Abstract
Infections and chronic diseases can alter the host’s immunological balance or result in immuno-deficiencies. We hypothesize that this may also affect the performance of vaccine adjuvants. Accordingly, the potency and adjuvanticity of eight adjuvant formulations based on Montanide ISA720, MF59, monophosphoryl lipid A (MPL), QS21 (saponin derivative), MPL-SE (stable emulsion of a MPL derivative), and MPL-AF (MPL in aqueous formulation) were studied in immune gene knockout mice, IFN-γ −/−, IL-4 −/−, and STAT6 −/−, using the P. falciparum MSP1 vaccine, P30P2MSP1-19 as a model immunogen. The adjuvants showed preferential requirements for the immune mediators to induce immune responses to MSP1-19, and the effects were formulation-specific. While emulsion-type adjuvants were highly effective in mice, their potency was more readily suppressed by immune knockouts; and additions of immunomodulators were required to restore efficacy. Formulated adjuvants had characteristics distinct from their individual components, and multi-components formulations were not necessarily superior. We conclude that perturbation of immune environments will have measurable impact on adjuvant’s potency. Evaluation of adjuvants in immune knockout models may be a supplementary approach to measure and compare adjuvants’ efficacy, and to further unveil their distinct biological activities.
INTRODUCTION
It is widely recognized that many vaccines will require the simultaneous administration of adjuvants to enhance immunogenicity and efficacy. In addition, immunity induced by vaccines often necessitate specific enhancement of a polarized immune response, eg. TH1 versus TH2, and this would require adjuvants possessing specialized mode of actions such as TLR ligation (reviewed in [1]). On the other hand, adjuvants can have pleomorphic effects on a variety of cell types and this is much less appreciated. For example, monophosphoryl lipid As (MPLs) and its more toxic parent compound, LPS are thought to interact mainly via TLR4 ligation, but these compounds have differing effects on T cells and cytokine production [2–10]. The different cell types stimulated by MPL and LPS further add to the complexity of the effects of the adjuvants [2–4, 9, 11]. Aluminum phosphates adjuvant (Alum) which is thought to primarily act as a depot for antigen release, has been shown to have additional immunomodulating capacities [12, 13]. However, at least some of the diverse biological and immunological activities of many adjuvants may contribute to harmful side effects, eg. IL-1, IL-6, and TNF-α production by LPS and MPL [8, 9]; and induction of IgE mediated allergic responses by Alum [14–16]. Thus, more in-depth understanding of the vaccine efficacy of an adjuvant formulation may necessitate not only knowledge on the overall immuno-biological activities, but also on the specific immunological environment(s) in which the adjuvant is able to potentiate a particular component of immunity, eg. TH1, CTL or antibody responses. The latter is of further relevance since there are many scenarios in which the host’s immune responses deviate from the norm. These include many types of genetic, infection, and drug induced immunodeficiencies, as well as immune response polarization and skewing due to chronic infections and aging. A recent study demonstrating reduced efficacy of a malaria vaccine in Titermax® adjuvant under a skewed TH2 environment that stemmed from nematode infections clearly demonstrates this phenomenon [17]
We have previously provided evidence that different liposomal formulations of muramyl dipeptide (MDP) and MPL have unique capacity to induce antibody responses to a blood stage malaria vaccine antigen, P. falciparum Merozoite Surface Protein 1, MSP1-19 (P30P2MSP1-19), under different immunological deficient environment, ie. IFN-γ or IL-4 knockout (KO) mouse models [18]. Some formulations have the ability to potentiate TH1 type antibody responses in IFN-γ KO mice; whereas other adjuvants can induce TH2 antibodies in the absence of IL-4. In the present study, we sought to further investigate the efficacy of adjuvants in the same IFN-γ, and IL-4 knockout settings using other types of adjuvants, including some compounds that are currently in or being considered for clinical use. Furthermore, we sought to investigate the effects of a different type of adjuvant carrier, ie. oil/water and oil/water emulsions, in the same cytokine knockout environment. We also began to study the effects of intracellular signaling pathways, ie. STAT6, as an additional approach to the cytokine KO studies. The STAT6 transcription factor has been shown to play critical roles in the development of TH2 responses [19, 20], particularly in a number of IL-4 mediated immune responses, including Ig gene transcriptions and switch recombination [21–23], B cell differentiation, maturation and survival [24–26]. Other studies have also shown some of IL-4’s positive effects on B cells are driven independent of STAT6 [27]. The importance of antibody responses in MSP1 vaccine induced immunity [28, 29] further supports investigations into the requirement of STAT6 in adjuvant-assisted MSP1 vaccine immunization. Analyses of cellular responses were also made to understand their relationships to the development of antibody responses. The significance of these results as relating to vaccine development and in terms of the understanding of adjuvant’s efficacy is discussed.
MATERIALS AND METHODS
Malaria vaccine antigen
The C-terminal 19 kDa fragment of Plasmodium falciparum Merozoite Surface Protein 1, MSP1-19 was used as the immunogen. The recombinant protein was expressed in Pichia pastoris as a fusion protein with the P30 and P2 universal T epitopes [30]. The production and purification of this vaccine antigen was described in detail in a previous study[30]. The utilization of the recombinant antigen with universal T helper epitopes was to help insure that any observed differences in immunogenicity is not due to preferential recognition of T epitopes regulated by immune response (IR) genes [31]. This immunogen was a kind gift from Dr. Anthony Stowers.
Adjuvant formulations
The following adjuvants were used. Montanide ISA720, a metabolizable oil adjuvant (Seppic Inc. Fairfield, NJ)[32]; MF59, squalene/oil emulsion (Chiron Corp. Emeryville, CA)[33]; QS21, a saponin derivative (Antigenics Inc. Lexington, MA)[34]; MPL (from E. coli F583 Rd mutant, Sigma-Aldrich, St Louis, MO); MPL-AF, monophosphoryl lipid A in aqueous formulation (Corixa Inc. Seattle, WA)[35]; MPL-SE, monophosphoryl lipid A in squalene emulsion (Corixa Inc. Seattle WA)[36]; and Freund’s Adjuvants, CFA/IFA (Gibco, Grand Island, NY). Adjuvant dosages were per manufacturers’ recommendations for usages in mice. Additional formulations comprised of combinations of above adjuvants were also used, ISA720/MPL, ISA720/QS21, ISA720/QS21/MPL. This is based on combining the carrier-type adjuvant (ISA720) with the immunomodulators. Due to stringent MTA (Material Transfer Agreement) restrictions, we were not able to study the combined formulations of QS21 with MPL-AF or with MPL-SE, or ISA720 with MPL-AF; instead, commercially available MPL (from E. coli F583 Rd mutant, Sigma-Aldrich, St Louis, MO) was used in the combined formulations. The MPL-AF (MPL in aqueous solution) was used as the “MPL alone” equivalent. Similarly, since MTA prohibits combining QS21 with MF59, only the ISA720 was used.
Formulation of antigen and adjuvants, and dosing
Each dose of the MSP1-19 antigen (P30P2MSP1-19) is 10 ug. The antigen was diluted in PBS (pH 7.0 or pH 6.8 for QS21 preparations). For MSP1-19/QS21, 10 ug of antigen was diluted in PBS (pH 6.8) and reconstituted with 5 ug QS21 to 100 ul. For MSP1-19/MF59, 10 ug MSP1-19 was vortexed with MF59 at a volume ratio of 1:1 in a final volume of 100 ul. For MSP1-19/MPL-SE, 10 ug of MSP1-19 in 150 ul PBS was vortexed with 50 ul MPL-SE (MPL content at 1000ug/ml). For MSP1-19/MPL-AF, 10 ug of MSP1-19 was mixed with 50 ug MPL-AF to a final volume of 100 ul. For MSP1-19/ISA720, 10 ug MSP1-19 was emulsified with ISA720 at a volume ratio of 7:3 (oil:water), as suggested by the manufacturer, in a total volume of 100 ul. The ISA720/MPL, ISA720/QS21, and ISA720/QS21/MPL were similarly emulsified with MSP1-19 except that the MPL was first dissolved in the ISA720 oil, and/or 5ug of QS21 were dissolved in the MSP1-19 antigen containing saline solution, before emulsification. The amount of MPL (50ug), or QS21 (5ug) given to each mouse is kept the same irrespective of the formulations. For CFA/IFA, 10 ug of MSP1-19 was emulsified with an equal volume of CFA or IFA to give a final volume of 100 ul per dose.
Immunization regimen
Mice (5 per group) were immunized intraperitoneally with a dose of 10 ug MSP1-19 (P30P3MSP1-19) in different adjuvant formulations as described above. In addition, three Balb/c mice were immunized with MSP1-19 in saline as negative controls. A total of three immunizations were given at 4 weeks interval. Mice were bled one week before the first and three weeks after the last immunization. The use of mice in this study has the full approval from the University of Hawaii Institutional Animal Care and Use Committee.
Mice
Female mice, 8–12 weeks old, of the following strains were purchased from Jackson Lab. (Bar Harbor, Maine). Interferon-γ deficient mice (IFN-γ −/−, Balb/c), Interleukin-4 deficient mice (IL-4 −/− Balb/c), STAT6 deficient mice (STAT6 −/−, Balb/c). Normal Balb/c mice were used as controls.
Serum antibody assays
The development of antibody responses to MSP1-19 in the immunized mice were evaluated by ELISAs according to established methods [18, 37]. Briefly, ELISA plates (Costar/Corning, Acton, MA) were coated with the test antigen, MSP1-19 [38] at 0.4ug/mL and blocked with 1% Bovine Serum Albumin (BSA) in Borate Buffered Saline (BBS). Test sera were serially diluted in 1% BSA/0.5% yeast extract (DIFCO/BD Biosciences, San Jose, CA) in BBS. Diluted sera were incubated in ELISA wells for 60 min. and washed 7 times with BBS. Washed wells were incubated for 60 min with horseradish peroxidase (HRP) conjugated, goat anti-mouse antibodies at a 1/1000 dilution (H & L chain specific, Kirkgaard and Perry Laboratories; ie. assay for total Ig). Peroxidase substrates, H202 and 2,2’azinobis (3-ethylbenzthiazolinesulfonic acid)/ABTS (Kirkgaard and Perry Laboratories, Gaithersburg, MD) were used for color development and the optical density (O.D.) determined at 410nm. End-point titers were calculated as the reciprocal serum dilutions giving an O.D. of 0.2, which is over four fold the mean O.D. value of baseline sera. The immunoglobulin isotypes of the anti-MSP1-19 antibodies were determined by ELISA as described [18]. Mouse sera were diluted to 1/500 for the assays, and HRP-conjugated, goat anti-mouse IgG1, IgG2a, IgG2b, IgG3, and IgM (SouthernBiotech Inc., Birmingham, AL) at a 1/4000 dilution were used as secondary antibodies. The O.D. was determined as above.
