus acute slices) and/or the age of animals examined. Considering a much larger area of the extrasynaptic surface membrane compared to that of synaptic surface membrane in neurons, the absolute number of the extrasynaptic NR2B subunit in the whole neuron could be larger than that of the synaptic NR2B subunit. Thus, it may be reasonable to assume that, in some experimental conditions, extracellular NR2B subunits contribute more predominantly to certain phenomena than synaptic NR2B subunits. Collectively, our present data strongly indicate that the NR2B subunit is targeted to the synaptic site and is involved in synaptic transmission and plasticity at the adult CNS synapse.
Lopez de Armentia & Sah (2003) have reported that the principal cell in the LA expresses a considerable number of synaptic NMDA receptors containing NR2B subunits, which is consistent with our present results; however, no apparent NMDA EPSCs sensitive to NR2B-selective antagonists were detected in the hippocampal CA1 pyramidal cells in the rat at the age of 4 weeks. In contrast to that report, our electrophysiological and morphological analysis in the present study has detected synaptic NR2B subunits (Fig. 1). Furthermore, in our previous study (Sakimura et al. 1995), NMDA EPSCs were clearly recorded in the CA1 pyramidal cell of mutant mice lacking the NR2A subunit and expressing NMDA receptors containing only NR1 and NR2B subunits. The discrepancy in the results on the expression of NR2B subunits in the CA1 region between the studies might be accounted for by the difference in the animal species (rats versus mice) and/or in the experimental conditions such as temperature (33–35°C versus 25–27°C in our study).
The NR2 subunit of NMDA receptors has a large carboxy-terminal domain (Ikeda et al. 1992; Kutsuwada et al. 1992; Meguro et al. 1992; Monyer et al. 1992), which plays essential roles in the regulation of the receptor localization, receptor channel property and downstream signalling via NMDA receptor-associated proteins such as scaffolding proteins, cytoskeletal proteins, signalling proteins, protein kinases and protein phosphatases (Kornau et al. 1997; Husi et al. 2000; Scannevin & Huganir, 2000; Sheng & Kim, 2002). The NR2B subunit interacts with αCaMKII and is phosphorylated by tyrosine kinases such as Fyn (Nakazawa et al. 2006), and mutant mice lacking these molecules exhibit impaired amygdala-dependent emotional behaviour (Chen et al. 1994; Miyakawa et al. 1994). In addition, knockin mutant mice in which Tyr-1472 of the NR2B subunit is mutated to phenylalanine to prevent its phosphorylation show impaired amygdala-dependent tone fear conditioning and amygdaloid synaptic plasticity (Nakazawa et al. 2006). The relatively more abundant expression of the NR2B subunit in the LA than in the CA1 region, which has been found in the present study, may provide characteristic properties to the LA synapse through the modulation by the associated proteins and make it possible to exert amygdala-dependent higher brain functions such as emotion.
The NMDA receptor is characterized by the voltage-dependent Mg2+ block of the receptor channel (Mayer et al. 1984; Nowak et al. 1984). We have found in this study that the sensitivity of NMDA EPSCs to 1.3 mm extracellular Mg2+ is significantly lower in the LA (Fig. 3A and B), and thus, it might account for the difference in the I–V relationship of NMDA EPSCs between the LA and the CA1 region; however, even in 0.1 mm Mg2+, where the Mg2+ sensitivity was not different between the LA and the CA1 region, the I–V curve of NMDA EPSCs is still significantly different (Fig. 3C and D). Thus, the difference in the I–V curve between the LA and the CA1 region is not explained by the difference in sensitivity of the NMDA receptor to the extracellular Mg2+. The precise mechanisms underlying the stronger voltage dependency of the Mg2+ blockade in the I–V relationship (Fig. 2C and D) and weaker Mg2+ sensitivity of the NMDA EPSCs (Fig. 3A and B) observed in the LA are still unknown. It is possible that the difference in the voltage dependency of the Mg2+ blockade might be attributable to the differential post-translational modification in the M2 domain of the NR2A subunit between the two brain regions, which has been suggested to be a determinant of the Mg2+ blockade (Kuner & Schoepfer, 1996). The lower Mg2+ sensitivity of NMDA EPSCs at thalamo-LA synapses discovered in the present study (Fig. 3E), allowing an easier entry of Ca2+ into spines, may make the threshold for synaptic plasticity in the LA lower than in the CA1 region.
