Abstract
Although gephyrin is an important postsynaptic scaffolding protein at GABAergic synapses, the role of gephyrin for GABAergic synapse formation and/or maintenance is still under debate. We report here that knocking down gephyrin expression with small hairpin RNAs (shRNAs) in cultured hippocampal pyramidal cells decreased both the number of gephyrin and GABA(A) receptor clusters. Similar results were obtained by disrupting the clustering of endogenous gephyrin by overexpressing a gephyrin-EGFP fusion protein that formed aggregates with the endogenous gephyrin. Disrupting postsynaptic gephyrin clusters also had transynaptic effects leading to a significant reduction of GABAergic presynaptic boutons contacting the transfected pyramidal cells. Consistent with the morphological decrease of GABAergic synapses, electrophysiological analysis revealed a significant reduction in both the amplitude and frequency of the spontaneous inhibitory postsynaptic currents (sIPSCs). However, no change in the whole-cell GABA currents was detected, suggesting a selective effect of gephyrin on GABA(A) receptor clustering at postsynaptic sites. It is concluded that gephyrin plays a critical role for the stability of GABAergic synapses.
Introduction
Gephyrin is a cytoplasmic protein that accumulates at the postsynaptic complex of GABAergic and glycinergic synapses where it forms submembranous lattices associated with postsynaptic clusters of GABAA receptors (GABAARs) and glycine receptors (GlyRs) respectively (Kneussel and Betz 2000). Studies with a gephyrin-deficient mouse mutant (geph-/-) have shown that while gephyrin is essential for the synaptic clustering of glycine receptors (Essrich et al., 1998; Feng et al., 1998; Levi et al., 2004), gephyrin is only essential for the clustering of some GABAARs (Kneussel et al., 1999, 2001; Levi et al., 2004).
The geph-/- mouse mutant dies soon after birth. Thus the study of GABAAR clusters in these mutants is normally done in embryonic tissue or neuronal cultures derived from embryonic tissue. In the gephyrin knockout mouse or in the corresponding neuronal cultures, some of the observed phenotypes (i.e. decreased number of GABAAR clusters) might result from developmental defects, while the absence of a phenotypic change might be due to compensatory mechanisms. Therefore, some of the conclusions reached with the geph-/- mouse need to be tested with other independent approaches. The RNA interference (RNAi, Dykxhoorn et al., 2003; Zeringue and Constantine-Paton 2004) is an alternative to the gene knockout technology. With the RNA interference approach, there is a knockdown (not a knockout from the day of gestation) of gephyrin, which is still expressed during the treatment. The knockdown by RNA interference is done during a short time-window (i.e. between 10 and 15 days in culture of E18 neurons). In such a short time and with gephyrin being present, it is considerably less likely that compensatory and/or silencing mechanisms occur. In the present study, we have used gephyrin RNAi to knock down the expression of gephyrin in cultured hippocampal pyramidal cells. We have also used the overexpression of a gephyrin-EGFP fusion protein construct, which forms aggregates and interferes with the normal clustering of endogenous gephyrin. The gephyrin RNAi and gephyrin-EGFP overexpression experiments indicate that gephyrin is essential for the postsynaptic clustering of many GABAARs.
Our approaches have also led to an observation that has not been uncovered by studying the geph-/- mouse mutant, namely that postsynaptic clustering of gephyrin is essential for the maintenance of the GABAergic synapses. We have previously shown that knocking down the γ2 GABAAR subunit in pyramidal cells leads to decreased density of both γ2 subunit-containing GABAAR (γ2-GABAAR) clusters and gephyrin clusters, and to reduced GABAergic innervation on pyramidal cells (Li et al., 2005b). Thus, the postsynaptic clustering of γ2-GABAARs and gephyrin is tightly linked to each other and is essential for the stability of presynaptic GABAergic contacts.
Results
Knocking down gephyrin with gephyrin shRNAs decreases the clustering of both gephyrin and GABAARs
In cultured hippocampal neurons, gephyrin forms clusters that highly colocalize with γ2 subunit-containing GABAAR (γ2-GABAAR) clusters (Craig et al., 1996; Christie et al., 2002b). In these cultures, about 90% of gephyrin clusters colocalized with γ2-GABAAR clusters and 95% of all γ2-GABAAR clusters colocalized with gephyrin (Christie et al., 2002b). We have investigated whether in these cultures, the clustering of gephyrin is essential for the clustering of GABAARs.
Transfection of cultured hippocampal pyramidal cells with shRNAs (Fig 1A) that target either the coding region of gephyrin (Geph CR) or the non-coding 3′ UTR region of gephyrin (Geph UTR) led to a large reduction of both gephyrin and γ2-GABAAR clusters in the transfected cells (Fig 1B and D), when compared to cells transfected with the corresponding mutated shRNAs Geph CR3m and Geph UTR3m (Fig 1C and E, respectively), as revealed by immunofluorescence with antibodies to gephyrin and to the γ2 GABAAR subunit respectively. Transfected pyramidal cells were identified by EGFP fluorescence signal that was co-transfected with shRNAs (green color, Fig 1 B-E).
Fig. 1. Gephyrin knock down by shRNAs reduces the density of both gephyrin and γ2-GABAAR clusters.

A, The gephyrin shRNAs used in this study. The three point mutations introduced in the control shRNAs are shown in red (see materials and methods); B-E, Cultured hippocampal neurons were co-transfected with pEGFP-N1 and Geph CR (B), or Geph CR3m (C), or Geph UTR (D), or Geph UTR3m (E) shRNAs. Triple-label immunofluorescence was done using mAb to gephyrin (red color) and rabbit anti-γ2 GABAAR antibodies (blue color). EGFP fluorescence of transfected neurons is shown in green color. The smaller panels at the right side of each figure show at higher magnification the corresponding boxed area. Arrows in B and D show dendrites of a non-transfected neuron which has much higher density of gephyrin clusters and γ2-GABAAR clusters than the dendrites of the sister neurons (green color) transfected with Geph CR or Geph UTR shRNAs respectively. Arrowheads show gephyrin clusters that colocalize with γ2-GABAAR clusters. Scale bar: 10 μm for large panels; 5 μm for the small panels. F, Immunoblots with anti-gephyrin and anti-α tubulin antibodies of hippocampal cultures transfected by nucleofection with Geph CR, Geph CR3m, Geph UTR, or Geph UTR3m shRNAs. G. Quantification of the immunoblots shown in F. Values for Geph CR and Geph UTR represent percentages of the normalized intensity of the gephyrin protein bands (normalized to the density of α-tubulin) when compared to that of the corresponding 100% control value (pyramidal cells transfected with Geph CR3m or Geph UTR3m shRNAs).
Quantitative immunofluorescence analysis showed that in pyramidal cells transfected with Geph CR shRNA, gephyrin cluster density was significantly reduced to 22.3±2.2% when compared to the gephyrin cluster density in non-transfected sister pyramidal cells from the same culture (Fig 1B, red fluorescence and Fig 2G). The arrow in Fig 1B shows dendrites from a non-transfected neuron with normal density of gephyrin clusters. Note that the neighbor transfected neuron (green) shows a high reduction in the number of gephyrin clusters. The pyramidal neurons transfected with Geph CR shRNA also showed a significant reduction in γ2-GABAAR cluster density (to 27.4±1.7%), as shown in Fig 1B blue fluorescence, and Fig 2G. The remaining gephyrin and γ2-GABAAR clusters showed a high level of co-localization in dendrites (Fig 1B, arrowheads), where 98.0±3.5% of the remaining gephyrin clusters colocalized with γ2-GABAAR clusters and 80.0±3.9% of the remaining γ2-GABAAR clusters colocalized with gephyrin. Neurons transfected with a control shRNA containing three point mutations (Geph CR3m) showed no significant reduction in gephyrin cluster density (93.9±3.8%) or γ2-GABAAR cluster density (91.6±3.3%) compared with sister non-transfected neurons (Fig 1C and Fig 2G).
Fig. 2. Gephyrin knock down reduces the clustering of various GABAAR subunits to different extent.
A-F, Cultured hippocampal neurons were co-transfected with pEGFP-N1 and Geph CR (A, C, and E) or Geph CR3m (B, D, and F) shRNAs. Triple label immunofluorescence was done by using combinations of the mouse mAb to gephyrin and rabbit anti-α1, or rabbit anti-α2 GABAAR subunit antibodies or the combination of rabbit anti-γ2 and the mouse mAb to β2/3. Note that the neurons transfected (EGFP fluorescence) with Geph CR (C and E) show a high reduction in the cluster density of gephyrin and α2, β2/3, and γ2-GABAARs when compared to neurons transfected with the control Geph CR3m (D and F). However, the reduction of α1 subunit containing GABAAR clusters (A) is small when compared with Geph CR3m (B). G and H: Quantification of the effect of the gephyrin shRNAs on gephyrin and the GABAAR cluster density. Values (mean±SEM) represent the percentages compared to the corresponding internal control (non-transfected sister cells in the same cultures). Significant differences with the corresponding internal control values are indicated by asterisks (**, p<0.01; ***, p<0.001 in Student's t-test). Comparisons between groups using one-way ANOVA Tukey test when compared at p<0.05 showed that the mutated shRNAs (Geph CR3m or Geph UTR3m) had no effect on the density of gephyrin and GABAAR clusters. The cluster densities (number of clusters/100 μm2; mean ± SEM) in sister non-transfected cells in the Geph CR shRNA transfection experiments were 21.1±0.9 (632) for gephyrin, 20.9±0.9 (628) for γ2, 23.9±0.8 (717) for α1, 15.7±0.8 (472) for α2, and 17.7±0.8 for β2/3 (531) (The number of counted clusters is shown in parenthesis). Similar values for the non-transfected cells were obtained in the other transfection experiments. Arrowheads show colocalizing gephyrin and GABAAR clusters. Scale bar: 5 μm.
Neurons transfected with a shRNA targeting the 3′ untranslated region of gephyrin mRNA (Geph UTR) also showed a large and significant reduction in gephyrin cluster density (to 36.3±3.0%) and γ2-GABAAR density (to 40.1±2.3%) as shown in Fig 1D and Fig 2H. Pyramidal cells transfected with the corresponding control shRNA containing three point mutations (Geph UTR3m) showed no significant reduction in gephyrin clusters (94.9±3.7%) or γ2-GABAAR clusters (91.5±4.0%) when compared to non-transfected sister pyramidal cells (Fig 1E and Fig 2H) from the same culture. The arrow in Fig 1D shows dendrites from a non-transfected neuron with normal density of gephyrin clusters.