Antigen stimulated ELISPOT assays
IFN-γ and IL-4 ELISPOT assays were performed using splenocytes from immunized mice according to described methods [39–42]. Briefly, 96 wells multiscreen filter plates (Milipore Inc., Bedford, MA) were coated with monoclonal antibodies to mouse IFN-γ (R4-6A2, 10ug/ml) and IL-4 (11B11, 5 ug/l) (BD Biosciences, San Diego, CA) overnight at room temp. Plates were washed and further incubated with DMEM/10% Fetal Bovine Serum at 370C for 1 hr. Spleens from immunized mice were removed 5 days after the last booster immunization and single cell suspensions of splenocytes were prepared. A total of 2.5 × 105 splenocytes were added to each antibody-coated well. The test assays were done in triplicate wells and P30P2MSP1-19 was added to each well at a final concentration of 2 ug/ml. This antigen concentration was previous determined to be optimal from a dose range of 1 ug to10 ug. Positive Control wells were incubated with phorbol myristate acetate (PMA, 5 ng/ml) and ionomycin (1 ng/ml). Negative Control wells were incubated with growth medium alone. After a 48 hr. incubation at 370C in 5% CO2, wells were washed, incubated with biotinylated monoclonal anti- IFN-γ (XMG1.2, 2 ug/ml) and anti-IL-4 (BVD6-24G2, 1 ug/ml) antibodies (BD Biosciences, San Diego, CA). Wells were then washed, incubated in a 1/800 dillution of peroxidase labeled strepstravidin (Kirkgaard and Perry Laboratories, Gaithersburg, MD), and spots were developed in a solution of 3’3’- diaminobenzidine tetrahydrochloride, DAB and 30% H2O2 (Sigma-Aldrich, St. Louis, MO). Spots were enumerated microscopically.
RESULTS
Antibody responses to MSP1-19 in IFN-γ deficient mice immunized with different adjuvant formulations
Figure 1 shows the ELISA antibody titers (ie. total MSP1-19 specific Ig) of IFN-γ KO mice immunized with P30P2MSP1-19 in different adjuvants as compared with wild type (WT) controls (Balb/c). In WT mice, the emulsion-type adjuvants, ISA720 and MF59 were able to induce similar or higher antibody titers to those formulations containing immunomodulators (ie. MPL, QS21). For example, ISA720 alone produced higher antibody titers (p<0.05) than most other formulations with the exception of MF59 and ISA720/QS21. MF59 induced higher antibody titer than MPL-AF, MPL-SE, QS21, and ISA720/MPL (p<0.05). Despite the observed differences, all the adjuvant formulations studied here showed considerable potency in light of the fact that the MSP1-19 antigen induced titers of <1/1250 in the three Balb/c mice immunized with MSP1-19/saline (data not shown), thus supporting previous findings that MSP1 is a poor immunogen [37, 43].
Figure 1.
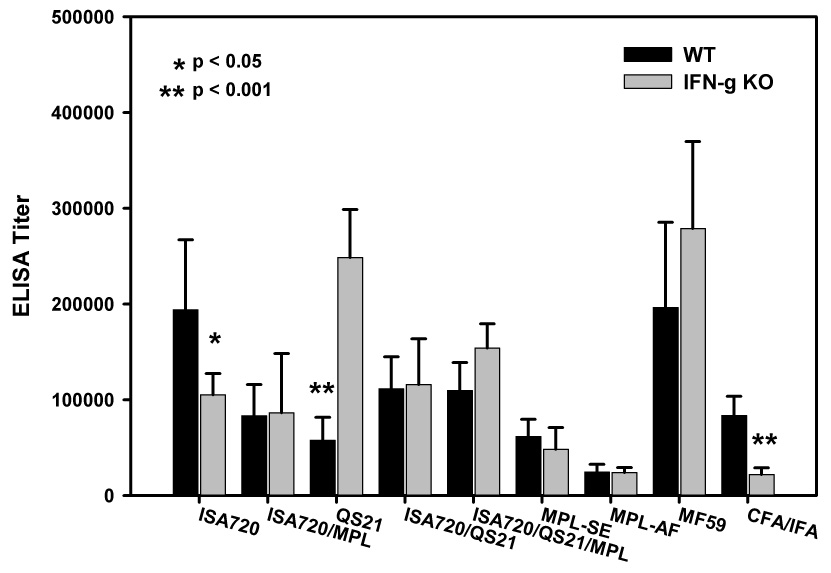
ELISA antibody titers to MSP1-19 of mice, IFN-γ KO and WT, immunized with P30P2MSP1-19 in different adjuvant formulations. Mouse sera were obtained at 21 days after tertiary immunizations. Symbols: Black bars: WT mice; Grey bars: IFN-γ KO mice. Significant differences (Student t test) between WT and IFN-γ KO mice of an adjuvant group are indicated by asterisks.
All adjuvant formulations potentiated anti-MSP1-19 antibodies in the absence of IFN-γ. The two groups with the highest antibody titers in the IFN-γ KO mice were MF59 (p<0.02) and QS21 (p<0.01). Most formulations were also effective in inducing antibody levels similar to those in WT mice. For the formulations, CFA/IFA and ISA720, antibody titers were significantly lowered in IFN-γ KO mice when compared with WT mice. This was also the case after the first booster immunization (data not shown). However, ISA720 formulated with additional adjuvant components induced antibody levels that are comparable to WT mice. Of further interest is the formulation, QS21, with which there was a significant increase in antibody responses in the IFN-γ KO mice. No similar increases were noted for other adjuvant formulations containing QS21, ie. ISA720/QS21 and ISA720/QS21/MPL.
Effects of adjuvants on the Immunoglobulin isotypes of the anti-MSP1-19 antibodies in IFN-γ KO and WT mice
Figure 2 (A and B) shows the isotype specific (IgG1 and IgG2a) anti-MSP1-19 antibodies induced by different adjuvants formulations in IFN-γ KO and WT mice. There were significant reductions of IgG2a antibodies in the IFN-γ KO mice receiving ISA720, CFA/IFA, QS21, and MPL-AF (Figure 2A). Of these four adjuvant groups, only the MPL-AF group had a reversal of IgG1/IgG2a ratio (Figure 2A). For other four formulations, ie MPL-SE, ISA720/MPL, ISA720/QS21, and ISA720/QS21/MPL (see Figure 2B), IFN-γ knockout had no effects on their ability to induce IgG2a antibodies. Thus, Ig class switching by these adjuvants to the TH1-type (IgG2a) is not linked to the TH1 cytokine, IFN-γ. The MF59 and ISA720/QS21adjuvants induced very low amounts of IgG2a in WT mice, and hence the effects of IFN-γ KO were, by default, negligible. There were little or no IgG3 antibodies detected in all mouse groups (data not shown); as well as no significant changes or increases in the IgM responses (data not shown).
Figure 2.
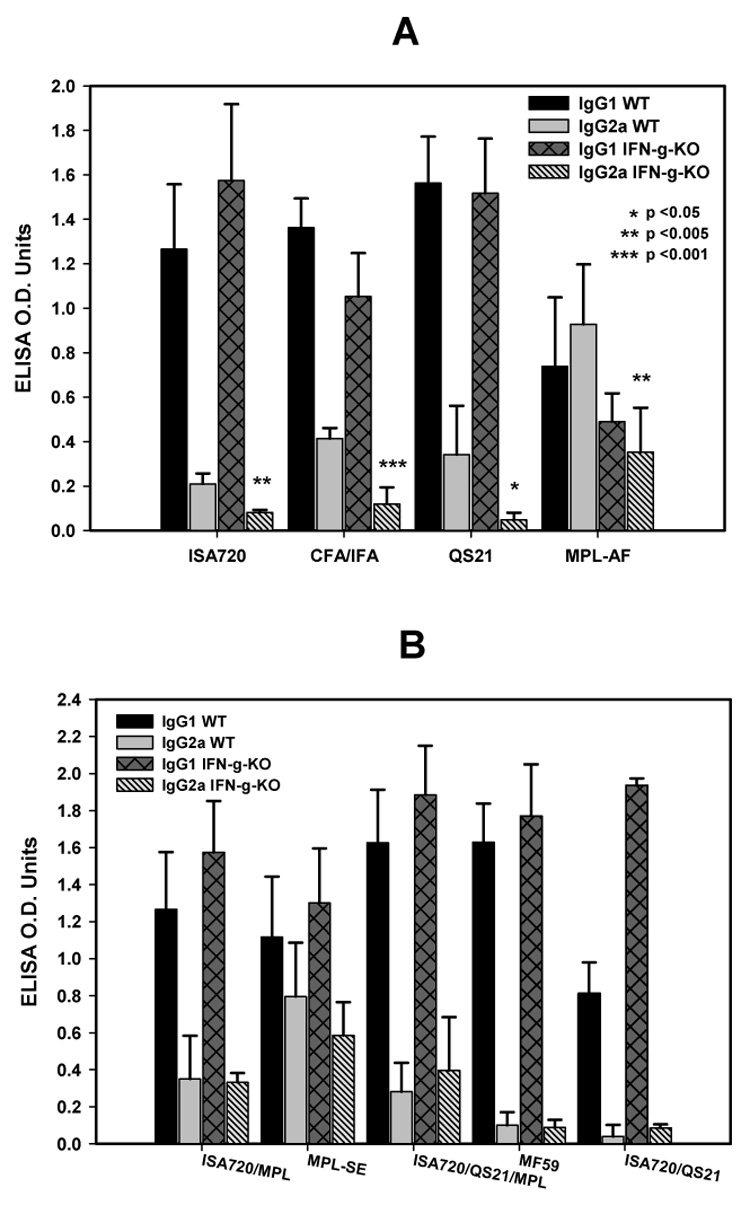
Immunoglobulin isotypes profiles, IgG1 and IgG2a, of anti-MSP1-19 antibodies from IFN-γ KO and WT mice immunized with P30P2MSP1-19 in different adjuvant formulations. Panel A, formulations, ISA720; CFA/IFA; QS21; MPL-AF. Panel B, ISA720/MPL, MPL-SE, ISA720/QS21/MPL, MF59, ISA720/QS21. Symbols: Black bars, WT, IgG1; grey bars, WT, IgG2a; dark grey cross-hatched bars, IFN-γ KO, IgG1; hatched bars, IFN-γ KO, IgG2a. Significant differences (Student t test) in the IgG2a levels between WT and IFN-γ KO mice of an adjuvant group are indicated by asterisks.
