Abstract
A bioassay-guided fractionation of the EtOH extract of the Madagascan plant Terminalia calcicola H. Perrier (Combretaceae) led to the isolation of two new cytotoxic xanthones, termicalcicolanone A (1) and termicalcicolanone B (2). The structures of the new compounds were established on the basis of one and two dimensional NMR spectroscopic data. Both compounds showed modest antiproliferative activity toward the A2780 human ovarian cancer cell line.
In our continuing search for bioactive molecules from the Madagascar rainforests as part of an International Cooperative Biodiversity Group (ICBG) program,1 we obtained extracts of various parts of the tree Terminalia calcicola H. Perrier (Combretaceae) collected in Madagascar. The EtOH extract of the immature fruit proved to have moderate antiproliferative activity, with an IC50 value of 14 µg/mL against the A2780 human ovarian cancer cell line. This extract was thus selected for bioassay-guided fractionation based on its cytotoxicity and also on the absence of any previous detailed phytochemical studies on this species.
Previous phytochemical studies have revealed the genus of Terminalia to be a rich source of secondary metabolites, such as lignans,2 flavonoids,3 terpenoids,4 and tannins.5 Some of these metabolites have shown a wide range of biological activities, including anti-HIV-1,2 antimalarial,2 antifungal,2,4 antibacterial,4,6 and cytotoxic5 activities. The anthelminthic and haemolytic properties of the terpene esters from T. macroptera have been studied.4 The antioxidant effects of aqueous extract of T. chebula have also been investigated.7
Activity-guided fractionation of the dichloromethane extract (IC50, 10 µg/mL) by passage over a C18 open column, followed by purification of active fractions using C18 HPLC, led to the isolation of the two new compounds, 1 and 2.
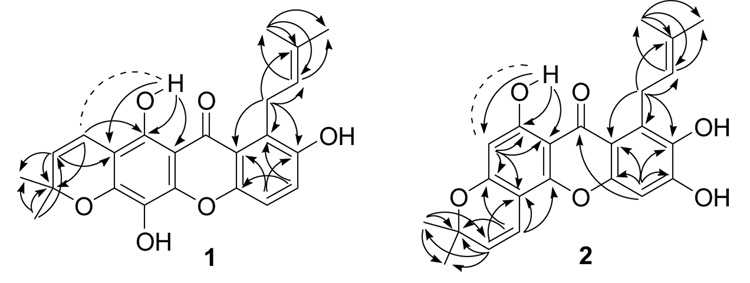
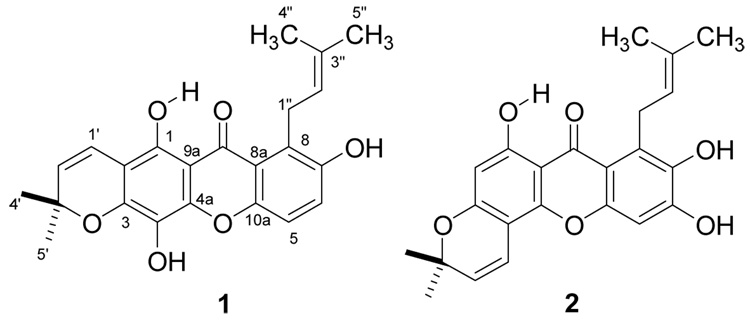
Termicalcicolanone A (1) was obtained as a yellow powder. Its positive HRFABMS revealed a pseudo-molecular ion [(M+H)+] consistent with the molecular formula C23H22O6. The IR spectrum for 1 revealed the existence of hydroxy (3435 cm−1) and conjugated ketone (1652 cm−1) functionalities. Its UV absorptions at 237, 262, 298, 401 nm suggested a xanthone skeleton as its base structure.8 The 1H NMR spectrum (pyridine-d5) of 1 revealed the presence of a hydrogen-bonded hydroxy group [δH 13.83 (1H, s)], two ortho-coupled protons [δH 7.51 (1H, d, J = 9.0 Hz), and 7.33 (1H, d, J = 9.0 Hz)], a dimethylchromene system [δH 7.07, 5.63 (1H each, d, J = 10.1 Hz), and 1.39 (6H, s)], and a 3-methyl-but-2-enyl group [δH 5.83 (1H, br t, J = 7.1 Hz), 