The Geph CR and the Geph UTR shRNAs reduced the levels of gephyrin expression in the culture as shown in immunoblots after nucleofection (Fig 1F). Quantification of the density of the gephyrin band in immunoblots after normalization with the density of the α-tubulin band, showed that Geph CR and Geph UTR shRNAs significantly reduced gephyrin expression in hippocampal cultures (to 60.3±3.1% and 67.1±2.1%) when compared with cultures transfected with Geph CR3m and Geph UTR3m shRNAs respectively (Fig 1G). The reduction of gephyrin expression in the whole culture was attenuated when compared to individual transfected pyramidal cells because only about 40% of the cells in the culture became transfected after nucleofection, and even the cells transfected with Geph CR or Geph UTR shRNAs did not show complete disappearance of the gephyrin clusters. About 20% of the gephyrin clusters remained in the transfected cells. Thus, in the whole culture, gephyrin protein is expected to decrease by approximately 32% (or 0.4×0.8) which is similar to the gephyrin reduction observed in the immunoblots after transfecting the cultures with Geph CR and Geph UTR shRNAs (Fig 1F and G).
Transfection of pyramidal cells with Geph CR or Geph UTR shRNAs also led to large and significant reductions in α2-GABAAR cluster density (to 32.2±4.1% and 47.6±3.7% respectively) and β2/3-GABAAR cluster density (to 47.8±3.1% and 56.6±2.5% respectively), but to little but significant reduction of α1-GABAAR cluster density (to 85.2 ±2.4% and 88.9±2.5% respectively) compared to non-transfected sister pyramidal cells (Fig 2). Similar to the remaining γ2-GABAAR clusters, the remaining α2-GABAAR and β2/3-GABAAR clusters frequently colocalized with the remaining gephyrin clusters (Fig 2C and E, arrowheads). In contrast, most of the remaining α1-GABAAR clusters did not colocalize with gephyrin (Fig 2A, arrowheads). Control experiments showed that transfection with mutated Geph CR3m and Geph UTR3m shRNAs had no significant effect on the cluster density of α2-GABAARs (91.9±3.0% and 93.9±4.1% respectively), β2/3-GABAARs (98.0±4.1% and 91.9±3.1% respectively), or α1-GABAARs (97.2±2.5% and 96.2±2.2% respectively) as shown in Fig 2.
These results showed that knocking down gephyrin led to a high reduction in gephyrin cluster density which was accompanied by a similar reduction in the density of γ2- and α2-GABAAR clusters. In contrast, only a small reduction in α1-GABAAR cluster density was observed. Moreover, most of the remaining γ2-GABAAR and α2-GABAAR clusters co-localized with the remaining gephyrin clusters, while most of the α1-GABAAR clusters did not. These results indicate that gephyrin or gephyrin clustering is essential for the clustering (and/or cluster maintenance) of most of the γ2-GABAAR and α2-GABAAR, but not for the clustering of the majority of α1-GABAARs. Our results agree with those reported by Jacob et al., (2005), which showed a 50% reduction of α2-GABAAR clusters after knocking down gephyrin. Moreover, we have expanded the study to additional GABAAR subunits, which has given us insights on the differential effects of knocking down gephyrin on α2- γ2- and α1-GABAARs as well as on the relationship between the remaining gephyrin and GABAAR clusters.
Overexpression of gephyrin-EGFP, a fusion protein that forms filamentous cytoplasmic aggregates, interferes with the clustering of endogenous gephyrin and GABAA receptors
Overexpression of gephyrin-EGFP in transfected pyramidal cells led to the formation of large intracellular filamentous gephyrin-EGFP aggregates in the perikaryon (Fig 3A, crossed arrows) and in the proximal segment of some dendrites (Fig 3B and C, crossed arrows), as identified by EGFP fluorescence (Fig 3A-C, green) or by an anti-EGFP antibody (not shown) or an anti-gephyrin mAb (Fig 3A-C, red). The anti-gephyrin mAb recognized not only the endogenous gephyrin but also gephyrin-EGFP as revealed by immunofluorescence of HEK293 cells transfected with gephyrin-EGFP (not shown). The clusters recognized by the anti-gephyrin antibody that showed no EGFP fluorescence (or anti-EGFP fluorescence) corresponded to clusters of endogenous gephyrin. Immunoblot of hippocampal cultures nucleofected with the gephyrin-EGFP fusion protein (with EGFP at the gephyrin C-terminal) confirmed that the transfected neurons expressed a 118 kD protein, corresponding to the gephyrin-EGFP fusion protein, as revealed by immunoblots with a rabbit anti-gephyrin antibody (not shown). The 118kD gephyrin-EGFP protein band was not detected in immunoblots of non-nucleofected hippocampal cultures. In addition, immunoblots showed that nucleofected and non-nucleofected cultures had similar expression levels of the endogenous 93 kD gephyrin protein. The pyramidal neurons that overexpressed gephyrin-EGFP showed reduced density of the endogenous gephyrin clusters (51.5±3.2% Fig 3A and E, red) one day after transfection when compared with non-transfected sister neurons from the same culture (100%). Further time-dependent reduction of gephyrin cluster density was observed three days (27.6±2.7%, Fig 3B and E) and five days after transfection (14.6±1.9%, Fig 3C and E). The results from Fig 3E also indicate that normal gephyrin clusters have a half-time turnover rate of about 1.5 days.
Fig. 3. Overexpression of gephyrin-EGFP decreases endogenous gephyrin and GABAAR -γ2 clusters.

Cultured hippocampal neurons were transfected with gephyrin-EGFP. A-D, Triple-label immunofluorescence, at 1 day (A), 3 days (B), and 5 days (C, D) after transfection, with a mouse mAb to gephyrin (red color) and rabbit anti-γ2 (blue color). Note that the pyramidal cells overexpressing gephyrin-EGFP form large filamentous aggregates of gephyrin-EGFP in the soma and proximal dendrites (crossed arrows). Arrows in C, D show that the dendrites of non-transfected neurons have normal density of gephyrin and γ2-GABAAR clusters, while transfected neurons expressing gephyrin-EGFP (green color) show reduced cluster density of gephyrin or γ2-GABAAR in cell dendrites. White arrowheads show colocalizing gephyrin clusters and γ2-GABAAR clusters. Black arrowheads show γ2-GABAARclusters without colocalizing gephyrin clusters. Gephyrin-EGFP fluorescence was not detected in gephyrin clusters of normal aspect, which are presumably formed by endogenous gephyrin (anti-Geph+/EGFP-). E-G, Time-course of the reduction of gephyrin and γ2-GABAAR clusters after transfection of pyramidal cells with gephyrin-EGFP. Overexpression of gephyrin-EGFP led to a reduction in the density of the endogenous gephyrin (Geph+/EGFP-) clusters to 51.5±3.2%, 31.8±3.2%, 14.6±1.9% (mean±SEM) at 1, 3, 5 days respectively after transfection when compared with control (100%) sister non-transfected neurons from the same culture (1 day 11.4±0.5; 3 day 14.1±0.5; 5 day 17.3±0.7 clusters/100 μm2, Fig 3F). Overexpression of gephyrin-EGFP also led to a delayed reduction (with respect to gephyrin clusters) in the density of the γ2-GABAAR clusters to 95.0±4.3%, 50.1±4.3%, and 22.4±2.5% at 1, 3, and 5 days respectively after transfection when compared with control (100%) sister non-transfected neurons from the same culture (1 day 12.6±0.5; 3 day 15.2±0.7; 5 day 17.7±0.6 clusters/100 μm2, Fig 3G). Significant differences with the corresponding control are indicated by asterisks (*** p<0.001 in Student's t test). Scale bar: 10 μm for the large panels; 5 μm for the small panels.
The time-dependent reduction in gephyrin cluster density was also observed when considering actual density values (5.9±0.4; 3.9±0.4 and 2.5±0.3 clusters/100 μm2 at 1, 3 and 5 days after transfection respectively, Fig 3F). It is worth noting that between 11 and 15 DIV (corresponding to 1 and 5 days after transfection respectively) non-transfected cells show an increase in the density of gephyrin (Fig 3F) and γ2-GABAAR clusters (Fig 3G) as part of the normal developmental maturation of GABAergic synapses. All these results show that gephyrin-EGFP expression disrupts existing gephyrin clusters and interferes with formation of new gephyrin clusters. The reductions in endogenous gephyrin clusters occurred in all dendrites of the pyramidal cells showing gephyrin-EGFP aggregates, even though the aggregates per se were often restricted to a few dendrites. The gephyrin-EGFP filamentous aggregates were not found in non-transfected neurons, which showed normal levels of endogenous gephyrin clusters (Fig 3C and D, arrows).
Overexpression of gephyrin-EGFP also decreased the density of the γ2-GABAAR clusters to 95.0±4.3%, 50.1±3.5%, and 22.4±2.5% (Fig 3A, B, C and E, blue) one, three and five days after transfection respectively, when compared with non-transfected sister neurons (100%). More importantly, these experiments also showed that the disappearance of γ2-GABAAR clusters was delayed about 1-2 days with respect to the disappearance of endogenous gephyrin clusters (Fig 3E), which is consistent with the notion that gephyrin clustering is essential for the stability of many of the existing γ2-GABAAR clusters. The remaining endogenous gephyrin clusters showed high colocalization with the remaining γ2-GABAAR clusters (Fig 3A and B, white filled arrowheads). However, there were many γ2-GABAAR clusters, particularly at one day and three days after transfection, that had no colocalizing gephyrin clusters (Fig 3A-C, black filled arrowheads), which is consistent with the disappearance of gephyrin clusters prior to the disappearance of γ2-GABAAR clusters. The time-dependent reduction in γ2-GABAAR cluster density was also observed when considering actual density values (11.9±0.5; 7.0±0.5 and 4.0±0.4 clusters/100 μm2 at 1, 3 and 5 days after transfection respectively, Fig 3G).
Transfection of pyramidal cells with gephyrin-EGFP, or gephyrin shRNAs was done at day 10 in culture, a time at which many but not all gephyrin and γ2-GABAARs clusters are formed in our cultures (Christie et al., 2002a, and legend to Fig 3). We have previously shown that gephyrin clusters and γ2-GABAAR clusters are already present in these cultures after 3.5 days and that a large number of these clusters are present after 8.5 days in culture (Christie et al. 2002a). Fig 3F and G show that although the density of both gephyrin and γ2-GABAAR clusters increased in non-transfected neurons from day 11 to 15, both densities decreased substantially from 1 day to 5 days after gephyrin-EGFP overexpression, suggesting that the formation of new gephyrin and γ2-GABAAR clusters is seriously compromised in addition to the reduction of existing clusters. These results support the notion that gephyrin clustering is critically important for both the formation and the maintenance of many γ2 subunit containing GABAAR clusters.
It has been proposed that normal gephyrin clustering occurs by homo-oligomerization of gephyrin molecules forming submembranous hexagonal lattices via the N terminal G-domain, which can form trimers, and the C-terminal E-domain, which can form dimers (Kim et al., 2006). This clustering of gephyrin would facilitate the accumulation of glycine receptors and GABAAR at the postsynaptic membrane (Kim et al., 2006). It has been reported that native EGFP can also form dimers (Yang et al., 1996). Thus the addition of EGFP to the C-terminal of gephyrin may alter the normal hexagonal clustering of gephyrin, leading to the formation of filamentous aggregates containing both gephyrin-EGFP and endogenous gephyrin. The decreased clustering of endogenous gephyrin likely results from being trapped by the gephyrin-EGFP aggregates that forms at the perikaryon and the proximal dendrites, thus preventing the delivery of both endogenous gephyrin and gephyrin-EGFP to synaptic sites.