Comparison of IgG/IgG2a responses in WT mice receiving QS21 or ISA720 (Figure 2A) to the WT mice receiving ISA720/QS21 (Figure 2B) showed that while the two singly formulated adjuvant preparations induced IgG2a antibodies, the combined formulation was significantly less effective (IgG1/IgG2a ratio: 6.0 for ISA720; 4.6 for QS21; and 20.1 for ISA720/QS21).
The relative levels of Ig isotypes when compared among the mouse groups (measured as OD values at a low serum dilution of 1/500) may not completely correspond to data of the ELISA end-point titers due to contributions of low avidity antibodies and the use of different secondary antibodies in the former assay.
Antibody responses to MSP1-19 in IL-4 deficient mice immunized with different adjuvant formulations
Figure 3 shows the ELISA antibody titers of IL-4 KO mice immunized with P30P2MSP1-19 in different adjuvants as compared with WT controls. All adjuvant formulations were able to induce anti-MSP1-19 antibodies in the absence of IL-4. There were significant reductions of antibody titers in the IL-4 KO mice receiving MF59 and CFA/IFA. Formulations, ISA720, QS21, ISA720/QS21, and MPL-AF were able to potentiate antibody responses to MSP1-19 in IL-4 KO mice at levels similar to WT mice. Furthermore, the formulations, ISA720/MPL, ISA720/QS21/MPL, and MPL-SE induced significantly higher levels of anti-MSP1-19 antibodies in IL-4 KO mice than in WT controls. The common characteristic of these adjuvant formulations is that they contained MPL derivatives that were formulated in emulsion-type carriers.
Figure 3.
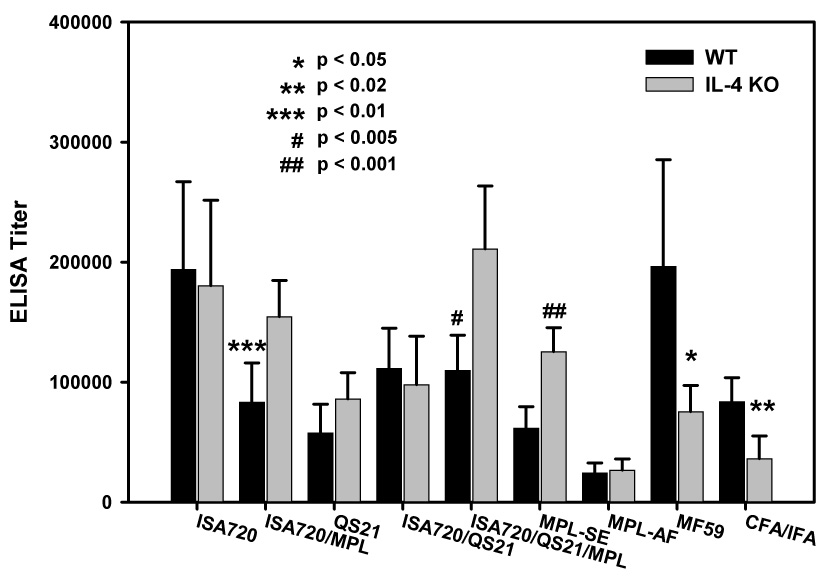
ELISA antibody titers to MSP1-19 of mice, IL-4 KO and WT, immunized with P30P2MSP1-19 in different adjuvant formulations. Mouse sera were obtained at 21 days after tertiary immunizations. Symbols: black bars: WT mice; grey bars: IIL-4 KO mice. Significant differences (Student t test) between WT and IL-4 KO mice of an adjuvant group are indicated by asterisks and the pound “#” symbol.
Antibody responses to MSP1-19 in STAT6 deficient mice immunized with different adjuvant formulations
Figure 4 shows the ELISA antibody titers of STAT6 KO mice immunized with P30P2MSP1-19 in different adjuvants as compared with WT controls. As with the previous KO mouse strains, all adjuvants were able to induce antibody responses to MSP1-19. However, formulations, ISA720, MF59, and CFA/IFA had significantly reduced ability to induce anti-MSP1-19 antibodies when compared with WT controls. In contrast, formulations MPL-AF, ISA720/MPL, and MPL-SE induced significantly higher antibody responses in the STAT6 KO mice. These three formulations contained a single type of immunomodulator, ie. MPL derivatives. The remaining formulations, QS21, ISA720/QS21, and ISA720/QS21/MPL showed equivalent potency in the STAT6 KO and WT mice. It is also of interest that each of these formulations contained QS21. Furthermore, the addition of QS21 to the ISA720/MPL formulation (ie. ISA720/SQ21/MPL) abrogated its ability to induced enhanced antibody responses in STAT6 KO mice.
Figure 4.
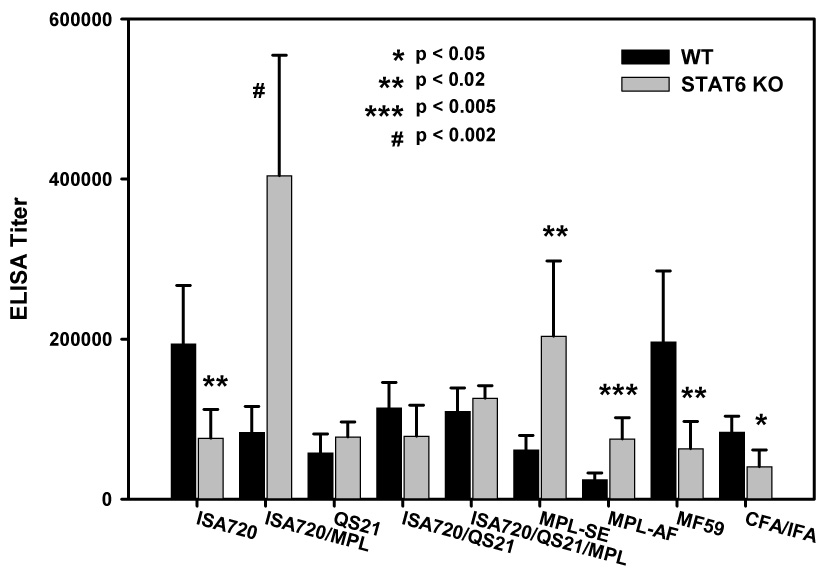
ELISA antibody titers to MSP1-19 of mice, STAT6 KO and WT, immunized with P30P2MSP1-19 in different adjuvant formulations. Mouse sera were obtained at 21 days after tertiary immunizations. Symbols: black bars: WT mice; grey bars: STAT6 KO mice. Significant differences (Student t test) between WT and STAT6 KO mice of an adjuvant group are indicated by asterisks and the pound “#” symbol.
Effects of adjuvants on the Immunoglobulin isotypes of the anti-MSP1-19 antibodies in IL-4 KO, STAT6 KO, and WT mice
Figure 5 (panels A to E) shows the IgG1/IgG2a profile of the anti-MSP1-19 antibodies in IL-4 KO, STAT6 KO and WT mice induced by QS21 (A), CFA/IFA (B), MPL-AF (C), MF59 (D), and MPL-SE (E); respectively. With the exception of MPL-AF, all formulations induced higher IgG2a responses in the two KO mouse strains, with concomitant changes in the IgG1/IgG2a ratio. This was not the case for MPL-AF, in which the dominance of IgG2a had already been established in the WT mice, and changed little in the two KO strains.
Figure 5.