4.68 (2H, d, J = 7.1 Hz), 2.04 (3H, s), and 1.75 (3H, s)] (Table 1). The 13C NMR spectrum (DMSO-d6) of 1 exhibited the 23 carbon signals reported in Table 1, including one carbonyl group, two aromatic rings with six oxygenated carbons, and two prenyl groups. The H-1″ resonance appeared at δH 4.68, which was a more deshielded value than that usually found for this functionality, due to the effect of the C-9 carbonyl group.9 Clearly, both the C-1 hydroxy and the C-8 3-methyl-but-2-enyl groups were peri to the carbonyl group. The ortho coupled aromatic H-5 and H-6 showed 3J HMBC correlations (Figure 1) to C-7 and C-8a, and C-8 and C-10a, respectively, while H-1″ correlated to C-7, C-8, C-8a, C-2″, and C-3″, which provided further evidence for the position of the 3-methyl-but-2-enyl group at C-8. The HMBC correlations between the hydrogen-bonded proton (1-OH) and C-1, C-2, and C-9a, H-1′ and C-1, and H-2′ and C-2 were also observed, indicating that the 3′,3′-dimethylpyrano ring was fused at C-2 and C-3, which was confirmed by a ROESY correlation between the hydrogen-bonded proton and H-1′. Based on the molecular formula of 1, the remaining two hydroxy groups must be located at C-4 and C-7, respectively. Thus, the structure of 1 was determined to be 5,8,12-trihydroxy-2,2-dimethyl-7-(3-methylbut-2-enyl)pyrano[3,2-b]xanthen-6(2H)-one.
Table 1.
1H and 13C NMR Data for Compounds 1 and 2a
| no | 1 | 2 | ||||
|---|---|---|---|---|---|---|
| 1Hb | 1Hc | 13Cb | 1Hb | 1Hc | 13Cb | |
| 1 | 149.1 | 162.5 | ||||
| 2 | 103.0 | 6.03 s | 6.51 s | 97.9 | ||
| 3 | 149.1 | 158.6 | ||||
| 4 | 144.6 | 99.7 | ||||
| 4a | 148.1 | 150.4 | ||||
| 5 | 7.35 br s | 7.33 d (9.0) | 115.1 | 6.59 s | 7.22 s | 99.9 |
| 6 | 7.35 br s | 7.51 d (9.0) | 123.4 | 155.3 | ||
| 7 | 151.5 | 142.0 | ||||
| 8 | 128.3 | 126.0 | ||||
| 8a | 117.8 | 108.6 | ||||
| 9 | 183.2 | 181.3 | ||||
| 9a | 103.3 | 102.9 | ||||
| 10a | 150.4 | 152.7 | ||||
| 1′ | 6.64 d (10.1) | 7.07 d (10.1) | 116.1 | 6.74 d (9.8) | 6.77 d (12.0) | 114.7 |
| 2′ | 5.76 d (10.1) | 5.63 d (10.1) | 127.0 | 5.67 d (9.8) | 5.52 d (12.0) | 127.2 |
| 3′ | 77.8 | 77.9 | ||||
| 4′ | 1.45 s | 1.39 | 27.7 | 1.41 s | 1.44 s | 27.8 |
| 5′ | 1.45 s | 1.39 | 27.7 | 1.41 s | 1.44 s | 27.8 |
| 1″ | 4.03 d (6.4) | 4.68 d (7.1) | 25.6 | 3.93 d (6.1) | 4.77 d (6.9) | 25.4 |
| 2″ | 5.18 br t (6.4) | 5.83 br t | 124.7 | 5.25 br t (6.1) | 5.92 br t (6.9) | 123.8 |
| (7.1) | ||||||
| 3″ | 130.3 | 130.0 | ||||
| 4″ | 1.78 s | 2.04 | 18.0 | 1.75 s | 2.08 s | 18.1 |
| 5″ | 1.61 s | 1.75 | 25.1 | 1.60 s | 1.76 s | 25.7 |
| 1-OH | 13.12 s | 13.83 s | 14.55 s | |||
δ (ppm), 500 MHz for 1H NMR and 125 MHz for 13C NMR; multiplicities; J values (Hz).