Overexpression of non-tagged gephyrin slightly increases the density of both gephyrin and GABAA receptor clusters
We transfected hippocampal cultures with non-tagged gephyrin, aiming to avoid the formation of gephyrin aggregates induced by the EGFP tagging of gephyrin. Neurons transfected with gephyrin were identified by cotransfection with EGFP. Fig 4A shows that pyramidal cells transfected with non-tagged gephyrin had normal gephyrin clusters (Fig 4, Geph column) instead of the abnormal aggregates that occurred when cells were transfected with gephyrin-EGFP. Pyramidal cells transfected with non-tagged gephyrin also showed γ2-GABAAR clusters of normal appearance (Fig 4A, γ2 column), similar to those of non-transfected sister cells. Quantification revealed that the transfected cells had slightly increased density of gephyrin clusters compared with non-transfected sister cells (Fig 4B). These statistically significant effects were observed as early as one day after transfection (transfected 12.0±0.5 vs non-transfected 10.7±0.4 clusters/100 μm2). The effect was also observed 3 days after transfection (15.1±0.7 vs 12.8±0.5 clusters/100 μm2) and 5 days after transfection (20.0±0.7 vs 17.6±0.7 clusters/100 μm2). Similar statistically significant but small increases in the density of γ2-GABAAR clusters were observed (Fig 4C) 1-day (13.0±0.5 vs 11.3±0.4 clusters/100 μm2), 3-day (15.7±0.7 vs 13.9±0.5 clusters/100 μm2), and 5-day (21.1±0.7 vs 18.8±0.7 clusters/100 μm2) after transfection. As indicated above, between day 11 and 15 (1 and 5 days after transfection respectively), non-transfected cells show an increase in the density of gephyrin and γ2-GABAAR clusters as part of their normal developmental maturation of these cultures. Thus, gephyrin overexpression leads only to a small increase in gephyrin cluster density in the transfected pyramidal cells, indicating that the maximum density of gephyrin clusters is tightly regulated. A similar small increase in the γ2-GABAAR clusters after gephyrin overexpression indicates that the clustering of γ2-GABAAR is precisely coupled to the clustering of gephyrin during synaptic development.
Fig. 4. Overexpression of non-tagged gephyrin increases the density of both gephyrin and GABAAR -γ2 clusters.
Cultured hippocampal neurons were co-transfected with EGFP and non-tagged gephyrin. A, Triple-label immunofluorescence was done at 1, 3 and 5 days after transfection, using a mAb to gephyrin and a rabbit anti-γ2 antibody. B and C, The density of gephyrin and γ2-GABAAR clusters is increased in the gephyrin transfected cells when compared with non-transfected sister cells at 1, 3 or 5 days after transfection respectively. (* p<0.05, in Student's t test). Scale bar: 5 μm.
In cultured pyramidal cells, decreased gephyrin clustering resulting from knocking down gephyrin by shRNA is accompanied by decreased GABAergic innervation of the targeted pyramidal cells
Pyramidal cells transfected with Geph CR or Geph UTR shRNA showed not only reduced gephyrin cluster density, as described above, but also reduced GABAergic innervation, as determined by the density of GAD+ presynaptic boutons contacting transfected pyramidal cells, compared with that of sister non-transfected neurons (Fig 5A and C respectively, green color). Similar results were obtained when we used an antibody to VIAAT (vesicular inhibitory amino acid transporter) to identify presynaptic GABAergic boutons (not shown). We have previously shown that the GAD+ boutons apposed to GABAAR clusters concentrate the GABAergic synaptic vesicle marker VIAAT (also named vesicular GABA transporter or vGAT), and the general synaptic vesicle marker SV2 (Li et al., 2005b). We have also shown that in these cultures, these GAD-containing boutons have actively recycling synaptic vesicles (Christie et al., 2002b).
Fig. 5. The knock down of gephyrin in pyramidal cells decreases the GABAergic innervation that these cells receive.
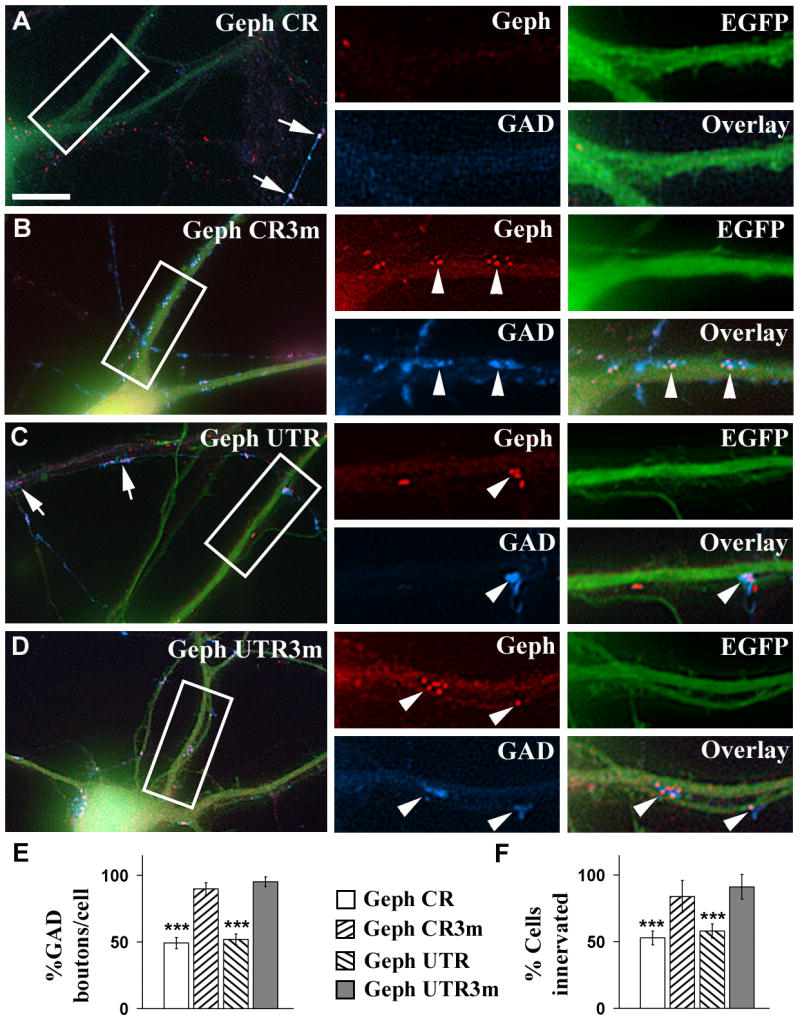
A-D, Cultured hippocampal neurons were co-transfected with pEGFP-N1 and Geph CR (A), or Geph CR3m (B), or Geph UTR (C), or Geph UTR3m (D) shRNAs. Triple-label immunofluorescence was done using a mouse mAb to gephyrin and a sheep anti-GAD antibody. Green color shows the EGFP fluorescence of transfected neurons. Arrows in A and C point to dendrites of non-transfected neurons, having a normal density of gephyrin clusters and GAD+ boutons. Arrowheads show presynaptic GAD+ boutons apposed to postsynaptic gephyrin clusters. Note that the pyramidal cells transfected with Geph CR (A) or Geph UTR (C) shRNAs show a lower density of gephyrin clusters and GAD+ boutons than the corresponding controls Geph CR3m (B) or Geph UTR3m (D) respectively. E, F, Quantification of the effect of the gephyrin shRNAs on the density of GAD+ boutons contacting pyramidal cells (E) and on the number of pyramidal cells showing synaptic GAD+ boutons (F) expressed as percentage of control (mean ± SEM). Control values were determined in non-transfected sister cells in the same cultures. The non-transfected pyramidal cells of the Geph CR shRNA transfection experiment had 57.5±4.5 GAD+ boutons/cell (mean ± SEM, n=30 cells). The percentage of innervated non-transfected pyramidal cells by GAD+ terminals was 89±6% (n=54 cells). There is significant decrease in the GAD+ terminals contacting the transfected pyramidal cells whose gephyrin has been knocked down, compared to non-transfected cells or cells transfected with the mutated shRNAs (***, p<0.001, Student's t test). However, there are no significant differences in the density of GAD+ boutons contacting the pyramidal cells transfected with gephyrin CR3m or gephyrin UTR3m shRNAs and the non-transfected cells (one-way ANOVA Tukey test when compared at p<0.05). Scale bar: 10 μm for large panels; 5 μm for small panels.
The non-transfected pyramidal cells showed normal levels of both gephyrin clusters and apposed GAD+ boutons (Fig 5A and C, arrows). In pyramidal cells transfected with Geph CR or Geph UTR shRNAs, the remaining GAD+ boutons from GABAergic axons innervating these cells were also apposed to remaining postsynaptic gephyrin clusters (Fig 5A and C, arrowheads). The dendrites of pyramidal cells transfected with control shRNA Geph CR3m and Geph UTR3m (Fig 5B, D respectively) showed normal density of GAD+ presynaptic boutons, which were apposed to postsynaptic gephyrin clusters (Fig 5B and D, arrowheads).
Quantification (Fig 5E) shows that the density of GAD+ boutons contacting pyramidal cells transfected with Geph CR or Geph UTR shRNAs was reduced to 49.2±4.2% and 51.9±4.0% respectively of the non-transfected sister neurons (100%). In contrast, pyramidal cells transfected with Geph CR3m or Geph UTR3m shRNAs did not show significant reduction in the density of GAD+ boutons (91.5±4.7% for Geph CR3m and 95.1±3.1% for Geph UTR3m). Similar results were obtained when we quantified the percentage of pyramidal cells that received GABAergic innervation (Fig 5F). In the pyramidal cells transfected with Geph CR or Geph UTR, the percentage of these neurons that received GABAergic innervation was reduced (to 52.8±5.1% and 57.9±5.3% respectively) compared to 83.9±11.9% and 91.1±9.4% for pyramidal cells transfected with Geph CR3m and Geph UTR3m.
We also performed control rescue experiments by co-transfecting neurons with Geph UTR shRNA and gephyrin mRNA that contained the coding region but not the 3′UTR. In this way, the Geph UTR shRNA targeted the endogenous gephyrin mRNA but not the exogenous gephyrin mRNA. Fig. 1 supplement shows that the exogenous gephyrin mRNA rescued gephyrin clustering, γ2-GABAAR clustering and GABAergic innervation by GAD+ boutons to control levels.