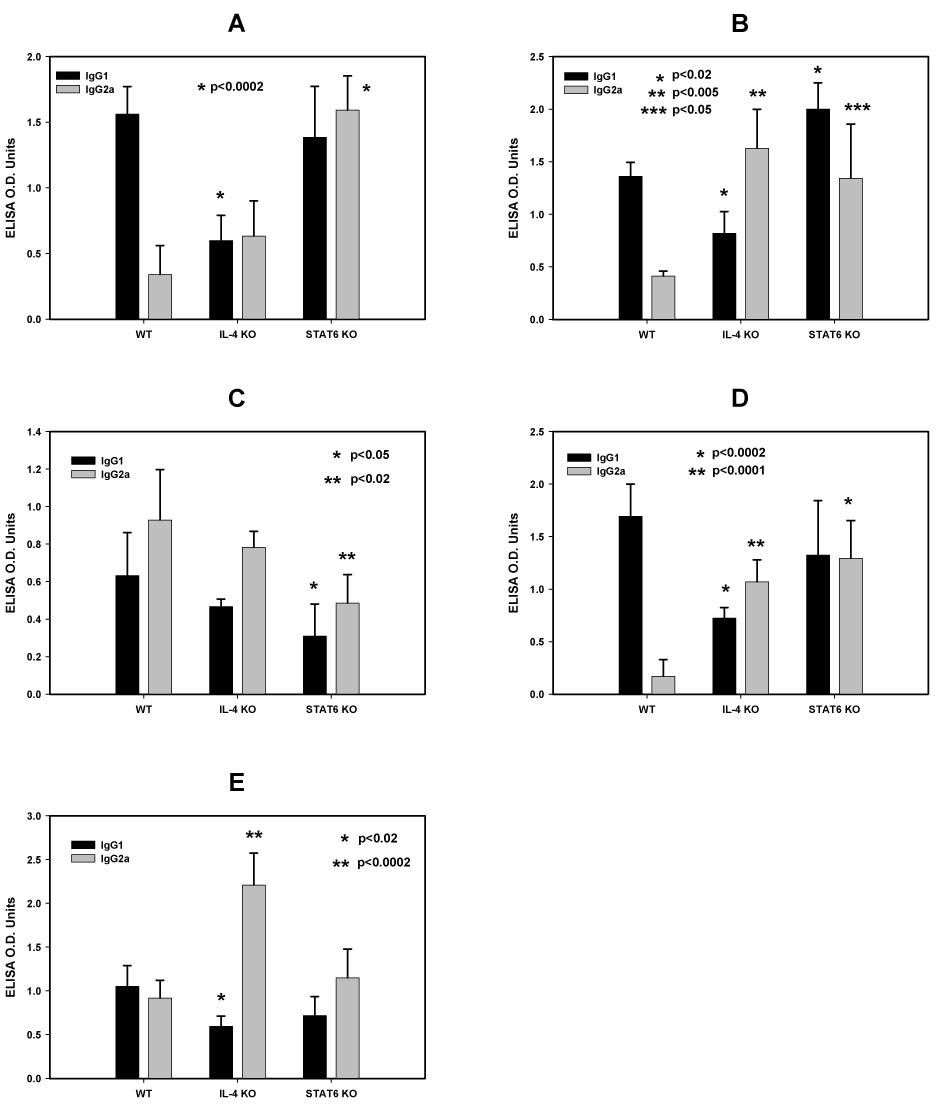
Immunoglobulin isotypes profiles, IgG1 and IgG2a, of anti-MSP1-19 antibodies from IL-4 KO, STAT6 KO and WT mice immunized with P30P2MSP1-19 in different adjuvant formulations. Panel A, QS21; Panel B, CFA/IFA; Panel C: MPL-AF; Panel D, MF59; Panel E, MPL-SE. Symbols: black bars, IgG1; light grey bars, IgG2a. Significant differences in the Ig isotype responses (Student t test) between the WT and KO mice of an adjuvant group are indicated by asterisks above the corresponding Ig isotype.
Figure 6 (panels A to D) compares the IgG1/IgG2a profiles of the four adjuvant formulations containing ISA720, ie. ISA720 (A), ISA720/QS21 (B), ISA720/MPL (C), and ISA720/QS21/MPL (D); respectively. All formulations were able to induce higher IgG2a antibodies in both KO strains. In the STAT6 KO mice receiving ISA720, the increased ratio of IgG1/IgG2a was at the expense of a drastically reduced IgG1 response. However, this reduction was fully compensated by the addition of either MPL and/or QS21 into the ISA720 emulsion. In all mouse groups, the profiles of IgG2b response followed closely to the corresponding IgG2a responses (data not shown).
Figure 6.
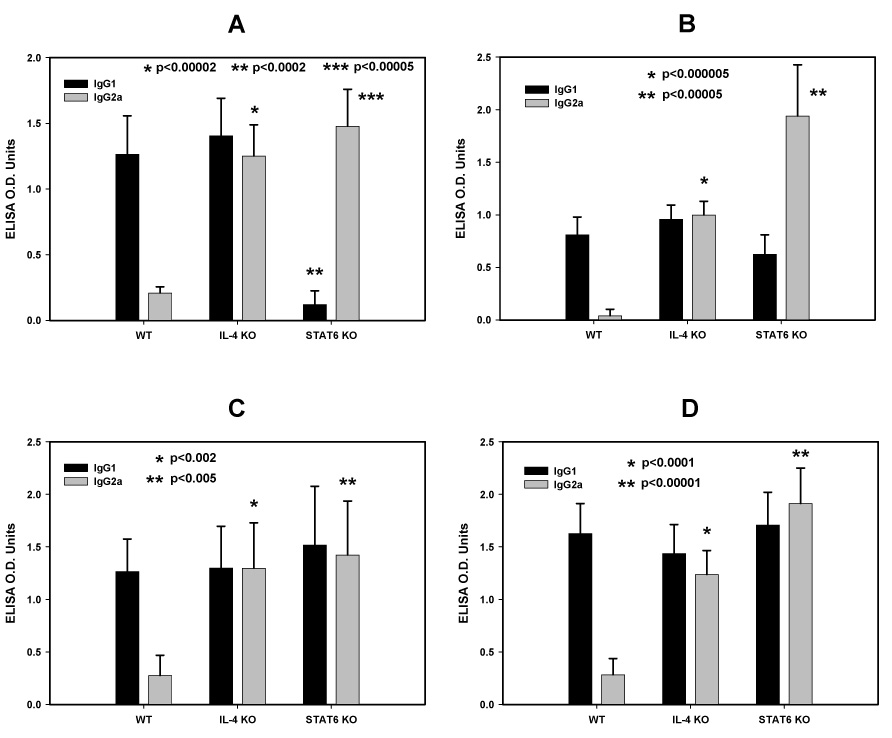
Immunoglobulin isotypes profiles, IgG1 and IgG2a, of anti-MSP1-19 antibodies from IL-4 KO, STAT6 KO and WT mice immunized with P30P2MSP1-19 in different adjuvant formulations. Panel A, ISA720; Panel B, ISA720/QS21 Panel C: ISA720/MPL; Panel D, ISA720/QS21/MPL. Symbols: black bars, IgG1; light grey bars, IgG2a. Significant differences in the Ig isotype responses (Student t test) between the WT and KO mice of an adjuvant group are indicated by asterisks above the corresponding Ig isotype.
ELISPOT analyses of IFN-γ KO mice immunized with P30P2MSP1-19 in different adjuvant formulations
Figure 7A compares the IL-4 production, as measured by ELISPOT, from antigen-stimulated splenocytes of IFN-γ KO and WT mice. Significant reduction in the frequency of IL-4 producing cells in IFN-γ KO mice were observed with ISA720, ISA720/QS21/MPL, and ISA720/QS21. In contrast, QS21 and ISA720/MPL showed enhanced ability to potentiate IL-4 responses. For the remaining adjuvants, IFN-γ KO had no significant effects on IL-4 production as compared with WT mice. The ability to induce IL-4 responses in the IFN-γ KO mice was reduced for most ISA720 formulated adjuvants. On the other hand, adjuvant formulations containing a MPL derivative as the only immunomodulator, ie. MPL-AF (equivalent to MPL alone), MPL-SE and ISA720/MPL, could resist the effects of IFN-γ KO. In the case with ISA720/QS21/MPL, the activities of MPL may have been dominated by the combined effects of ISA720 and QS21, since the formulation ISA720/QS21 showed a reduction of IL-4 producing cells.
Figure 7.
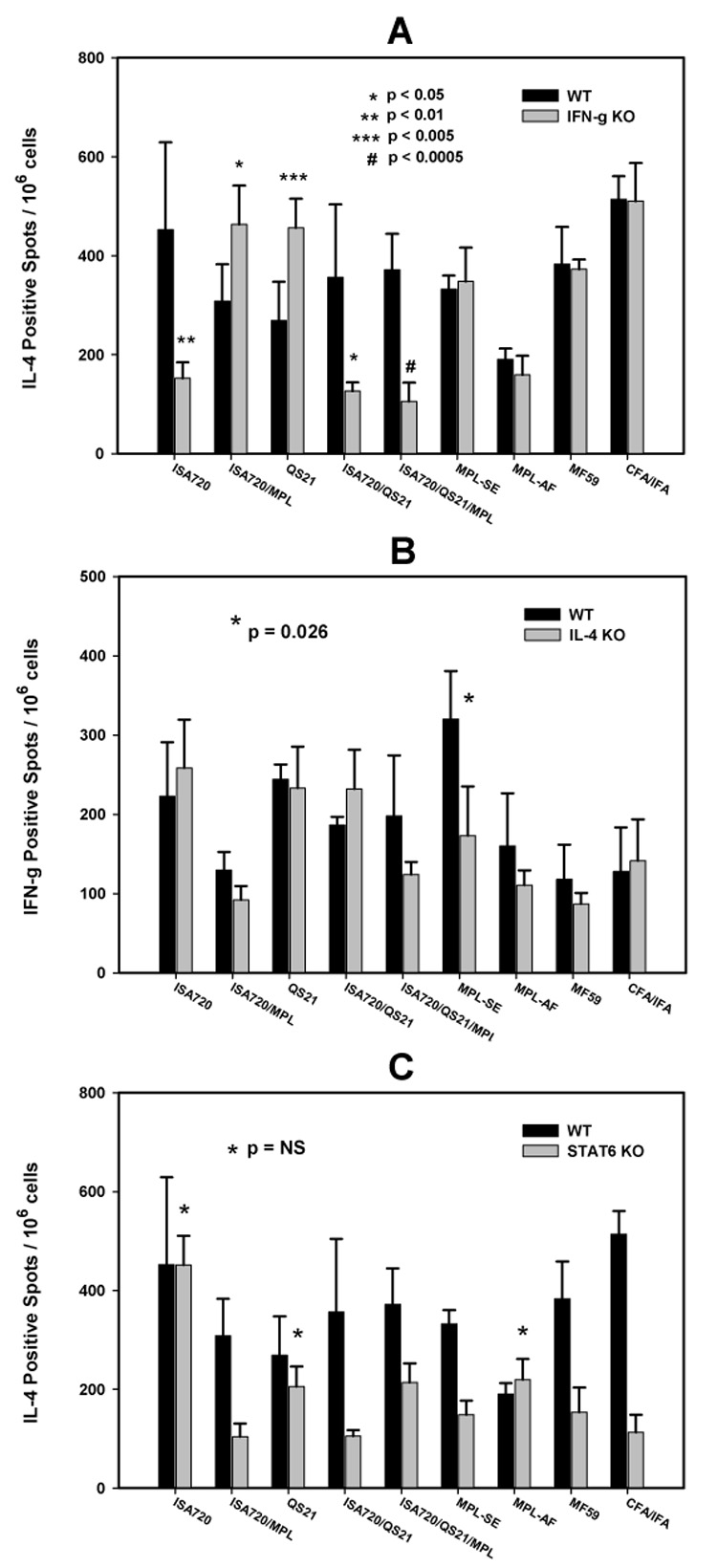
ELISPOT (IFN-γ and IL-4) of antigen stimulated splenocytes from WT (black bars) and immune gene KO (grey bars) mice immunized with P30P2MSP1-19 in different adjuvant formulations. Panel A: IL-4 production from WT and IFN-γ KO mice. Panel B: IFN-γ production from WT and IL-4 KO mice. Significant differences (Student t test) between WT and KO mice of an adjuvant group are indicated by asterisks and the pound “#” symbol. Panel C: IL-4 production from WT and STAT6 KO mice. Adjuvant groups marked by asters indicate no significant differences (Student t test) between WT and KO mice. All other groups showed significant differences between WT and KO (p<0.005).