DMSO-d6
pyridine-d5
Figure 1.
Key HMBC (arrows) and ROESY (dashed) correlations of 1 and 2
Termicalcicolanone B (2) was also obtained as a yellow powder. It was deduced to have a molecular formula of C23H22O6 by HRFABMS and 13C NMR spectroscopy. A spectroscopic comparison of 2 and 1 indicated that both compounds had a hydrogen-bonded hydroxy group, a 3-methyl-but-2-enyl group, a 3′,3′-dimethylpyrano ring, and two aromatic protons. The hydrogen-bonded hydroxy and the 3-methyl-but-2-enyl groups of 2 were located at C-1 and C-8, respectively, by the HMBC correlations (Figure 1) between the hydrogen-bonded proton (1-OH) and C-1, C-2 and C-9a, and between H-1″ and C-7, C-8, and C-8a. The 3′,3′-dimethylpyrano ring of 2 was fused at C-3 and C-4, rather than at C-2 and C-3, as indicated by the HMBC correlations (Figure 1) between H-2 and C-3 and C-4, between H-1′ and C-3, C-4, and C-4a, and between H-2′ and C-4. In the HMBC spectrum, proton H-2 correlated to C-1, C-3, C-4, and C-9a, while H-5 correlated to C-6, C-7, C-8a, C-9, and C-10a. A ROESY correlation between the hydrogen-bonded proton and H-2 confirmed its location. Therefore, the structure of 2 was determined to be 6,9,10-trihydroxy-3,3-dimethyl-8-(3-methylbut-2-enyl)pyrano[2,3-c]xanthen-7(3H)-one.
Both compounds 1 and 2 were evaluated for antiproliferative activity in the A2780 assay, and had IC50 values of 40.6 and 8.1 µM respectively.
Experimental Section
General Experimental Procedures
IR and UV spectra were measured on a Spectrum One FT-IR Spectrometer (Perkin-Elmer Instruments) and a Shimadzu UV-1201 spectrophotometer, respectively. NMR spectra were obtained on a JEOL Eclipse 500 and a Varian INOVA 400 spectrometer in DMSO-d6 or pyridine-d5. The chemical shifts are given in δ (ppm), and coupling constants are reported in Hz. Mass spectra were obtained on a JEOL JMS-HX-110 instrument, in the positive ion mode. HPLC was performed on a Shimadzu LC-10AT instrument with a semi-preparative C18 Varian Dynamax column (5 µm, 250 × 10 mm) and a preparative C18 Varian Dynamax column (8 µm, 250 × 21.4 mm).
Antiproliferative Bioassays
Antiproliferative activities were obtained at Virginia Polytechnic Institute and State University against the drug - sensitive A2780 human ovarian cancer cell line.10 In brief, human A2780 ovarian cancer cells grown to 95% confluency were harvested and resuspended in growth medium (RPMI1640 supplemented with 10% fetal bovine serum and 2 mM L-glutamine). Cells were counted using a hemacytometer and a solution containing 2.7×105 cells per mL was prepared in growth media. Eleven columns of a 96 well microtiter plate were seeded with 180 µL of cell suspension per well, and the remaining column contained media only (one hundred percent inhibition control). The plate was incubated for 3 h at 37 °C/5%CO2 to allow the cells to adhere to the wells. Following this incubation, potential cytotoxic agents, prepared in 50/50 H2O/DMSO, were added to the wells in an appropriate series of concentrations, 20 µL per well. One column of wells was left with no inhibitor (zero percent inhibition control), and 4 dilutions of a known compound (paclitaxel or actinomycin D) were included as a positive control. The plate was incubated for 2 days at 37°C/5%CO2, then the media gently shaken from the wells and replaced with reaction media (supplemented growth medium containing 1% AlamarBlue), and incubated for another 3 h. The level of AlamarBlue converted to a fluorescent compound by living cells was then analyzed using a Cytofluor Series 4000 plate reader (Perseptive Biosystems) with an excitation wavelength of 530 nm, an emission wavelength of 590 nm, and gain of 45. The percent inhibition of cell growth was calculated using the zero percent and one hundred percent controls present on the plate, and an IC50 value (concentration of agent which produces 50% cell growth inhibition) was calculated using a linear extrapolation of the data which lay on either side of the 50% inhibition level. Samples were analyzed in triplicate on at least two separate occasions to produce a reliable IC50 value.