We have previously shown that knocking down the GABAAR γ2 subunit in pyramidal cells with γ2 shRNAs led not only to reduced clustering of both postsynaptic GABAARs and gephyrin but also to a reduced presynaptic GABAergic innervation of these neurons by GABAergic interneurons (Li et al., 2005b). We are now showing that knocking down gephyrin with gephyrin shRNA also leads to reduced gephyrin and GABAAR clustering and to decreased GABAergic innervation of the pyramidal cells. What is common in both experimental conditions is the reduced clustering of both gephyrin and γ2-GABAARs. Therefore, the postsynaptic clustering of gephyrin and γ2-GABAARs is mutually dependent on each other, and the presynaptic GABAergic innervations rely upon intact postsynaptic gephyrin and γ2-GABAAR clusters. The notion that the postsynaptic clustering of gephyrin is essential for maintaining the normal level of GABAergic innervation of the pyramidal cells is also supported by the gephyrin-EGFP overexpression experiments shown next.
In cultured pyramidal cells, decreased clustering of endogenous gephyrin resulting from overexpression and aggregation of gephyrin-EGFP is also accompanied by decreased GABAergic innervation of the targeted pyramidal cells
As shown in above, overexpression of gephyrin-EGFP in transfected pyramidal cells led to the formation of filamentous aggregates of gephyrin-EGFP (anti-gephyrin+/EGFP+, crossed arrows, Fig 6A), and to decreased density of endogenous gephyrin clusters (anti-gephyrin+/EGFP-, arrowheads, Fig 6A), when compared with sister non-transfected cells (Fig 6B). Now we are showing that the transfected pyramidal cells also have reduced GABAergic innervation from interneurons as shown by decreased density of GAD+ presynaptic boutons contacting these cells (arrowheads, Fig 6A, blue) when compared to non-transfected cells (arrowheads, Fig 6B, blue). In both transfected and non-transfected cells the GABAergic terminals were apposed to endogenous gephyrin clusters (arrowheads, Fig 6A, red) that showed no EGFP fluorescence. However, the GAD+ terminals were not apposed to the gephyrin-EGFP aggregates (crossed arrows, Fig 6A), indicating that the presynaptic GABAergic terminal didn't form synaptic contacts with the dendritic areas where gephyrin-EGFP aggregates were localized. Quantification showed that pyramidal cells overexpressing gephyrin-EGFP had 43.6±2.5 GAD+ boutons/cell (n=30 cells) compared with 97.8±3.0 GAD+ boutons/cell in sister non-transfected pyramidal cells (Fig 6C). The proportion of transfected neurons that received GABAergic innervations was 54.8±3.1% (n=62 cells) compared with 88.6±3.6% for non-transfected neurons (n=70 cells, Fig 6D). These experiments further support the notion that the normal postsynaptic clustering of gephyrin, rather than the level of expression, is essential for maintaining the normal levels of presynaptic GABAergic innervation. As indicated above, immunoblots of hippocampal cultures transfected with gephyrin-EGFP did not show significant changes in the expression of endogenous gephyrin
Fig. 6. Transfection of pyramidal cells with gephyrin-EGFP leads to decreased GABAergic innervation while transfection with non-tagged gephyrin leads to little or no effect on GABAergic innervation.

A-B, Triple label immunofluorescence of cultured hippocampal pyramidal cells were transfected with gephyrin-EGFP using a mouse mAb to gephyrin (red) and a sheep anti-GAD antibody (blue). Crossed arrows in A show aggregates of gephyrin-EGFP. There is a significant reduction of gephyrin clusters and GAD+ boutons contacting the pyramidal neurons overexpressing gephyrin-EGFP (A), when compared with the sister non-transfected cells from the same culture (B). Arrowheads show presynaptic GAD+ boutons apposed to postsynaptic gephyrin clusters. There is no apposition of gephyrin-EGFP aggregates with GAD+ boutons. C, D, Quantification shows that overexpression of gephyrin-EGFP reduces the density of GAD+ boutons contacting the transfected pyramidal cells and the number of these cells that are contacted by GAD+ terminals (*** p<0.001, in Student's t test). E, F, Triple-label immunofluorescence of pyramidal cells co-transfected with EGFP and non-tagged gephyrin. Arrowheads show gephyrin clusters apposed to GAD+ boutons. G, H, Quantification shows that pyramidal cells transfected with non-tagged gephyrin had little or no increase in either the density of GAD+ boutons (* p=0.041, Student's t test) or the percentage of pyramidal cells innervated by GAD+ terminals (p=0.063). Scale bar: 10 μm for large panels; 5 μm for small panels.
Transfection of pyramidal cells with non-tagged gephyrin leads to slight increase in the GABAergic innervations of these cells
We have shown in Fig 4 that transfecting pyramidal cells with non-tagged gephyrin leads to a small increase in gephyrin and γ2-GABAAR cluster density over non-transfected sister neurons. We have investigated whether this small increase in gephyrin and γ2-GABAAR clusters affects the GABAergic innervation of these cells. Pyramidal cells transfected with non-tagged gephyrin (Fig 6E, green) showed a slight increase in GABAergic innervation (Fig 6E, blue) when compared with sister non-transfected pyramidal cells (Fig 6F) of the same culture measured as GAD+ boutons contacting these cells. Quantification showed that there was small but significant increase in the GABAergic innervation that the transfected cells received when compared to sister non-transfected cells (72.3±2.5 vs 66.0±1.8 boutons/cell respectively, n=30 cells, p=0.041, Fig 6G). Nevertheless, there was no significant change in the percentage of the transfected pyramidal cells that received GABAergic innervation (92.9±2.3% vs 85.7±2.5%, p=0.063, n=30, Fig 6H). Thus overexpression of non-tagged gephyrin in pyramidal cells leads to little or no effect on the density of GABAergic innervation of these cells.
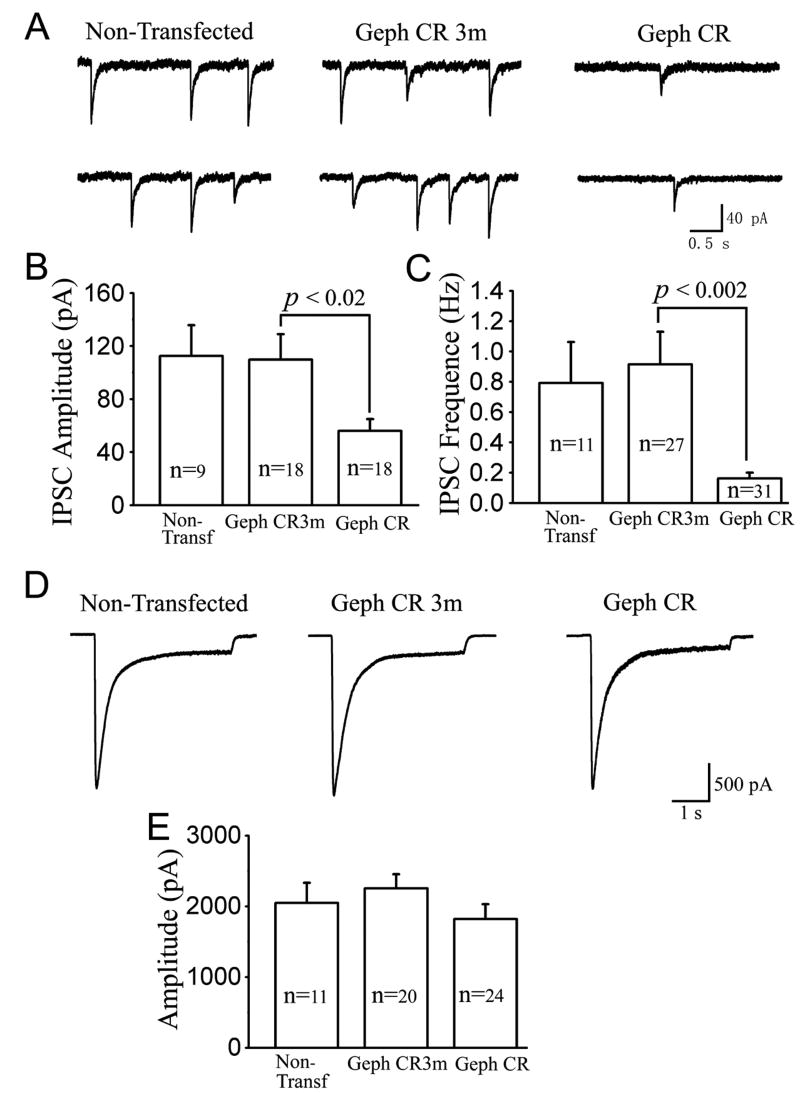
Knocking down gephyrin by gephyrin shRNA decreases inhibitory synaptic currents
To further investigate whether knocking down gephyrin will cause any functional changes of GABAergic synaptic transmission, we performed electrophysiological recordings on neurons transfected with either Geph CR or Geph CR3m shRNA (together with EGFP). We examined the frequency and amplitude of spontaneous IPSCs in both transfected groups as well as non-transfected neurons. We found that the sIPSC amplitude significantly decreased in cells expressing Geph CR shRNA in comparison with either non-transfected or Geph CR3m-transfected cells (Fig. 7A & B). The significant decrease of the sIPSC amplitude after knocking down gephyrin is in accordance with the reduction in γ2-GABAAR clusters observed in our immunofluorescence experiments and with the notion that gephyrin plays a critical role in stabilizing postsynaptic GABAA receptors. Moreover, in addition to the amplitude change, we also found that the sIPSC frequency was significantly reduced in the Geph CR group but not in cells transfected with Geph CR3m (Fig. 7A & C). The large frequency decrease observed in neurons transfected with Geph CR cannot be solely explained by a decrease in the amplitude and suggests a potential functional deficit in the presynaptic GABA release mechanism. This presynaptic deficit is also consistent with our immunofluorescence data, which show that the density of presynaptic GABAergic boutons was significantly reduced after knocking down gephyrin. To further examine whether the knockdown of gephyrin affected the expression level of GABAARs on cell membranes, we recorded whole-cell GABA currents in cells from non-transfected, Geph CR3m-transfected, and Geph CR-transfected groups. No significant difference was found in the whole-cell GABA currents among all three different groups (Fig. 7D and E). These data suggest that although gephyrin is critical for the clustering of many GABAARs at postsynaptic sites, knocking down gephyrin may not affect the total expression and insertion level of GABAARs in neuronal plasma membranes.
Fig. 7. The knock down of gephyrin by gephyrin shRNA decreases inhibitory synaptic currents.
A, Representative recordings of spontaneous synaptic responses from non-transfected (left panel), Geph CR3m-transfected (middle panel), and Geph CR-transfected neurons (right panel). Cells with typical sIPSCs are presented. B, Bar graphs showing that the average sIPSC amplitude was similar between non-transfected (112.5 ± 23.2 pA) and Geph CR3m groups (109.8 ± 19.0 pA; p>0.9). In contrast, the sIPSC amplitude in the Geph CR group was greatly decreased (56.0 ± 9.0 pA; p<0.02) comparing to the Geph CR3m group. C, Bar graphs showing that the average sIPSC frequency was similar between non-transfected (0.79 ± 0.27 Hz) and the Geph CR3m group (0.92 ± 0.22 Hz; p>0.7), but the sIPSC frequency in the Geph CR group was significantly reduced (0.16 ± 0.04 Hz; p<0.002). D, Typical recordings showing whole-cell currents induced by rapid application of GABA (20 μM) from non-transfected (left panel), Geph CR3m-transfected (middle panel), and Geph CR-transfected neurons (right panel). E, Summarized data showing no difference in the GABA current amplitude among non-transfected (2045 ± 288 pA), Geph CR3m transfected (2252 ± 202 pA; p>0.6), and Geph CR transfected neurons (1823 ± 207 pA; p>0.1).