ELISPOT analyses of IL-4 KO and STAT6 KO mice immunized with P30P2MSP1-19 in different adjuvant formulations
Figure 7B compares the IFN-γ production by ELISPOT from splenocytes of IL-4 KO and WT mice receiving different adjuvants. All adjuvant formulations were able to induce IFN-γ responses similar to WT mice. The exception was the MPL-SE group, where a modest but significant decrease of IFN-γ production was noted. Figure 7C compares the IL-4 production in STAT6 KO versus WT mice. Most adjuvant formulations showed significantly lowered IL-4 responses in STAT6 KO as compared to WT mice. STAT6 KO mice receiving ISA720, MPL-AF, or QS21 showed no significant changes in the IL-4 responses. The production of IFN-γ was also investigated by ELISPOT assays and there were no significant changes between STAT6 KO and WT strains for all adjuvant formulations (data not shown).
DISCUSSION
One of the interesting findings of this study is the efficacy of emulsion-type adjuvants, ISA720 and MF59. These emulsion-type adjuvant formulations induced some of the highest antibody responses in wild type mice. Furthermore, in the IFN-γ and IL-4 KO models, when considering either ISA720 or MF59, the emulsion-type adjuvants also produced higher antibody responses than most other formulations (Figure 1 and 3). In previous studies using multi-lamellar liposomes in adjuvant-assisted immunization with P30P2MSP1-19 [18], the liposomal MSP1-19 also induced considerable antibody responses as compared to other strong adjuvants such as CFA/IFA. Moreover, inclusion of immunomodulators such as MPL or QS21 to these carrier-type adjuvants did not provide a substantial increase in antibody titer. Thus, for the induction of total antibody responses, the additional activation of immune cells (eg. APC activation via TLR by MPL) and/or enhanced cytokine production brought about by these immunomodulators apparently have no additive effects. The recombinant antigen in these emulsions may have been very efficiently internalized and presented by APCs that further immune activation is not required. We further hypothesize that this is likely due to the small sizes (~1 um for ISA720; ~0.2 um for MF59) of the emulsion droplets [44, 45], the resulting high surface/volume ratio for the exposure of antigen and/or immunomodulator, and the depot effect of the emulsions.
On the other hand, the advantages of the immunomodulators became apparent during immunizations in hosts with immunological deficits. As observed with IFN-γ −/−, IL-4 −/−, and STAT6 −/− mice, emulsion-type adjuvant formulations without immunomodulators were much more prone to reduced potency in inducing antibody responses when compared to the wild-type counter-parts (Table 1). The present results with ISA720 and MF59 further extend our previous observations with multilamellar liposomes encapsulated MSP1-19 [18], and thus these findings may be common for carrier-type adjuvants.
Table 1.
Potency of Adjuvant Formulations in Inducing Anti-MSP1-19 Antibodies in IFN-γ KO, IL-4 KO and STAT6 KO Mice as compared to Wild Type Controls @
| ISA720/ MPL | MPL-SE | MPL-AF | ISA720/QS21/MPL | ISA720 | ISA720/QS21 | QS21 | MF59 | CFA/IFA | |
|---|---|---|---|---|---|---|---|---|---|
| IFN-γ KO | o | o | o | o | − | o | + | o | − |
| IL-4 KO | + | + | o | + | o | o | o | − | − |
| STAT6 KO | + | + | + | o | − | o | o | − | − |
significant increase in antibody levels as compared to WT mice
significant decrease in antibody levels as compared to WT mice
no significant change in antibody levels as compared to WT mice
Our results with CFA/IFA suggest that the formulation is similar to emulsion-type adjuvants, despite the fact that it contains Mycobacterial components. However, our immunization regimen called for initial priming with CFA and followed by two repeated boosters with IFA only. This may lead to skewing of the immune responses characteristic of those with IFA. Indeed, our previous results with IFN-γ −/−, IL-4 −/− mice using CFA/IFA show little dependency of these cytokines in inducing anti-MSP1-19 antibodies [18] which seems to contradict the present study. This apparent discrepancy is likely due to the fact that the secondary and tertiary booster immunizations in the previous study utilized successively reduced CFA/IFA ratios (1:1 and 1:3), while the present study used only IFA for boosting.
The role of the immunomodulators, MPLs and QS21 can be further appreciated when comparing the adjuvants’ potency in TH2 deficient environment created by IL-4 KO and STAT6 KO (summarized in Table 1). MPL containing formulations are most likely to have enhanced potency in these KO strains, whereas QS21 containing formulations are most likely to retain the level of potency observed in WT mice. Previous studies with QS21 have demonstrated a requirement of IL-4 to induce serum antibodies via the oral immunization route [46]. Our data showed that parental immunizations may overcome this dependency. It is likely that the different types of immune cells that are encountered during oral versus parental immunizations contributed to the observed differences in IL-4 requirement.
The ability of the adjuvant formulations to maintain potency in the IFN-γ KO environment cannot be categorically attributed to an enhancement of compensatory TH2 pathways since only QS21 and ISA720/MPL induced elevated levels of IL-4 production. In fact, most of the adjuvant formulations induced equivalent or even lower levels of IL-4 production in IFN-γ KO mice. Since IFN-γ is important in the activation and maturation of antigen presenting cells [47], these adjuvants may substitute for the biological functions of this cytokine in APC activation, which in turn may be sufficient to potentiate a robust antibody response.
The fact that most of the adjuvants here could sustain antibody responses in the IL-4 KO and STAT6 KO environment strongly suggests that the TH2 cytokines IL-4 and IL-13, which share the common STAT6 signaling pathway [48, 49], are not obligatory for this particular aspect of adjuvanticity. Furthermore, since these adjuvants induced similar levels of IFN-γ producing cells in the KO and WT mice, compensatory TH1 pathways are not involved in maintaining adjuvant potency. The possibility that the adjuvants may drive compensatory production of another TH2 cytokine, IL-5 to maintain potency remains to be determined, although the role of IL-5 in antibody production has not been demonstrated to be significant and independent of IL-4 [50]. It is known that TH2 cytokines such as IL-4 also play a role in the growth and differentiation of myeloid cells in addition to lymphocytes, as well as in the regulation of hematopoiesis including fibroblasts and endothelial cells [51, 52]. Thus, in the KO environment the immunomodulators may act by enhancing these biological activities, which in turn may lead to an overall improvement of immune responses. The cell sources of the IFN-γ and IL-4 production in immunized mice are currently under investigation. Preliminary studies showed that at least for some adjuvants, the production of IL-4 were from non-CD4+ cell populations (G. Hui and C. Hashimoto, unpublished data). Since the present study employed the intra-peritoneal route (IP) for injections, the possibility that mast cells; which are abundant in the injection sites, may at least in part provide a driving force towards TH2 dominance [53–55] in some mouse or adjuvant groups is worth exploring. The availability of mast cell deficient mice, ie. Kit W-sh mutant [56, 57], should allow direct examination of this hypothesis. A further consideration is the fact that we utilized the Balb/c instead of C57BL6 background in our studies, which represented a TH2 (Balb/c) rather than a TH1 (C57BL6) bias in the host’s immune responses [58]. It will be interesting to determine the extent of the host’s normal immunological background in affecting the adjuvants’ efficacy.
Our data also showed that the induction of IgG2a was not tightly linked to the IFN-γ response, as some adjuvants were able to maintain IgG2a production in IFN-γ KO mice. This is further evident in the IL-4 KO and STAT6 KO mice in which increases in IgG2a response were not accompanied by an enhanced IFN-γ production. We believe that in these cases, a suppression of TH2 environment alone that was brought about by IL-4 or STAT6 KOs in the Balbc mice, which have a TH2-biased immune response, may be sufficient to allow for the expression of TH1 phenotypes, ie. IgG2a production, without significant increases in the production of TH1 cytokines such as IFN-γ.
We investigated the effects of formulating combinations of adjuvant components, ISA720, QS21, and MPL. The results showed that the adjuvanticity of a combination of adjuvants can be distinct from those of its individual components. For example, the ability to induce IgG2a antibodies observed with ISA720 and QS21 was lost in the ISA720/QS21 formulation. The ability to retain potency to induce IL-4 response in STAT6 KO mice by ISA720 and by QS21 was lost in the ISA720/QS21 formulation. The amphiphilic or surfactant nature of QS21 [34] may have significantly influenced the overall characteristic of the emulsion formulation, which may in turn contributed to the observed changes in adjuvanticity. Since we have previously demonstrated the unique characteristics of adjuvant combinations with liposomes, MDP and MPL [18], the distinctive nature of adjuvant combinations may be a general phenomenon.
We compared the triple adjuvant combination, ISA720/QS21/MPL with the double adjuvant combinations, ISA720/QS21 and ISA720/MPL. Though not identical, the ISA720/QS21/MPL formulation is similar to the ASO2A adjuvant used in recent malaria vaccine clinical trials of MSP1 [59–62]. From the antibody and cellular responses in the three KOs and WT mouse strains, it is apparent that the triple combination did not offer any additional advantage in terms of overall potency nor unique adjuvanticity beyond those that could be obtained with ISA720/QS21 or ISA720/MPL. Furthermore, ISA720/QS21/MPL did not possess all of the characteristics of the two double -adjuvant combinations. Thus, the ISA720/QS21/MPL formulation has no practical advantage especially in light of the increased potential for toxicity.