The properties of NMDA receptors in the LA were distinct from those in the CA1 region: a greater contribution of the NR2B subunit to synaptic NMDA responses (Fig. 1A and B); a higher NR2B/NR2A ratio (Fig. 1C and E) and a higher ratio of NMDA to AMPA receptor-mediated synaptic transmission (Fig. 2A and B); smaller synaptic currents in the I–V curve of NMDA receptors (Fig. 2C and D); and lower Mg2+ sensitivity (Fig. 3A, B and E). These properties of NMDA receptors in the LA are similar to those in the neonatal hippocampal CA1 region (Kirson & Yaari, 1996; Hsia et al. 1998; Kirson et al. 1999). In fact, the thalamo-LA synapses show paired-pulse depression (data not shown), suggesting higher release probability (Manabe et al. 1993), which is consistent with much higher release probability at the CA1 synapse of neonatal rats than that of rats at the age of 2–3 weeks (Bolshakov & Siegelbaum, 1995). Recent studies have shown that smaller spines, but not larger spines, preferentially contain NR2B subunits (Sobczyk et al. 2005), and LTP is easier to induce in smaller spines (Matsuzaki et al. 2001). Because thalamo-LA synapses contain more NR2B subunits (Fig. 1), these synapses might have a large number of small spines and it might be easier to induce LTP at these synapses. Whereas there are several differences in the properties of NMDA receptors between the LA and CA1 region, the kinetics of NMDA EPSCs in the control external solution was not significantly different between the two brain regions in spite of the clear difference in the effect of ifenprodil on NMDA EPSCs (Fig. 1). Although this may occur if the influence of the difference in the NR2B/NR2A ratio on the current kinetics is smaller than that on the sensitivity of NMDA EPSC amplitudes to ifenprodil for some reason, it might be explained by the presence of NR1–NR2A–NR2B trimers. To clarify this issue, further examination would be required.
It is still an open question what is the physiological significance of the synaptic NMDA receptor subunit composition. Previous reports showed that the NR2B/NR2A ratio in the visual cortex of dark-reared rats was rapidly decreased by light exposure (Quinlan et al.1999a,b), suggesting that the ratio can be regulated by experiences. The change in the NR2B/NR2A ratio may dynamically regulate the threshold of the induction of synaptic plasticity and thus contribute to metaplasticity that controls the following synaptic plasticity (Abraham & Bear, 1996). The difference in the NR2B/NR2A ratio between the LA and the CA1 region might reflect intrinsic variability in synaptic activities among brain regions and introduce the difference in the liability to synaptic metaplasticity. Therefore, dynamic regulation of the NR2B/NR2A ratio in the amygdala and the hippocampus may play a critical role in memory formation in adult animals, and it is interesting to examine possible causal relationship between activity-dependent modification of the LTP threshold and the NMDA receptor subunit composition in future studies.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (A.W., M.W. and T.M.) and Center for Brain Medical Science, 21st Century COE Program (H.M. and T.M.) from the Ministry of Education, Science, Sports, Culture and Technology of Japan, by The Uehara Memorial Foundation (T.M.), by The Ichiro Kanehara Foundation (A.W.), by RISTEX, Japan Science and Technology Agency (JST) (T.M.), and by The Novartis Foundation (Japan) for the Promotion of Science (T.M.). We are grateful to Drs Yuko Sekino and Shizuka Kobayashi for helpful comments on the manuscript.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Bauer EP, Schafe GE, LeDoux JE. NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci. 2002;22:5239–5249. doi: 10.1523/JNEUROSCI.22-12-05239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bolshakov VY, Siegelbaum SA. Regulation of hippocampal transmitter release during development and long-term potentiation. Science. 1995;269:1730–1734. doi: 10.1126/science.7569903. [DOI] [PubMed] [Google Scholar]
- Chen C, Rainnie DG, Greene RW, Tonegawa S. Abnormal fear response and aggressive behavior in mutant mice deficient for α-calcium-calmodulin kinase II. Science. 1994;266:291–294. doi: 10.1126/science.7939668. [DOI] [PubMed] [Google Scholar]
- Choi DW. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988;11:465–469. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Bliss TVP. Memories of NMDA receptors and LTP. Trends Neurosci. 1995;18:54–56. [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Cur Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Fukaya M, Kato A, Lovett C, Tonegawa S, Watanabe M. Retention of NMDA receptor NR2 subunits in the lumen of endoplasmic reticulum in targeted NR1 knockout mice. Proc Natl Acad Sci U S A. 2003;100:4855–4860. doi: 10.1073/pnas.0830996100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia AY, Malenka RC, Nicoll RA. Development of excitatory circuitry in the hippocampus. J Neurophysiol. 1998;79:2013–2024. doi: 10.1152/jn.1998.79.4.2013. [DOI] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Nagasawa M, Mori H, Araki K, Sakimura K, Watanabe M, Inoue Y, Mishina M. Cloning and expression of the ɛ4 subunit of the NMDA receptor channel. FEBS Lett. 1992;313:34–38. doi: 10.1016/0014-5793(92)81178-o. [DOI] [PubMed] [Google Scholar]
- Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, Nakanishi S. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- Kirson ED, Schirra C, Konnerth A, Yaari Y. Early postnatal switch in magnesium sensitivity of NMDA receptors in rat CA1 pyramidal cells. J Physiol. 1999;521:99–111. doi: 10.1111/j.1469-7793.1999.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirson ED, Yaari Y. Synaptic NMDA receptors in developing mouse hippocampal neurones: functional properties and sensitivity to ifenprodil. J Physiol. 1996;497:437–455. doi: 10.1113/jphysiol.1996.sp021779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhr G, Seeburg PH. Subtype-specific regulation of recombinant NMDA receptor-channels by protein tyrosine kinases of the src family. J Physiol. 1996;492:445–452. doi: 10.1113/jphysiol.1996.sp021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau HC, Seeburg PH, Kennedy MB. Interaction of ion channels and receptors with PDZ domain proteins. Curr Opin Neurobiol. 1997;7:368–373. doi: 10.1016/s0959-4388(97)80064-5. [DOI] [PubMed] [Google Scholar]
- Kuner T, Schoepfer R. Multiple structural elements determine subunit specificity of Mg2+ block in NMDA receptor channels. J Neurosci. 1996;16:3549–3558. doi: 10.1523/JNEUROSCI.16-11-03549.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, Mishina M. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:55–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Choi JS, Brown TH, Kim JJ. Amygdalar NMDA receptors are critical for the expression of multiple conditioned fear responses. J Neurosci. 2001;21:4116–4124. doi: 10.1523/JNEUROSCI.21-11-04116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J Neurosci. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Lopez de Armentia M, Sah P. Development and subunit composition of synaptic NMDA receptors in the amygdala: NR2B synapses in the adult central amygdala. J Neurosci. 2003;23:6876–6883. doi: 10.1523/JNEUROSCI.23-17-06876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJA, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara A, Laake JH, Davanger S, Usami S, Ottersen OP. Organization of AMPA receptor subunits at a glutamate synapse: a quantitative immunogold analysis of hair cell synapses in the rat organ of corti. J Neurosci. 1996;16:4457–4467. doi: 10.1523/JNEUROSCI.16-14-04457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Rev. 1990;15:41–70. doi: 10.1016/0165-0173(90)90011-c. [DOI] [PubMed] [Google Scholar]
- Meguro H, Mori H, Araki K, Kushiya E, Kutsuwada T, Yamazaki M, Kumanishi T, Arakawa M, Sakimura K, Mishina M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992;357:70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- Miserendino MJ, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Watanabe S, Niki H. Increased fearfulness of Fyn tyrosine kinase deficient mice. Mol Brain Res. 1994;27:179–182. doi: 10.1016/0169-328x(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Mohrmann R, Hatt H, Gottmann K. Developmental regulation of subunit composition of extrasynaptic NMDA receptors in neocortical neurones. Neuroreport. 2000;11:1203–1208. doi: 10.1097/00001756-200004270-00012. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Mori H, Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology. 1995;34:1219–1237. doi: 10.1016/0028-3908(95)00109-j. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Watabe AM, Kiyama Y, Fukaya M, Arima-Yoshida F, Horai R, Sudo K, Ebine K, Delawary M, Goto J, Umemori H, Tezuka T, Iwakura Y, Watanabe M, Yamamoto T, Manabe T. NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity. EMBO J. 2006;25:2867–2877. doi: 10.1038/sj.emboj.7601156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci U S A. 1999a;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci. 1999b;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J Neurosci. 2001;21:6889–6896. doi: 10.1523/JNEUROSCI.21-17-06889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Clugnet MC, Bordi F, LeDoux JE. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci. 1993;107:444–450. doi: 10.1037//0735-7044.107.3.444. [DOI] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, Mishina M. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor ɛ1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- Scannevin RH, Huganir RL. Postsynaptic organization and regulation of excitatory synapses. Nat Rev Neurosci. 2000;1:133–141. doi: 10.1038/35039075. [DOI] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298:776–780. doi: 10.1126/science.1075333. [DOI] [PubMed] [Google Scholar]
- Sobczyk A, Scheuss V, Svoboda K. NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. J Neurosci. 2005;25:6037–6046. doi: 10.1523/JNEUROSCI.1221-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receprots in developing rat cortical neurons. J Physiol. 1998;507:13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Fukaya M, Sakimura K, Manabe T, Mishina M, Inoue Y. Selective scarcity of NMDA receptor channel subunits in the stratum lucidum (mossy fibre-recipient layer) of the mouse hippocampal CA3 subfield. Eur J Neurosci. 1998;10:478–487. doi: 10.1046/j.1460-9568.1998.00063.x. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport. 1992;3:1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Bauer EP, LeDoux JE. L-type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J Neurosci. 1999;19:10512–10519. doi: 10.1523/JNEUROSCI.19-23-10512.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Zhong J, Carrozza DP, Williams K, Pritchett DB, Molinoff PB. Expression of mRNAs encoding subunits of the NMDA receptor in developing rat brain. J Neurochem. 1995;64:531–539. doi: 10.1046/j.1471-4159.1995.64020531.x. [DOI] [PubMed] [Google Scholar]