Treatments that lead to decreased density of gephyrin clusters, γ2-GABAAR clusters and GABAergic innervation do not affect the density of glutamatergic synapses
As shown above, pyramidal cells transfected with Geph CR shRNA showed significant decreased density in gephyrin clusters (Fig 8A and C, blue panels, black arrowheads) when compared with cells transfected with Geph CR3m (Fig 8B and D respectively, blue panels, black arrowheads). However, the same transfected cell (Geph CR shRNA) showed no apparent changes in the density of GluR1 AMPA receptor clusters (Fig 8A vs B, red panels, white arrowheads) or vGlut1-contaning presynaptic glutamatergic boutons (Fig 8 C vs D, red panels, white arrowheads). Quantification (Fig 8E and F) showed that Geph CR or Geph UTR shRNA had no significant effect on the density of AMPA receptor GluR1 and GluR2/3 subunit clusters, NMDA receptor NR1 subunit clusters, PSD-95 clusters, or vGlut1-containing presynaptic glutamatergic boutons, when compared with sister non-transfected cells from the same culture (100%) or with cells transfected with Geph CR3m or Geph UTR3m respectively.
Fig. 8. The knock down of gephyrin with gephyrin shRNA doesn't affect the density of glutamatergic synapses.

A-D, Cultured hippocampal neurons were co-transfected with EGFP and Geph CR (A, C) or Geph CR3m (B, D) shRNAs. Triple-label immunofluorescence was done using a combination of a mAb to gephyrin (blue color) and a rabbit anti-GluR1 (A and B, red color), or a guinea pig anti-vGlut1 (C and D, red color). Transfected neurons show green fluorescence. Pyramidal cells transfected with Geph CR shRNAs show a high reduction of gephyrin clusters (A, blue, black arrowheads), compared to neurons transfected with Geph CR3m (B, blue, black arrowheads). However, the same neurons showed no obvious difference in the density of GluR1 clusters (A, red, white arrowheads) or vGlut1+ boutons (C, red, white arrowheads), compared to the neurons transfected with Geph CR3m (B and D, respectively). E-F, Quantification of the density of GluR1, GluR2/3, NR1, PSD-95 clusters and vGlut1+ boutons in pyramidal cells transfected with gephyrin shRNA. Values (mean±SEM) represent the percentages compared to the corresponding internal control (non-transfected sister cells in the same culture). There was no significant difference (p>0.05, Student's t test) in the cluster density of various glutamatergic markers between transfected and non-transfected cells. Comparisons between groups using one-way ANOVA Tukey test showed that the Geph CR, Geph UTR, and the mutated shRNAs (Geph CR3m and Geph UTR3m) had no effect on the density of GluR1, GluR2/3, NR1, PSD-95 clusters or vGlut1 boutons (when compared at p<0.05). The cluster densities (number of clusters/100 μm2; mean ± SEM, 100%) in the controls of the Geph CR shRNA transfection experiments were 16.0±0.5 (481) for GluR1, 16.5±0.7 (508) for GluR2/3, 16.2±0.1 (501) for NR1, 15.7±0.7 (471) for PSD-95, 13.4±0.5 (402) for vGlut1 (the number of clusters or vGlut1+ boutons is shown in parentheses). Similar control values were obtained in the other transfection experiments. Scale bar: 10 μm for large panels; 5 μm for small panels.
We have shown above that pyramidal cells transfected with gephyrin-EGFP showed gephyrin-EGFP aggregates and reduced density of gephyrin clusters, γ2-GABAAR clusters and GABAergic innervation. However, the gephyrin-EGFP transfected cells also showed no changes in the density of PSD-95 or vGlut1 presynaptic terminals (not shown). These experiments further indicate that the disruption of gephyrin clusters by either gephyrin shRNAs or gephyrin-EGFP leads to a specific disruption of the stability of GABAergic synapses but not of glutamatergic synapses.
Discussion
We have addressed the function of gephyrin at GABAergic synapses by using experimental approaches independent of the gephyrin knockout mouse which had generated somewhat controversial results previously (Kneussel et al., 1999; Kneussel and Betz 2000; Kneussel et al., 2001; Levi et al., 2004). With the use of both gephyrin RNAi and gephyrin-EGFP aggregation to disrupt gephyrin clustering, we have found that not only the number of gephyrin clusters are greatly reduced in transfected hippocampal pyramidal cells, but also the number of γ2, α2 and β2/3 GABAAR subunit clusters are all significantly reduced correspondingly in these cells. In contrast, there was little reduction in the number of α1 GABAAR subunit clusters. Our approach has also allowed us to reveal some functional roles of gephyrin that had not been previous observed by studying the gephyrin knockout mouse. Thus the time-course following the disruption of gephyrin clusters has shown that gephyrin clustering is essential for the maintenance of existing postsynaptic GABAAR clusters. More interestingly, we have also shown that disrupting normal postsynaptic gephyrin clustering in these cells leads to a significant reduction in the total number of GABAergic synapses. Thus our results support the notion that gephyrin clustering is essential for the stability of GABAergic synapses.
By studying a gephyrin knockout mouse (geph-/-), others have concluded that gephyrin is essential for the clustering of GABAARs (Kneussel et al., 1999; Kneussel and Betz 2000). Nevertheless, although using the same mouse mutant line, others have reported the existence of many GABAAR synaptic clusters in the geph-/- mouse mutant (Levi et al., 2004). Thus, it has been reported that the intact spinal cord or cultured hippocampal neurons from the gephyrin-deficient (geph -/-) mouse show a large reduction in the number of clusters of the γ2, α2 and β2/3 GABAAR subunits while the clustering of the α1 GABAAR subunit was not affected (Kneussel et al., 2001; Levi et al., 2004) when compared with littermate control mice (geph +/+ or geph +/-) which agrees with our results using a different experimental approach.
It has been proposed that the interaction with synaptic gephyrin reduces the lateral diffusion of GABAARs and glycine receptors, thus facilitating their accumulation at GABAergic synapses and glycinergic synapses respectively (Meier et al., 2001; Jacob et al., 2005; Charrier et al., 2006). This type of mechanism would explain why disrupting gephyrin clusters by shRNAs or gephyrin-EGFP aggregation, leads to the disruption of many GABAAR clusters. The disruption of the GABAAR clusters by gephyrin-EGFP overexpression was delayed by approximately 1-2 days following the disruption of the gephyrin clusters. Our results support the notion that gephyrin clustering is essential for maintenance of many GABAAR clusters at GABAergic synapses.
An important observation derived from our studies is that interfering with the normal postsynaptic clustering of gephyrin and/or disrupting existing gephyrin clusters in pyramidal neurons (by either gephyrin shRNAs or overexpression and aggregation of the gephyrin-EGFP) also leads to reduced presynaptic GABAergic innervation that these pyramidal neurons received, as shown by the decreased number of GAD-containing (or VIAAT-containing) presynaptic terminals contacting these pyramidal cells. We have previously shown that knocking down the GABAAR γ2 subunit in pyramidal cells with γ2 shRNAs not only disrupted GABAAR clusters and gephyrin clusters in pyramidal cells but also decreased the GABAergic innervation that these cells received (Li et al., 2005b). These results and the results presented in this communication indicate that: I) the disruption of the postsynaptic γ2-GABAARs clusters is accompanied by disruption of gephyrin clusters; II) the disruption of gephyrin clusters is accompanied by the disruption of many GABAARs clusters; and III) the coordinated disruption of the postsynaptic clustering of GABAARs and gephyrin has transynaptic effects that lead to reduced innervation of pyramidal cells by GABAergic interneurons. The results obtained by immunofluorescence were confirmed by measuring inhibitory synaptic currents in hippocampal cultures transfected with gephyrin shRNAs. Moreover, the disruption of the GABAARs and gephyrin clustering and GABAergic innervation by gephyrin shRNAs, or γ2 GABAAR subunit shRNAs or gephyrin-EGFP overexpression does not affect the density of AMPA receptor clusters, PSD-95 clusters or presynaptic vGlut1-containing glutamatergic boutons (Fig 8 and Li et al., 2005b).
It has been reported that in the geph-/- knockout mouse there was no difference in the number of VIAAT containing terminals in the spinal cord (Kneussel et al., 2001) or in GAD+-containing terminals in hippocampal cultures (Levi et al., 2004) when compared to the geph+/+ or the geph+/- littermates. The apparent discrepancy in the results between the knockout mouse and the RNAi experiments could be explained because in the geph-/- mouse none of the neurons express gephyrin at any stages of development while in the RNAi experiments some neurons have gephyrin knocked down, while other sister neurons in the same culture express normal levels of gephyrin and clustering. In the latter configuration, GABAergic synapses on pyramidal cells that have low levels of postsynaptic gephyrin and GABAAR clusters might be at a competitive disadvantage with the synapses that the same interneurons form with neighboring pyramidal cells that have normal levels of gephyrin and GABAAR clusters (see Li et al., 2005b for a more extensive discussion and other possible explanations for the different results obtained with the mouse knockout and RNAi technologies). Another possible explanation is that, although the density of presynaptic GABAergic terminals in the geph-/- mutant is similar to that of the geph+/+ mouse, in the mutant mouse some GABAergic terminals could be mislocalized. Another possible explanation is the off-target effects of the gephyrin shRNAs (Alvarez et al., 2006). However, this is unlikely since I) the mutated shRNAs show no effect on GABAergic synapses and II) the effects of Geph UTR on GABAergic synapses could be reversed by exogenous gephyrin mRNA that did not contain the 3′-UTR (rescue experiment). Moreover, the same neurons that showed decreased density of several GABAergic pre- and post-synaptic markers showed little effect on the density of α1-GABAAR clusters and no effect on the density of glutamatergic synaptic markers. Moreover, overexpression of gephyrin-EGFP interfered with endogenous gephyrin clustering and had effects similar to those of gephyrin shRNAs, namely decreased density of both postsynaptic GABAAR clusters and presynaptic GABAergic terminals.