It is apparent from analyses of antibody responses and cytokines production in the various KO mouse models that there is no definitive or absolute dependence of the adjuvant formulations on either TH1 or TH2 immune pathways. This is likely due to the redundancy of immune pathways that come into play, the multiple roles that immune mediators can serve, and the considerable cross-talk among different arms of the immune system. The recently established role of IL-4 in the generation of effector and memory CD8+ T cells is a clear example [63]. Thus, it may be more befitting to characterize adjuvant formulations in terms of their relative efficiency or potency under TH1 or TH2 environments. Accordingly, MPL-containing adjuvants were more effective in TH2 deficient environment s, but they could also perform well in specific TH1 deficient background (ie. IFN-γ−/−), possibly due to strong compensatory mechanisms (Table 1). Adjuvants containing QS21 could withstand perturbations in the TH1/TH2 balance, reflecting their efficient utilization of the reciprocal immune pathways. This is in direct contrast to emulsion-type adjuvants (ISA720, MF59) where, in the absence of other immunomodulators to drive compensatory/redundant pathways, their potency was more readily affected in a negative manner by the same immunological perturbations. Along the same line, little is known about the expression of TLRs in these KO mouse strains, and this is of substantial relevance since at least one adjuvant, ie. MPL, is known to act via TLR (TLR4) ligation [1]. Studies have shown that cytokines can down regulate TLR expression [64]; whereas a strong TLR ligand activation of DCs can overcome deficiencies in IFN-γ production due to weak TCR signaling and/or sub-optimal levels of IL-12 or IL-18 [65]. Thus, the potential interplay or interdependence of TLR signaling and cytokine production that can be driven by adjuvants in the KO models will likely affect the nature and/or the degree of compensatory immune mechanisms that can be elicited.
Although the focus of this study is on adjuvants’ basic mode of action, our data also revealed obvious differences in efficacy among the different adjuvant formulations to potentiate antibody responses to the MSP1 vaccine. Of significance is the performance of CFA/IFA which was inferior to many of the formulations we studied, eg. ISA720, MF59, ISA720/MPL, and MPL-SE. This contrasts the clear superiority of the CFA/IFA as the only efficacious adjuvant for MSP1 in the non-human primate (Aotus monkeys) malaria vaccine model for P. falciparum [66–69]. We believe that this apparent paradox is likely due to animal species differences in response to adjuvants since in the rodent malaria MSP1 vaccine model system, strong protective immunity can be induced by other less toxic adjuvant formulations [70–72]. For the P. falciparum MSP1 vaccine antigen described here, protective immunity is associated not with the level of total antibody responses but with the ability to induce parasite inhibitory antibodies [66, 73, 74]. We have recently conducted a comprehensive analysis (Hui et al, submitted) on the ability of the nine adjuvant formulations to induce parasite inhibitory, anti-MSP1-19 antibodies in different cytokine and co-stimulatory molecule gene KO mice [75, 76], including those described in this study. Results suggest that formulations based on MPL and emulsion carriers were most efficacious.
Our present study is a supplementary approach to dissect and evaluate the potency and immuno-activities of adjuvant formulations. Many of the differences in adjuvanticity would not be apparent without rendering the host immunodeficient. When combined with our previous findings with other adjuvants [18], the results suggest that the differential requirements of immune mediators to induce humoral and cellular responses, as well as the ability to potentiate compensatory responses can be effective indicators for an adjuvant’s efficacy. The data presented here indicate that at a minimum, all adjuvants studied were able to retain a degree of potency in the KO background. Infections and co-infections with microbial agents, as well as many genetic and environmental factors, may cause down modulation or polarization of immune mediators (or surface ligands) not so dissimilar from many immune KO models [17, 77–88]. The ability of adjuvants to sustain their potentiating activities in these skewed environments may determine the efficacy of vaccines.
ACKNOWLEDGEMENTS
We thank Mrs. Lynn Torigue for her excellent technical assistance. We also thank Antigenics Inc., Chiron Corp., and Corixa Inc. for providing adjuvants. This work was supported by NIH grant RO1AI45768.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11:s63–s68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 2.Ismaili J, Rennesson J, Aksoy E, Vekemans J, Vincart B, Amraoui Z, et al. Monophosphoryl lipid A activates both human dendritic cells and T cells. J Immunol. 2002;168(2):926–932. doi: 10.4049/jimmunol.168.2.926. [DOI] [PubMed] [Google Scholar]
- 3.Johnson DA, Keegan DS, Sowell CG, Livesay MT, Johnson CL, Taubner LM, et al. 3-O-Desacyl monophosphoryl lipid A derivatives: synthesis and immunostimulant activities. J Med Chem. 1999;42(22):4640–4649. doi: 10.1021/jm990222b. [DOI] [PubMed] [Google Scholar]
- 4.Johnson DA, Sowell CG, Johnson CL, Livesay MT, Keegan DS, Rhodes MJ, et al. Synthesis and biological evaluation of a new class of vaccine adjuvants: aminoalkyl glucosaminide 4-phosphates (AGPs) Bioorg.Med Chem.Lett. 1999;9(15):2273–2278. doi: 10.1016/s0960-894x(99)00374-1. [DOI] [PubMed] [Google Scholar]
- 5.Maxwell JR, Ruby C, Kerkvliet NI, Vella AT. Contrasting the roles of costimulation and the natural adjuvant lipopolysaccharide during the induction of T cell immunity. J Immunol. 2002;168(9):4372–4381. doi: 10.4049/jimmunol.168.9.4372. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell TC, Hildeman D, Kedl RM, Teague TK, Schaefer BC, White J, et al. Immunological adjuvants promote activated T cell survival via induction of Bcl-3. Nat Immunol. 2001;2(5):397–402. doi: 10.1038/87692. [DOI] [PubMed] [Google Scholar]
- 7.Okemoto K, Kawasaki K, Hanada K, Miura M, Nishijima M. A Potent Adjuvant Monophosphoryl Lipid A Triggers Various Immune Responses, but Not Secretion of IL-1{beta} or Activation of Caspase-1. J Immunol. 2006;176(2):1203–1208. doi: 10.4049/jimmunol.176.2.1203. [DOI] [PubMed] [Google Scholar]
- 8.Thompson BS, Chilton PM, Ward JR, Evans JT, Mitchell TC. The low-toxicity versions of LPS, MPL(R) adjuvant and RC529, are efficient adjuvants for CD4+ T cells. J Leukoc Biol. 2005;78(6):1273–1280. doi: 10.1189/jlb.0305172. [DOI] [PubMed] [Google Scholar]
- 9.Ulrich JT, Myers KR. Monophosphoryl lipid A as an adjuvant. Past experiences and new directions. Pharm.Biotechnol. 1995;6:495–524. [PubMed] [Google Scholar]
- 10.Vella AT, McCormack JE, Linsley PS, Kappler JW, Marrack P. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity. 1995;2(3):261–270. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 11.Kotani S. Bacterial cell surface biological response modifiers and their synthetic counterparts. Adv.Exp.Med Biol. 1992;319:145–164. doi: 10.1007/978-1-4615-3434-1_16. [DOI] [PubMed] [Google Scholar]
- 12.Gupta RK, Chang AC, Griffin P, Rivera R, Siber GR. In vivo distribution of radioactivity in mice after injection of biodegradable polymer microspheres containing 14C-labeled tetanus toxoid. Vaccine. 1996;14(15):1412–1416. doi: 10.1016/s0264-410x(96)00073-4. [DOI] [PubMed] [Google Scholar]
- 13.Ulanova M, Tarkowski A, Hahn-Zoric M, Hanson LA. The Common vaccine adjuvant aluminum hydroxide up-regulates accessory properties of human monocytes via an interleukin-4-dependent mechanism. Infect.Immun. 2001;69(2):1151–1159. doi: 10.1128/IAI.69.2.1151-1159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goto N, Kato H, Maeyama J, Eto K, Yoshihara S. Studies on the toxicities of aluminium hydroxide and calcium phosphate as immunological adjuvants for vaccines. Vaccine. 1993;11(9):914–918. doi: 10.1016/0264-410x(93)90377-a. [DOI] [PubMed] [Google Scholar]
- 15.Gupta RK, Rost BE, Relyveld E, Siber GR. Adjuvant properties of aluminum and calcium compounds. Pharm.Biotechnol. 1995;6:229–248. doi: 10.1007/978-1-4615-1823-5_8. [DOI] [PubMed] [Google Scholar]
- 16.Relyveld EH, Bizzini B, Gupta RK. Rational approaches to reduce adverse reactions in man to vaccines containing tetanus and diphtheria toxoids. Vaccine. 1998;16(9–10):1016–1023. doi: 10.1016/s0264-410x(97)00288-0. [DOI] [PubMed] [Google Scholar]