In another study using gephyrin shRNA, the density of VIAAT-containing terminals in hippocampal cells although reduced, it was reported not to be significant (Jacob et al., 2005). In the latter study, neurons were nucleofected at day 0 and the synaptic markers were tested by immunofluorescence at 14 DIV. In our study, neurons were transfected at 10 DIV and synaptic markers were assayed at 15 DIV. Knocking down gephyrin from day 0 (Jacob et al., 2005) might lead to a compensatory mechanism as it could be the case in the gephyrin knockout mouse. Another explanation is that Jacob et al. 2005 used high-density neuronal cultures, which complicates the determination of whether a presynaptic terminal is contacting the dendrite of a transfected cell or the dendrite of a non-transfected cell from the same bundle, since dendrites from several neurons tend to bundle together, particularly in the high-density cultures. We purposely used low-density cultures where the detection of individual synapses on transfected cells can be assessed more accurately. In experimental conditions similar to the ones we have used in the present communication, and in agreement with our results, Fang et al., 2006 have reported that knocking down the palmitoyl acyltransferase GODZ with GODZ RNAi in cultured pyramidal cells, led to reduced number of GABAAR clusters and to decreased GABAergic innervation of these cells. All of these experiments suggest that the postsynaptic clustering of gephyrin and GABAAR is required for the normal formation and/or maintenance of the GABAergic presynaptic contacts and functional synapses.
It is conceivable that the disruption of postsynaptic clustering of gephyrin and GABAARs also disrupts the postsynaptic clustering of a cell recognition molecule that transynaptically interacts with a presynaptic cell recognition partner. Candidate cell recognition molecules are postsynaptic neuroligins (NLs) which transynaptically interact with presynaptic neurexins (NRXs) and laterally with postsynaptic NRXs (Taniguchi et al., 2007). The NLs-NRXs interactions play a central role in the formation and/or maintenance of both glutamatergic and GABAergic synapses (Scheiffele et al., 2000; Dean et al., 2003; Graf et al., 2004; Prange et al., 2004; Boucard et al., 2005; Chih et al., 2005, 2006; Levinson et al., 2005; Dean and Dresbach 2006; Graf et al., 2006; Varoqueaux et al., 2006). Of particular interest are NL-1A and NL-2A, which are preferentially localized at GABAergic synapses while NL-1B, NL-1AB, NL-3 and NL-4 preferentially localize at glutamatergic synapses (Boucard et al., 2005; Chih et al., 2006). Knocking down postsynaptic NLs by RNAi in hippocampal pyramidal cells leads to preferential decreased number of GABAergic over glutamatergic presynaptic contacts (Chih et al., 2005). Moreover, gephyrin overexpression enhances the clustering of NL-2 at GABAergic synapses (Levinson et al., 2006). NL-2 has been demonstrated to be critical in molecular reconstitution of GABAergic synapses in HEK293 cells (Dong et al., 2007). Transynaptic effects leading to the reduction in the number of presynaptic GABAergic terminals that innervate the postsynaptic neuron occur after I) disruption of the postsynaptic gephyrin clusters (as shown in the present communication); II) or disruption of the postsynaptic γ2–GABAAR clusters (Li et al., 2005b); III) or by preventing the trafficking of postsynaptic GABAARs at the synapse (Fang et al., 2006). These transynaptic effects could result from the simultaneous disruption of the clustering of postsynaptic neuroligins at GABAergic synapses. A preliminary meeting report suggests that accumulation of NL-2 at GABAergic synapses is strictly dependent on postsynaptic GABAARs (Luscher B, unpublished observations). The unclustering of postsynaptic NLs would result in weakened interaction with presynaptic neurexins at GABAergic synapses followed by the withdrawal of presynaptic GABAergic innervation.
Other postsynaptic cell recognition molecules that could be involved in these transynaptic effects are EphB receptors. Some EphB receptors interact with glutamate receptor interacting proteins or GRIPs (Bruckner et al., 1999; Contractor et al., 2002). We have recently found that some splice variants of GRIP1, a family of molecules containing up to 7-PDZ domains, are postsynaptically localized at GABAergic synapses (Charych et al., 2004b; Kittler et al., 2004; Li et al., 2005a; Charych et al., 2006). Postsynaptic EphB receptors transynaptically interact with presynaptic ephrinBs.
Thus, it is conceivable that the clustering of postsynaptic neuroligins, EphB receptors and other transynaptic molecules together with the concentration of their interacting partners at the presynaptic GABAergic terminals lead to the strengthening of the transynaptic interactions and the stabilization of the GABAergic synaptic contacts.
Experimental methods
Antibodies
The following GABAAR antibodies raised in our laboratory were used: the rabbit and guinea pig anti-rat α1 GABAAR subunit antibodies were to amino acids 1-15 (QPSQDELKDNTTVFT); the rabbit anti-rat α2 GABAAR subunit antibody was to amino acids 417-423 (PVLGVSP); the mouse monoclonal (mAb) anti β2/3 GABAAR subunit antibody (clone 62-3G1) was raised to the affinity purified GABAA receptor (De Blas et al.1988; Vitorica et al.1988), which recognizes an N-terminal epitope present in both β2 and β3 subunits but not in β1 subunit (Ewert et al., 1992); rabbit and guinea pig anti-rat γ2 GABAAR subunit antibodies were to amino acids 1-15 (QKSDDDYEDYASNKT). These anti-GABAAR antibodies have been thoroughly characterized and their specificities determined previously by ELISA, immunoblotting, displacement by antigenic peptide, rat brain immunochemistry at the light microcopy and electron microscopy levels, immunofluorescence of hippocampal cultures and of HEK293 cells transfected with various GABAAR subunits and some knockout and knockdown mutant mice (De Blas et al., 1988; Vitorica et al.,1988; Homanics et al., 1999; Miralles et al.,1999; Christie et al., 2002a, b; Riquelme et al., 2002; Christie and De Blas 2003; Charych et al., 2004a, b; Chandra et al., 2005; Li et al., 2005a, b; Christie et al., 2006; Serwanski et al., 2006). The mouse mAb to gephyrin (clone mAb 7a) used in immunocytochemistry was from Cedarlane (Accurate Chemical & Scientific Corp., Westbury, NY). The rabbit anti-gephyrin antibody used in immunoblots was from Dr. Ben Bahr (University of Connecticut, Storrs, CT). The mouse anti-PSD-95 (postsynaptic density protein 95) antibody was from Upstate Biotechnology (Lake Placid, NY). Guinea pig anti-vGlut1 (vesicular glutamate transporter 1) and rabbit anti-GluR1 antibodies were from Chemicon (Temecula, CA). Rabbit anti-GluR2/3 was a gift from Dr. Robert J. Wenthold (NIDCD, Bethesda, MD). Sheep anti-GAD was a gift from Dr. Irwin J. Kopin (NINDS, Bethesda, MD). Mouse anti-α tubulin was from Sigma (Saint Louis, MI). All fluorophore-conjugated secondary antibodies were from Jackson ImmunoResearch (West Grove, PA).
Construction of various gephyrin plasmid vectors
Two gephyrin small hairpin RNAs (shRNAs) were made (Fig 1A), one targeting the gephyrin coding region (Geph CR, nucleotides 739-763) corresponding to the seventh exon of the gephyrin gene (GeneBank accession number NM_022865), and the second one targeting nucleotides 2531-2555 of the gephyrin 3′ UTR non-coding region (Geph UTR) corresponding to the twenty-first exon. Control shRNAs for each (Geph CR3m and Geph UTR3m, respectively) were made carrying three point mutations in the sense and antisense strands (Fig 1A). These shRNA were inserted in the mU6 vector and synthesized under the control of RNA U6 polymerase III promoter (Yu et al.2002; Li et al., 2005). Gephyrin hairpin DNA oligonucleotides and their corresponding complemental oligonucleotides were synthesized and purified by PAGE (Integrated DNA Technologies Inc. Coralville, IA). The DNA oligonucleotides were designed to create compatible overhangs on each end after annealing. The double-stranded DNA was inserted into the mU6pro vector between the Bbs I and Xba I sites. The antisense strand matched the target gene perfectly. A mismatched nucleotide was introduced in the sense strand to facilitate sequencing of the hairpin DNA (Fig 1A).
The human gephyrin cDNA clone FJ06168 (Gene named KIAA1385) was kindly provided by Dr. Nobumi Kusuhara (Kazusa DNA Research Institute, Japan). This gephyrin isoform, without the c5 cassette, is involved in clustering GABAARs (Meier and Grantyn, 2004). The full-length gephyrin coding sequence was amplified by PCR and inserted into the Hind III and Xho I sites of pcDNA3.1(+) or Nhe I and Sac II sites of pEGFP-N1, aiming to generate non-tagged gephyrin or a gephyrin-EGFP fusion protein with the EGFP tag located at the C-terminal of gephyrin, respectively. The quality of the constructs was confirmed by DNA sequencing and protein expression was detected by both immunofluorescence and immunobloting after transfection of HEK293 cells and hippocampal neurons.
Transfection of hippocampal cultured neurons for immunofluorescence
Primary hippocampal cultures were prepared from embryonic day 18 (E18) Sprague-Dawley rat brains by the method of Goslin et al., (1998) as described previously (Christie et al., 2002a, b; Christie and De Blas 2003; Charych et al., 2004a, b; Li et al., 2005a, b) and maintained in glial cell conditioned medium containing 1% N2 supplement (Invitrogen, Carlsbad, CA). Cultured hippocampal neurons (10 day old in culture) were co-transfected with 3 μg of the shRNA vector and 1 μg of the pEGFP-N1 vector (molar ratio 4:1), or with 3 μg of gephyrin vector and 1 μg of the pEGFP-N1 vector (molar ratio 2:1) or 4 μg of gephyrin-EGFP vectors using the CalPhos Mammalian Transfection Kit (BD Biosciences, San Jose, CA), according to the instructions provided by the company. Fluorescence immunocytochemistry was performed five days after transfection with shRNA or 1-5 days after transfection with gephyrin-EGFP or gephyrin.
Nucleofection of hippocampal cultured neurons and protein expression
Nucleofection was used to determine the effect of shRNAs on protein expression. Primary rat hippocampal neurons, prepared by the method of Goslin et al., 1998 as described above, were transfected with the shRNAs at high culture density using the Rat Neuron Nucleofector Kit (Amaxa GmbH, Koln, Germany) according to the instructions provided by the manufacturer. Transfection was done at day 0 before plating. Twenty four hours later, the medium was changed to Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 5% horse serum, 1% N2 supplement and 1mM sodium pyruvate solution (Invitrogen) in a 5% CO2 atmosphere. Neurons were collected at day 16, dissolved in sample dissociation buffer and subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting with anti-gephyrin and anti-α tubulin antibodies, and detected by chemiluminescence (ECL; Amersham Biosciences, England). Intensity of the protein bands was quantified after scanning the X-ray film optical density with a BioRad Gel Doc 2000 imaging system driven by Quantity One 4.4.0 software (BioRad, Hercules, CA).
Transfection of hippocampal cultured neurons for electrophysiology experiments
Hippocampal cultures were generated from E16-18 rat embryos as recently described (Chen et al., 2003; Yao et al., 2006). Briefly, the hippocampal CA1–CA3 region was dissected, cut into ∼1 mm3 cubes and incubated with 0.05% trypsin–EDTA in Hanks' Balanced Salt Solution (HBSS) for 20 min at 37°C. The tissue blocks were triturated in HBSS with 10% horse serum. Dissociated cells were plated onto a monolayer of astrocytes, in MEM, 5% fetal bovine serum, 20% B27 (Invitrogen, Carlsbad, California, USA), 0.5mM L-glutamine, and 25 unit ml−1 penicillin/streptomycin, 4 μM arabinosyl cytosine in a 5% CO2 atmosphere. Cells were transfected at 7-8 DIV by using a modified Ca2+-phosphate transfection protocol as described elsewhere (Jiang and Chen, 2006). Electrophysiology was done 48-72 hr after transfection.
Immunofluorescence of hippocampal cultures
Immunofluorescence was done as described elsewhere (Christie et al., 2002a, b; Charych et al., 2004a, b; Li et al., 2005a, b). Except where mentioned, all steps were done at room temperature. Briefly, neurons were fixed in 4% paraformaldehyde/4% sucrose/phosphate-buffered saline (PBS, pH 7.4) for 15 minutes followed by washing with PBS. Aldehyde groups were quenched by incubation with 50 mM NH4Cl in PBS for 10 minutes followed by permeabilization with 0.25% Triton X-100 in PBS for 10 minutes. Cells were incubated with 5% normal donkey serum in PBS for 30 min followed by incubation with a mixture of primary antibodies diluted in 0.25% Triton X-100 in PBS overnight at 4°C. Cells were washed and incubated with a mixture of secondary antibodies (anti-species specific IgG all made in donkey, Jackson Immunochemicals, West Grove, PA) conjugated to fluorescein isothiocyanate (FITC), Texas Red, or aminomethylcoumarin (AMCA) fluorophores (1:200 dilution in 0.25% Triton X-100 in PBS) for one hour at 37°C. The coverslips were washed with PBS followed by washing with PBS (pH8.5) and mounting using Prolong Gold anti-fade mounting solution (Molecular Probes, Eugene, OG).
Image acquisition and analysis
Digital images for hippocampal cultures were collected using a 60 × pan-fluor objective on a Nikon Eclipse T300 microscope with a Sensys KAF 1401E CCD camera driven by IPLab 3.0 acquisition software (Scanalytics, Fairfax, VA). Images obtained from different channels were processed and merged with PhotoShop 7.0 (Adobe) for analysis. Brightness and contrast were adjusted, the image was changed from 16 bits/channel to 8 bits/channel (1315×1035 pixel resolution), sharpened using the unsharp mask tool (setting: amount=125%, radius=1.5 pixel, threshold = 0 level), color was added to each channel and the images were merged for color co-localization. Fluorescent images in figures were presented before subtraction of the diffuse background signal in dendrites.
Quantification of clusters
For quantification of cluster density in neurons, the background fluorescence of each channel seen in the dendrites was subtracted and the maximum intensities of the fluorophore channels were normalized. Three independent immunofluorescent experiments were performed for each combination of antibodies. A total of 30 dendritic fields were analyzed from 20 – 30 randomly selected pyramidal neurons. Each measurement was taken from a 25-μm long, 4-μm wide dendritic segment. Density values were calculated as number of clusters per 100 μm2 of dendritic surface. The number of gephyrin clusters analyzed for each experiment was in the range of 374-750. Nevertheless, when clustering was highly inhibited by shRNAs, the number of clusters analyzed ranged 141-178. For quantifying the number of neurons that received GABAergic innervation, 31-62 transfected and 54-70 nontransfected pyramidal neurons were randomly selected from three individual experiments and the number of neurons that were contacted by GAD+ presynaptic terminal was recorded. The density of the GAD+ presynaptic boutons was calculated as the number of boutons per cell.
Whole-cell patch-clamp recordings
Whole-cell recordings of sIPSCs and GABA application-induced currents were made in voltage-clamp mode using a MultiClamp 700A amplifier (Molecular Devices Corporation, Sunnyvale, CA, USA). Patch pipettes were pulled from borosilicate glass and fire polished (4–6 MΩ). The recording chamber was continuously perfused with a bath solution consisting of (mM): 128 NaCl, 30 glucose, 25 Hepes, 5 KCl, 2 CaCl2, 1 MgCl2, pH 7.3 adjusted with NaOH. Patch pipettes were filled with (mM): 135 KCl, 10 Tris-phosphocreatine, 2 EGTA, 10 HEPES, 4 MgATP, 0.5 Na2GTP, pH 7.3 adjusted with KOH. The series resistance was typically 10–20 M′Ω. The membrane potential was held at −70 mV. Data were acquired using pClamp 9 software (Molecular Devices Corporation, Sunnyvale, CA, USA), sampled at 2–10 kHz, and filtered at 1 kHz. Offline analysis was done with Clampfit 9 software. The spontaneous IPSC (sIPSC) events were separated from sEPSCs according to their distinguishable decay time constants (τ = 2–5 ms for sEPSCs; τ = 9–18 ms for sIPSCs) and analyzed using MiniAnalysis software (Synaptosoft). The decay time constants were obtained from pharmacological isolation using CNQX (10 μM) to inhibit sEPSCs or using BIC (20 μM) to inhibit sIPSCs. All data were expressed as mean ± SEM and Student's t test was used for statistical analysis.
Supplementary Material
Fig. 1, supplement. Gephyrin mRNA without the 3′-UTR rescues the gephyrin clustering, the γ2-GABAAR clustering and the GABAergic innervation blocked by gephyrin RNA interference. Cultured hippocampal neurons were co-transfected with pEGFP-N1, Geph UTR shRNA and gephyrin cDNA containing the coding region of the mRNA (Geph) without the 3′-UTR (A and C) or transfected only with pEGFP-N1 (B and D). A-D, Triple-label immunofluorescence was done using a mAb to gephyrin (red color) and a rabbit anti-γ2 GABAAR antibody (blue color) or a sheep anti-GAD antibody (blue color). EGFP fluorescence of transfected neurons is shown in green color. Arrowheads show gephyrin clusters that colocalize with γ2-GABAAR clusters. The examples show that there is no apparent difference in the number of gephyrin clusters, γ2-GABAAR clusters and GAD+ boutons contacting the transfected neurons between the cells rescued with the Geph mRNA and the control neurons (neurons transfected only with EGFP). Scale bar: 10 μm for large panels; 5 μm for the small panels. E-G, Quantification of the rescue effect of the Geph mRNA on the density of gephyrin clusters (E), γ2-GABAAR clusters (F), and GAD+ boutons contacting transfected pyramidal cells (G). Values are mean ± SEM. The Geph UTR shRNA co-transfected with EGFP led to a significant decrease (p<0.001) in the density of gephyrin clusters (7.7±0.5 clusters/100 μm2), γ2-GABAAR clusters (8.8±0.5 clusters/100 μm2) and GAD+ boutons contacting the transfected cells (39.2±2.0 boutons/cell) when compared with rescued or control neurons. This effect was reversed (rescued) by the Geph mRNA (Geph), which led to neurons having the same density as control neurons transfected only with EGFP: 19.7±0.9 vs. 20.4±1.1 respectively, p=0.58 for gephyrin clusters; 19.3±0.6 vs. 21.0±1.0, p= 0.17 for γ2-GABAAR clusters and 71.3±2.7 vs. 69.1±3.3, p=0.65 for GAD+ boutons contacting the transfected cells. (***, p<0.001, Student's t test).
Acknowledgments
This work was supported by The National Institute of Neurological Disorders and Stroke grants NS38752 and NS39287 to Angel De Blas, and Johnson & Johnson / Pennsylvania State University Innovative Technology Research Grant to Gong Chen. We would like to thank Dr. Nobumi Kusuhara for the gephyrin construct. We would also like to thank Dr. Ben Bahr for the rabbit anti-gephyrin antibody, Dr. Irwin J. Kopin for the sheep anti-GAD antibody, and Dr. Robert J. Wenthold for the rabbit anti GluR2/3 antibody.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez VA, Ridenour DA, Sabatini BL. Retraction of synapses and dendritic spines induced by off-target effects of RNA interference. J Neurosci. 2006;26:7820–7825. doi: 10.1523/JNEUROSCI.1957-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Bruckner K, Pablo Labrador J, Scheiffele P, Herb A, Seeburg PH, Klein R. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron. 1999;22:511–524. doi: 10.1016/s0896-6273(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Chandra D, Korpi ER, Miralles CP, De Blas AL, Homanics GE. GABAA receptor gamma 2 subunit knockdown mice have enhanced anxiety-like behavior but unaltered hypnotic response to benzodiazepines. BMC Neurosci. 2005;6:30. doi: 10.1186/1471-2202-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier C, Ehrensperger MV, Dahan M, Levi S, Triller A. Cytoskeleton regulation of glycine receptor number at synapses and diffusion in the plasma membrane. J Neurosci. 2006;26:8502–8511. doi: 10.1523/JNEUROSCI.1758-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charych EI, Li R, Serwanski DR, Li X, Miralles CP, Pinal N, De Blas AL. Identification and characterization of two novel splice forms of GRIP1 in the rat brain. J Neurochem. 2006;97:884–898. doi: 10.1111/j.1471-4159.2006.03795.x. [DOI] [PubMed] [Google Scholar]
- Charych EI, Yu W, Miralles CP, Serwanski DR, Li X, Rubio M, De Blas AL. The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the beta subunits of the GABA receptors. J Neurochem. 2004a;90:173–189. doi: 10.1111/j.1471-4159.2004.02481.x. [DOI] [PubMed] [Google Scholar]
- Charych EI, Yu W, Li R, Serwanski DR, Miralles CP, Li X, Yang BY, Pinal N, Walikonis R, De Blas AL. A four PDZ domain-containing splice variant form of GRIP1 is localized in GABAergic and glutamatergic synapses in the brain. J Biol Chem. 2004b;279:38978–38990. doi: 10.1074/jbc.M405786200. [DOI] [PubMed] [Google Scholar]
- Chen Y, Deng L, Maeno-Hikichi Y, Lai M, Chang S, Chen G, Zhang JF. Formation of an endophilin-Ca2+ channel complex is critical for clathrin-mediated synaptic vesicle endocytosis. Cell. 2003;115:37–48. doi: 10.1016/s0092-8674(03)00726-8. [DOI] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Chih B, Gollan L, Scheiffele P. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron. 2006;51:171–178. doi: 10.1016/j.neuron.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Christie SB, De Blas AL. GABAergic and glutamatergic axons innervate the axon initial segment and organize GABA(A) receptor clusters of cultured hippocampal pyramidal cells. J Comp Neurol. 2003;456:361–374. doi: 10.1002/cne.10535. [DOI] [PubMed] [Google Scholar]
- Christie SB, Li RW, Miralles CP, Riquelme R, Yang BY, Charych E, Yu W, Daniels SB, Cantino ME, De Blas AL. Synaptic and extrasynaptic GABAA receptor and gephyrin clusters. Prog Brain Res. 2002a;136:157–180. doi: 10.1016/s0079-6123(02)36015-1. [DOI] [PubMed] [Google Scholar]