- 17.Su Z, Segura M, Stevenson MM. Reduced Protective Efficacy of a Blood-Stage Malaria Vaccine by Concurrent Nematode Infection. Infect.Immun. 2006;74(4):2138–2144. doi: 10.1128/IAI.74.4.2138-2144.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hui GS, Hashimoto CN. Pathways for potentiation of immunogenicity during adjuvant-assisted immunizations with Plasmodium falciparum major merozoite surface protein 1. Infect.Immun. 1998;66(11):5329–5336. doi: 10.1128/iai.66.11.5329-5336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agnello D, Lankford CS, Bream J, Morinobu A, Gadina M, O'Shea JJ, et al. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J.Clin.Immunol. 2003;23(3):147–161. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- 20.Hebenstreit D, Wirnsberger G, Horejs-Hoeck J, Duschl A. Signaling mechanisms, interaction partners, and target genes of STAT6. Cytokine Growth Factor Rev. 2006;17(3):173–188. doi: 10.1016/j.cytogfr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Linehan LA, Warren WD, Thompson PA, Grusby MJ, Berton MT. STAT6 is required for IL-4-induced germline Ig gene transcription and switch recombination. J.Immunol. 1998;161(1):302–310. [PubMed] [Google Scholar]
- 22.Stutz AM, Woisetschlager M. Functional synergism of STAT6 with either NF-kappa B or PU.1 to mediate IL-4-induced activation of IgE germline gene transcription. J.Immunol. 1999;163(8):4383–4391. [PubMed] [Google Scholar]
- 23.Kim RJ, Kim HA, Park JB, Park SR, Jeon SH, Seo GY, et al. IL-4-induced AID expression and its relevance to IgA class switch recombination. Biochem.Biophys.Res.Commun. 2007;361(2):398–403. doi: 10.1016/j.bbrc.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Davey EJ, Greicius G, Thyberg J, Severinson E. STAT6 is required for the regulation of IL-4-induced cytoskeletal events in B cells. Int.Immunol. 2000;12(7):995–1003. doi: 10.1093/intimm/12.7.995. [DOI] [PubMed] [Google Scholar]
- 25.Schroder AJ, Pavlidis P, Arimura A, Capece D, Rothman PB. Cutting edge: STAT6 serves as a positive and negative regulator of gene expression in IL-4-stimulated B lymphocytes. J.Immunol. 2002;168(3):996–1000. doi: 10.4049/jimmunol.168.3.996. [DOI] [PubMed] [Google Scholar]
- 26.Dufort FJ, Bleiman BF, Gumina MR, Blair D, Wagner DJ, Roberts MF, et al. Cutting edge: IL-4-mediated protection of primary B lymphocytes from apoptosis via Stat6-dependent regulation of glycolytic metabolism. J.Immunol. 2007;179(8):4953–4957. doi: 10.4049/jimmunol.179.8.4953. [DOI] [PubMed] [Google Scholar]
- 27.Mori M, Morris SC, Orekhova T, Marinaro M, Giannini E, Finkelman FD. IL-4 promotes the migration of circulating B cells to the spleen and increases splenic B cell survival. J.Immunol. 2000;164(11):5704–5712. doi: 10.4049/jimmunol.164.11.5704. [DOI] [PubMed] [Google Scholar]
- 28.Holder AA, Riley EM. Human immune response to MSP-1. Parasitol.Today. 1996;12(5):173–174. doi: 10.1016/0169-4758(96)20009-2. [DOI] [PubMed] [Google Scholar]
- 29.Holder AA, Guevara Patino JA, Uthaipibull C, Syed SE, Ling IT, Scott-Finnigan T, et al. Merozoite surface protein 1, immune evasion, and vaccines against asexual blood stage malaria. Parassitologia. 1999;41(1–3):409–414. [PubMed] [Google Scholar]
- 30.Brady CP, Shimp RL, Miles AP, Whitmore M, Stowers AW. High-level production and purification of P30P2MSP1(19), an important vaccine antigen for malaria, expressed in the methylotropic yeast Pichia pastoris. Protein Expr.Purif. 2001;23(3):468–475. doi: 10.1006/prep.2001.1526. [DOI] [PubMed] [Google Scholar]
- 31.Chang SP, Hui GS, Kato A, Siddiqui WA. Generalized immunological recognition of the major merozoite surface antigen (gp195) of Plasmodium falciparum. Proc.Natl.Acad.Sci.U.S.A. 1989;86(16):6343–6347. doi: 10.1073/pnas.86.16.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrence GW, Saul A, Giddy AJ, Kemp R, Pye D. Phase I trial in humans of an oil-based adjuvant SEPPIC MONTANIDE ISA 720. Vaccine. 1997;15(2):176–178. doi: 10.1016/s0264-410x(96)00150-8. [DOI] [PubMed] [Google Scholar]
- 33.Ott G, Barchfeld GL, Chernoff D, Radhakrishnan R, van Hoogevest P, Van Nest G. MF59. Design and evaluation of a safe and potent adjuvant for human vaccines. Pharm.Biotechnol. 1995;6:277–296. doi: 10.1007/978-1-4615-1823-5_10. [DOI] [PubMed] [Google Scholar]
- 34.Kensil CR, Wu JY, Soltysik S. Structural and immunological characterization of the vaccine adjuvant QS-21. Pharm.Biotechnol. 1995;6:525–541. doi: 10.1007/978-1-4615-1823-5_22. [DOI] [PubMed] [Google Scholar]
- 35.Childers NK, Miller KL, Tong G, Llarena JC, Greenway T, Ulrich JT, et al. Adjuvant activity of monophosphoryl lipid A for nasal and oral immunization with soluble or liposome-associated antigen. Infect.Immun. 2000;68(10):5509–5516. doi: 10.1128/iai.68.10.5509-5516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skeiky YA, Coler RN, Brannon M, Stromberg E, Greeson K, Crane RT, et al. Protective efficacy of a tandemly linked, multi-subunit recombinant leishmanial vaccine (Leish-111f) formulated in MPL adjuvant. Vaccine. 2002;20(27–28):3292–3303. doi: 10.1016/s0264-410x(02)00302-x. [DOI] [PubMed] [Google Scholar]
- 37.Hui GS, Chang SP, Gibson H, Hashimoto A, Hashiro C, Barr PJ, et al. Influence of adjuvants on the antibody specificity to the Plasmodium falciparum major merozoite surface protein, gp195. J.Immunol. 1991;147(11):3935–3941. [PubMed] [Google Scholar]
- 38.Kaslow DC, Hui G, Kumar S. Expression and antigenicity of Plasmodium falciparum major merozoite surface protein (MSP1(19)) variants secreted from Saccharomyces cerevisiae. Mol.Biochem.Parasitol. 1994;63(2):283–289. doi: 10.1016/0166-6851(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 39.Favre N, Bordmann G, Rudin W. Comparison of cytokine measurements using ELISA, ELISPOT and semi-quantitative RT-PCR. J Immunol Methods. 1997;204(1):57–66. doi: 10.1016/s0022-1759(97)00033-1. [DOI] [PubMed] [Google Scholar]
- 40.Nagabhushanam V, Cheers C. Non-major histocompatibility complex control of antibody isotype and Th1 versus Th2 cytokines during experimental infection of mice with Mycobacterium avium. Infect.Immun. 2001;69(3):1708–1713. doi: 10.1128/IAI.69.3.1708-1713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su HC, Cousens LP, Fast LD, Slifka MK, Bungiro RD, Ahmed R, et al. CD4+ and CD8+ T cell interactions in IFN-gamma and IL-4 responses to viral infections: requirements for IL-2. J Immunol. 1998;160(10):5007–5017. [PubMed] [Google Scholar]
- 42.Miyahira Y, Murata K, Rodriguez D, Rodriguez JR, Esteban M, Rodrigues MM, et al. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181(1):45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 43.Hui GS, Tam LQ, Chang SP, Case SE, Hashiro C, Siddiqui WA, et al. Synthetic low-toxicity muramyl dipeptide and monophosphoryl lipid A replace Freund complete adjuvant in inducing growth-inhibitory antibodies to the Plasmodium falciparum major merozoite surface protein, gp195. Infect.Immun. 1991;59(5):1585–1591. doi: 10.1128/iai.59.5.1585-1591.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miles AP, McClellan HA, Rausch KM, Zhu D, Whitmore MD, Singh S, et al. Montanide ISA 720 vaccines: quality control of emulsions, stability of formulated antigens, and comparative immunogenicity of vaccine formulations. Vaccine. 2005;23(19):2530–2539. doi: 10.1016/j.vaccine.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 45.Singh M, Ugozzoli M, Kazzaz J, Chesko J, Soenawan E, Mannucci D, et al. A preliminary evaluation of alternative adjuvants to alum using a range of established and new generation vaccine antigens. Vaccine. 2006;24(10):1680–1686. doi: 10.1016/j.vaccine.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 46.Boyaka PN, Marinaro M, Jackson RJ, van Ginkel FW, Cormet-Boyaka E, Kirk KL, et al. Oral QS-21 requires early IL-4 help for induction of mucosal and systemic immunity. J Immunol. 2001;166(4):2283–2290. doi: 10.4049/jimmunol.166.4.2283. [DOI] [PubMed] [Google Scholar]
- 47.Schreiber GH, Schreiber RD, Thomson AW, Lotze MT, editors. The Cytokine Handbook. 4th ed. San Diego, CA: Academic Press; 2003. pp. 567–602. Interferon-γ. [Google Scholar]
- 48.Jiang H, Harris MB, Rothman P. IL-4/IL-13 signaling beyond JAK/STAT. J Allergy Clin.Immunol. 2000;105(6 Pt 1):1063–1070. doi: 10.1067/mai.2000.107604. [DOI] [PubMed] [Google Scholar]
- 49.Murata T, Taguchi J, Puri RK, Mohri H. Sharing of receptor subunits and signal transduction pathway between the IL-4 and IL-13 receptor system. Int. J Hematol. 1999;69(1):13–20. [PubMed] [Google Scholar]
- 50.Kok CC, Schwenger GT, Osmond RIW, Urwin DL, Sanderson CJ, Thomson AW, Lotze MT, editors. The Cytokine Handbook. 4th ed. San Diego, CA: Academic Press; 2003. pp. 263–280. Interleukin-5. [Google Scholar]
- 51.Grunig G, de Vries JE, de Waal Malefyt R, Thomson AW, Lotze MT, editors. The Cytokine Handbook. 4th ed. San Diego, CA: Academic Press; 2003. pp. 409–430. Interleukin-13. [Google Scholar]
- 52.Okada H, Banchereau J, Lotze MT, Thomson AW, Lotze MT, editors. The Cytokine Handbook. 4th ed. San Diego, CA: Academic Press; 2003. pp. 227–262. Interleukin-4. [Google Scholar]
- 53.Boyce JA. The biology of the mast cell. Allergy Asthma Proc. 2004;25(1):27–30. [PubMed] [Google Scholar]
- 54.Maezawa Y, Nakajima H, Kumano K, Kubo S, Karasuyama H, Iwamoto I. Role of IgE in Th2 cell-mediated allergic airway inflammation. Int.Arch.Allergy Immunol. 2003;131 Suppl 1:2–6. doi: 10.1159/000070473. [DOI] [PubMed] [Google Scholar]
- 55.Ogawa Y, Grant JA. Mediators of anaphylaxis. Immunol.Allergy Clin.North Am. 2007;27(2):249–260. doi: 10.1016/j.iac.2007.03.013. vii. [DOI] [PubMed] [Google Scholar]
- 56.Tono T, Tsujimura T, Koshimizu U, Kasugai T, Adachi S, Isozaki K, et al. c-kit Gene was not transcribed in cultured mast cells of mast cell-deficient Wsh/Wsh mice that have a normal number of erythrocytes and a normal c-kit coding region. Blood. 1992;80(6):1448–1453. [PubMed] [Google Scholar]
- 57.Berrozpe G, Timokhina I, Yukl S, Tajima Y, Ono M, Zelenetz AD, et al. The W(sh), W(57), and Ph Kit expression mutations define tissue-specific control elements located between -23 and -154 kb upstream of Kit. Blood. 1999;94(8):2658–2666. [PubMed] [Google Scholar]
- 58.Charles PC, Weber KS, Cipriani B, Brosnan CF. Cytokine, chemokine and chemokine receptor mRNA expression in different strains of normal mice: implications for establishment of a Th1/Th2 bias. J.Neuroimmunol. 1999;100(1–2):64–73. doi: 10.1016/s0165-5728(99)00189-7. [DOI] [PubMed] [Google Scholar]
- 59.Bojang KA, Milligan PJ, Pinder M, Vigneron L, Alloueche A, Kester KE, et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet. 2001;358(9297):1927–1934. doi: 10.1016/S0140-6736(01)06957-4. [DOI] [PubMed] [Google Scholar]
- 60.MAL-36, A phase IIB in Kenya, MSP-1 with AS02A. Recent results of Phase I and II clinical trials of three candidate malaria antigens. 55th Annual Meeting of the Americal Society of Tropical Medicine and Hygiene; 2006 Nov 15.2006. [Google Scholar]
- 61.Thera MA, Doumbo OK, Coulibaly D, Diallo DA, Sagara I, Dicko A, et al. Safety and Allele-Specific Immunogenicity of a Malaria Vaccine in Malian Adults: Results of a Phase I Randomized Trial. PLoS Clinical Trials. 2006;1(7):e34. doi: 10.1371/journal.pctr.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Withers MR, McKinney D, Ogutu BR, Waitumbi JN, Milman JB, Apollo OJ, et al. Safety and Reactogenicity of an MSP-1 Malaria Vaccine Candidate: A Randomized Phase Ib Dose-Escalation Trial in Kenyan Children. PLoS Clinical Trials. 2006;1(7):e32. doi: 10.1371/journal.pctr.0010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Acacia de Sa PA, Morrot A, Chakravarty S, Overstreet M, Bream JH, Irusta PM, et al. IL-4 induces a wide-spectrum intracellular signaling cascade in CD8+ T cells. J Leukoc Biol. 2007;81(4):1102–1110. doi: 10.1189/jlb.0906583. [DOI] [PubMed] [Google Scholar]
- 64.Mueller T, Terada T, Rosenberg IM, Shibolet O, Podolsky DK. Th2 cytokines down-regulate TLR expression and function in human intestinal epithelial cells. J.Immunol. 2006;176(10):5805–5814. doi: 10.4049/jimmunol.176.10.5805. [DOI] [PubMed] [Google Scholar]
- 65.Nembrini C, Abel B, Kopf M, Marsland BJ. Strong TCR signaling, TLR ligands, and cytokine redundancies ensure robust development of type 1 effector T cells. J.Immunol. 2006;176(12):7180–7188. doi: 10.4049/jimmunol.176.12.7180. [DOI] [PubMed] [Google Scholar]
- 66.Chang SP, Case SE, Gosnell WL, Hashimoto A, Kramer KJ, Tam LQ, et al. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect.Immun. 1996;64(1):253–261. doi: 10.1128/iai.64.1.253-261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar S, Collins W, Egan A, Yadava A, Garraud O, Blackman MJ, et al. Immunogenicity and efficacy in aotus monkeys of four recombinant Plasmodium falciparum vaccines in multiple adjuvant formulations based on the 19-kilodalton C terminus of merozoite surface protein 1. Infect.Immun. 2000;68(4):2215–2223. doi: 10.1128/iai.68.4.2215-2223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siddiqui WA, Tam LQ, Kan SC, Kramer KJ, Case SE, Palmer KL, et al. Induction of protective immunity to monoclonal-antibody-defined Plasmodium falciparum antigens requires strong adjuvant in Aotus monkeys. Infect.Immun. 1986;52(1):314–318. doi: 10.1128/iai.52.1.314-318.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stowers AW, Cioce V, Shimp RL, Lawson M, Hui G, Muratova O, et al. Efficacy of two alternate vaccines based on Plasmodium falciparum merozoite surface protein 1 in an Aotus challenge trial. Infect.Immun. 2001;69(3):1536–1546. doi: 10.1128/IAI.69.3.1536-1546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daly TM, Long CA. Influence of adjuvants on protection induced by a recombinant fusion protein against malarial infection. Infect.Immun. 1996;64(7):2602–2608. doi: 10.1128/iai.64.7.2602-2608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumar S, Jones TR, Oakley MS, Zheng H, Kuppusamy SP, Taye A, et al. CpG oligodeoxynucleotide and Montanide ISA 51 adjuvant combination enhanced the protective efficacy of a subunit malaria vaccine. Infect.Immun. 2004;72(2):949–957. doi: 10.1128/IAI.72.2.949-957.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ling IT, Ogun SA, Momin P, Richards RL, Garcon N, Cohen J, et al. Immunization against the murine malaria parasite Plasmodium yoelii using a recombinant protein with adjuvants developed for clinical use. Vaccine. 1997;15(14):1562–1567. doi: 10.1016/s0264-410x(97)00076-5. [DOI] [PubMed] [Google Scholar]
- 73.Hui GS, Siddiqui WA. Serum from Pf195 protected Aotus monkeys inhibit Plasmodium falciparum growth in vitro. Exp.Parasitol. 1987;64(3):519–522. doi: 10.1016/0014-4894(87)90068-3. [DOI] [PubMed] [Google Scholar]
- 74.Singh S, Miura K, Zhou H, Muratova O, Keegan B, Miles A, et al. Immunity to Recombinant Plasmodium falciparum Merozoite Surface Protein 1 (MSP1): Protection in Aotus nancymai Monkeys Strongly Correlates with Anti-MSP1 Antibody Titer and In Vitro Parasite-Inhibitory Activity. Infect.Immun. 2006;74(8):4573–4580. doi: 10.1128/IAI.01679-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hui G, Hashimoto C. Interleukin-6 has differential influence on the ability of adjuvant formulations to potentiate antibody responses to a Plasmodium falciparum blood-stage vaccine. Vaccine. 2007;25(36):6598–6603. doi: 10.1016/j.vaccine.2007.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hui G, Hashimoto C. The requirement of CD80, CD86, and ICAM-1 on the ability of adjuvant formulations to potentiate antibody responses to a Plasmodium falciparum blood-stage vaccine. Vaccine. 2007;25(51):8549–8556. doi: 10.1016/j.vaccine.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo JT, Hayashi J, Seeger C. West Nile virus inhibits the signal transduction pathway of alpha interferon. J Virol. 2005;79(3):1343–1350. doi: 10.1128/JVI.79.3.1343-1350.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin RJ, Liao CL, Lin E, Lin YL. Blocking of the alpha interferon-induced Jak-Stat signaling pathway by Japanese encephalitis virus infection. J Virol. 2004;78(17):9285–9294. doi: 10.1128/JVI.78.17.9285-9294.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Munoz-Jordan JL, Sanchez-Burgos GG, Laurent-Rolle M, Garcia-Sastre A. Inhibition of interferon signaling by dengue virus. Proc.Natl.Acad.Sci.U.S.A. 2003;100(24):14333–14338. doi: 10.1073/pnas.2335168100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jankovic D, Steinfelder S, Kullberg MC, Sher A. Mechanisms underlying helminth- induced Th2 polarization: default, negative or positive pathways? Chem.Immunol Allergy. 2006;90:65–81. doi: 10.1159/000088881. [DOI] [PubMed] [Google Scholar]
- 81.Araujo MI, Bliss SK, Suzuki Y, Alcaraz A, Denkers EY, Pearce EJ. Interleukin-12 promotes pathologic liver changes and death in mice coinfected with Schistosoma mansoni and Toxoplasma gondii. Infect.Immun. 2001;69(3):1454–1462. doi: 10.1128/IAI.69.3.1454-1462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bermudez LE, Covaro G, Remington J. Infection of murine macrophages with Toxoplasma gondii is associated with release of transforming growth factor beta and downregulation of expression of tumor necrosis factor receptors. Infect.Immun. 1993;61(10):4126–4130. doi: 10.1128/iai.61.10.4126-4130.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bermudez LE. Production of transforming growth factor-beta by Mycobacterium avium-infected human macrophages is associated with unresponsiveness to IFN-gamma. J Immunol. 1993;150(5):1838–1845. [PubMed] [Google Scholar]
- 84.Ferreira AP, Aguiar AS, Fava MW, Correa JO, Teixeira FM, Teixeira HC. Can the efficacy of bacille calmette-guerin tuberculosis vaccine be affected by intestinal parasitic infections? J Infect.Dis. 2002;186(3):441–442. doi: 10.1086/341656. [DOI] [PubMed] [Google Scholar]
- 85.Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, et al. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6(5):536–542. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 86.Marshall AJ, Brunet LR, van Gessel Y, Alcaraz A, Bliss SK, Pearce EJ, et al. Toxoplasma gondii and Schistosoma mansoni synergize to promote hepatocyte dysfunction associated with high levels of plasma TNF-alpha and early death in C57BL/6 mice. J Immunol. 1999;163(4):2089–2097. [PubMed] [Google Scholar]
- 87.Haga IR, Bowie AG. Evasion of innate immunity by vaccinia virus. Parasitology. 2005;130 Suppl:S11–S25. doi: 10.1017/S0031182005008127. [DOI] [PubMed] [Google Scholar]
- 88.Liu L, Xu Z, Fuhlbrigge RC, Pena-Cruz V, Lieberman J, Kupper TS. Vaccinia virus induces strong immunoregulatory cytokine production in healthy human epidermal keratinocytes: a novel strategy for immune evasion. J Virol. 2005;79(12):7363–7370. doi: 10.1128/JVI.79.12.7363-7370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