- Christie SB, Miralles CP, De Blas AL. GABAergic innervation organizes synaptic and extrasynaptic GABAA receptor clustering in cultured hippocampal neurons. J Neurosci. 2002b;22:684–697. doi: 10.1523/JNEUROSCI.22-03-00684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, Li RW, Miralles CP, Yang BY, De Blas AL. Clustered and non-clustered GABAA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2006;31:1–14. doi: 10.1016/j.mcn.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Contractor A, Rogers C, Maron C, Henkemeyer M, Swanson GT, Heinemann SF. Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science. 2002;296:1864–1869. doi: 10.1126/science.1069081. [DOI] [PubMed] [Google Scholar]
- Craig AM, Banker G, Chang W, McGrath ME, Serpinskaya AS. Clustering of gephyrin at GABAergic but not glutamatergic synapses in cultured rat hippocampal neurons. J Neurosci. 1996;16:3166–3177. doi: 10.1523/JNEUROSCI.16-10-03166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Dresbach T. Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006;29:21–29. doi: 10.1016/j.tins.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Blas AL, Vitorica J, Friedrich P. Localization of the GABAA receptor in the rat brain with a monoclonal antibody to the 57,000 Mr peptide of the GABAA receptor/benzodiazepine receptor/Cl- channel complex. J Neurosci. 1988;8:602–614. doi: 10.1523/JNEUROSCI.08-02-00602.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N, Qi J, Chen G. Molecular reconstitution of functional GABAergic synapses with expression of neuroligin-2 and GABA(A) receptors. Mol Cell Neurosci. 2007;35:14–23. doi: 10.1016/j.mcn.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Dykxhoorn DM, Novina CD, Sharp PA. Killing the messenger: short RNAs that silence gene expression. Nat Rev Mol Cell Biol. 2003;4:457–467. doi: 10.1038/nrm1129. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Ewert M, De Blas AL, Mohler H, Seeburg PH. A prominent epitope on GABAA receptors is recognized by two different monoclonal antibodies. Brain Res. 1992;569:57–62. doi: 10.1016/0006-8993(92)90368-j. [DOI] [PubMed] [Google Scholar]
- Fang C, Deng L, Keller CA, Fukata M, Fukata Y, Chen G, Luscher B. GODZ-mediated palmitoylation of GABA-A receptors is required for normal assembly and function of GABAergic inhibitory synapses. J Neurosci. 2006;26:12758–12768. doi: 10.1523/JNEUROSCI.4214-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Tintrup H, Kirsch J, Nichol MC, Kuhse J, Betz H, Sanes JR. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science. 1998;282:1321–1324. doi: 10.1126/science.282.5392.1321. [DOI] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Kang Y, Hauner AM, Craig AM. Structure function and splice site analysis of the synaptogenic activity of the neurexin-1 beta LNS domain. J Neurosci. 2006;26:4256–4265. doi: 10.1523/JNEUROSCI.1253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K, Asmussen H, Banker G. Rat hippocampal neurons in low density culture. In: Banker G, Goslin K, editors. Culturing Nerve Cells. 2nd. MIT Press; Cambridge, MA: 1998. pp. 339–370. [Google Scholar]
- Homanics GE, Harrison NL, Quinlan JJ, Krasowski MD, Rick CE, De Blas AL, Mehta AK, Kist F, Mihalek RM, Aul JJ, Firestone LL. Normal electrophysiological and behavioral responses to ethanol in mice lacking the long splice variant of the gamma2 subunit of the gamma-aminobutyrate type A receptor. Neuropharmacology. 1999;38:253–265. doi: 10.1016/s0028-3908(98)00177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Chen G. High Ca(2+)-phosphate transfection efficiency in low-density neuronal cultures. Nature Protocols. 2006;1:695–700. doi: 10.1038/nprot.2006.86. [DOI] [PubMed] [Google Scholar]
- Kim EY, Schrader N, Smolinsky B, Bedet C, Vannier C, Schwarz G, Schindelin H. Deciphering the structural framework of glycine receptor anchoring by gephyrin. EMBO J. 2006;25:1385–1395. doi: 10.1038/sj.emboj.7601029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Arancibia-Carcamo IL, Moss SJ. Association of GRIP1 with a GABA(A) receptor associated protein suggests a role for GRIP1 at inhibitory synapses. Biochem Pharmacol. 2004;68:1649–1654. doi: 10.1016/j.bcp.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Betz H. Receptors, gephyrin and gephyrin-associated proteins: novel insights into the assembly of inhibitory postsynaptic membrane specializations. J Physiol. 2000;525:1–9. doi: 10.1111/j.1469-7793.2000.t01-4-00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Gasnier B, Feng G, Sanes JR, Betz H. Gephyrin-independent clustering of postsynaptic GABA(A) receptor subtypes. Mol Cell Neurosci. 2001;17:973–982. doi: 10.1006/mcne.2001.0983. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Laube B, Stahl S, Muller U, Betz H. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S, Logan SM, Tovar KR, Craig AM. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J Neurosci. 2004;24:207–217. doi: 10.1523/JNEUROSCI.1661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson JN, Chery N, Huang K, Wong TP, Gerrow K, Kang R, Prange O, Wang YT, El-Husseini A. Neuroligins mediate excitatory and inhibitory synapse formation: involvement of PSD-95 and neurexin-1beta in neuroligin-induced synaptic specificity. J Biol Chem. 2005;280:17312–17319. doi: 10.1074/jbc.M413812200. [DOI] [PubMed] [Google Scholar]
- Levinson JN, Li R, El-Husseini A. Mechanisms underlying the effects of neuroligin 2 at inhibitory synapses. Society for Neuroscience. 2006 abstract, Online. [Google Scholar]
- Li RW, Serwanski DR, Miralles CP, Li X, Charych E, Riquelme R, Huganir RL, De Blas AL. GRIP1 in GABAergic synapses. J Comp Neurol. 2005a;488:11–27. doi: 10.1002/cne.20566. [DOI] [PubMed] [Google Scholar]
- Li RW, Yu W, Christie SB, Miralles CP, Bai J, LoTurco JJ, De Blas AL. Disruption of postsynaptic GABAA receptor clusters leads to decreased GABAergic innervation of pyramidal neurons. J Neurochem. 2005b;95:756–770. doi: 10.1111/j.1471-4159.2005.03426.x. [DOI] [PubMed] [Google Scholar]
- Meier J, Grantyn R. A gephyrin-related mechanism restraining glycine receptor anchoring at GABAergic synapses. J Neurosci. 2004;24:1398–1405. doi: 10.1523/JNEUROSCI.4260-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meie J, Vannier C, Serge A, Triller A, Choquet D. Fast and reversible trapping of surface glycine receptors by gephyrin. Nat Neurosci. 2001;4:253–260. doi: 10.1038/85099. [DOI] [PubMed] [Google Scholar]
- Miralles CP, Li M, Mehta AK, Khan ZU, De Blas AL. Immunocytochemical localization of the beta(3) subunit of the gamma-aminobutyric acid(A) receptor in the rat brain. J Comp Neurol. 1999;413:535–548. [PubMed] [Google Scholar]
- Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci. 2004;101:13915–13920. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme R, Miralles CP, De Blas AL. Bergmann glia GABA(A) receptors concentrate on the glial processes that wrap inhibitory synapses. J Neurosci. 2002;22:10720–10730. doi: 10.1523/JNEUROSCI.22-24-10720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li X, De Blas AL. Synaptic and nonsynaptic localization of GABAA receptors containing the alpha5 subunit in the rat brain. J Comp Neurol. 2006;499:458–470. doi: 10.1002/cne.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Gollan L, Scholl FG, Mahadomrongkul V, Dobler E, Limthong N, Peck M, Aoki C, Scheiffele P. Silencing of neuroligin function by postsynaptic neurexins. J Neurosci. 2007;27:2815–2824. doi: 10.1523/JNEUROSCI.0032-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- Vitorica J, Park D, Chin G, De Blas AL. Monoclonal antibodies and conventional antisera to the GABAA receptor/benzodiazepine receptor/Cl- channel complex. J Neurosci. 1988;8:615–622. doi: 10.1523/JNEUROSCI.08-02-00615.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Moss LG, Phillips GN., Jr The molecular structure of green fluorescent protein. Nat Biotechnol. 1996;14:1246–1251. doi: 10.1038/nbt1096-1246. [DOI] [PubMed] [Google Scholar]
- Yao J, Qi J, Chen G. Actin-dependent activation of presynaptic silent synapses contributes to long-term synaptic plasticity in developing hippocampal neurons. J Neurosci. 2006;26:8137–8147. doi: 10.1523/JNEUROSCI.1183-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JY, DeRuiter SL, Turner DL. RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci. 2002;99:6047–6052. doi: 10.1073/pnas.092143499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeringue HC, Constantine-Paton M. Post-transcriptional gene silencing in neurons. Curr Opin Neurobiol. 2004;14:654–659. doi: 10.1016/j.conb.2004.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1, supplement. Gephyrin mRNA without the 3′-UTR rescues the gephyrin clustering, the γ2-GABAAR clustering and the GABAergic innervation blocked by gephyrin RNA interference. Cultured hippocampal neurons were co-transfected with pEGFP-N1, Geph UTR shRNA and gephyrin cDNA containing the coding region of the mRNA (Geph) without the 3′-UTR (A and C) or transfected only with pEGFP-N1 (B and D). A-D, Triple-label immunofluorescence was done using a mAb to gephyrin (red color) and a rabbit anti-γ2 GABAAR antibody (blue color) or a sheep anti-GAD antibody (blue color). EGFP fluorescence of transfected neurons is shown in green color. Arrowheads show gephyrin clusters that colocalize with γ2-GABAAR clusters. The examples show that there is no apparent difference in the number of gephyrin clusters, γ2-GABAAR clusters and GAD+ boutons contacting the transfected neurons between the cells rescued with the Geph mRNA and the control neurons (neurons transfected only with EGFP). Scale bar: 10 μm for large panels; 5 μm for the small panels. E-G, Quantification of the rescue effect of the Geph mRNA on the density of gephyrin clusters (E), γ2-GABAAR clusters (F), and GAD+ boutons contacting transfected pyramidal cells (G). Values are mean ± SEM. The Geph UTR shRNA co-transfected with EGFP led to a significant decrease (p<0.001) in the density of gephyrin clusters (7.7±0.5 clusters/100 μm2), γ2-GABAAR clusters (8.8±0.5 clusters/100 μm2) and GAD+ boutons contacting the transfected cells (39.2±2.0 boutons/cell) when compared with rescued or control neurons. This effect was reversed (rescued) by the Geph mRNA (Geph), which led to neurons having the same density as control neurons transfected only with EGFP: 19.7±0.9 vs. 20.4±1.1 respectively, p=0.58 for gephyrin clusters; 19.3±0.6 vs. 21.0±1.0, p= 0.17 for γ2-GABAAR clusters and 71.3±2.7 vs. 69.1±3.3, p=0.65 for GAD+ boutons contacting the transfected cells. (***, p<0.001, Student's t test).