Plant Material
Samples of roots, bark, wood, leaves, and immature fruit of Terminalia calcicola H. Perrier (Combretaceae) were collected by Randrianaivo et al. (collection #1086) on September 8th, 2004 in the forest of Andranonakomba, Andavakoera, in the Montagne des Français, Province of Antsiranana Madagascar (12. 21. 00 S / 49. 21. 34E, elevation 112 m). The plant was a tree 12 m in height, diameter 40 cm, with green fruits. Duplicate voucher specimens were deposited at the Centre National d’Application des Recherches Pharmaceutiques (CNARP) and the Parc Botanique et Zoologique de Tsimbazaza (PBZT) herbarium (TAN) in Antananarivo Madagascar, at the Missouri Botanical Garden, St. Louis, Missouri (MO), and at the Muséeum National d’Histoires Naturelles (MNHN), Paris, France (P). The specimens were collected from a tree 12 m in height, with a diameter of 40 cm.
Extraction and Isolation
Dried leaves of T. calcicola (420 g) were ground in a hammer mill, then extracted with EtOH by percolation for 24 h at rt to give the crude extract MG 2581 (36.6 g), of which 7.8 g was shipped to Virginia Polytechnic Institute and State University (VPISU) for fractionation. Extract MG 2581 (IC50: 14 µg/mL, 96.2 mg) was suspended in aqueous MeOH (MeOH−H2O, 9:1, 10 mL) and extracted with hexane (3 × 10 mL portions). The aqueous layer was then diluted to 70% MeOH (v/v) with H2O and extracted with CH2Cl2 (3 × 10 mL portions). Both the hexane and the CH2Cl2 extracts were evaporated in vacuo to leave 43.9 mg and 35.9 mg of residues (IC50: 11 and 10 µg/mL, respectively). The aqueous MeOH extract (16.4 mg) was inactive. The CH2Cl2 extract was first fractionated using a SPE cartridge over C18, and five fractions were collected. Fractions I, II, III, IV, and V (19.1, 3.5, 6.1, 1.2, and 0.3 mg) had IC50 values of 14, 11, 6, 18, 17 µg/mL, respectively. Fraction III was separated by C-18 HPLC (80% MeOH−H2O), and compounds 1 (0.8 mg, tR 29 min) and 2 (0.8 mg, tR 41 min) were isolated.
Termicalcicolanone A (1)
yellow powder; IR (film) νmax 3435, 2975, 2955, 1652, 1614, 1580, 1485, 1380, 1258, 1139, 1061, 1026, 897, 819 cm−1; UV (MeOH) λmax (log ε) 237 (4.21), 262 (4.22), 298 (4.48), 330 (sh), 401 (3.45) nm; 1H NMR (500 MHz, DMSO-d6 and pyridine-d5) and 13C NMR (125 MHz, DMSO-d6) see Table 1; HRFABMS m/z 395.1504 (calcd for C23H23O6, 395.1495).
Termicalcicolanone B (2)
yellow powder; IR (film) νmax 3401, 3293, 2973, 2927, 1647, 1618, 1575, 1556, 1484, 1460, 1375, 1275, 1177, 1156, 1113, 833 cm−1; UV (MeOH) λmax (log ε) 262 (4.48), 287 (4.38), 321 (sh), 377 (3.83) nm; 1H NMR (500 MHz, DMSO-d6 and pyridine-d5) and 13C NMR (125 MHz, DMSO-d6) see Table 1; HRFABMS m/z 395.1488 (calcd for C23H23O6, 395.1495).
Acknowledgments
This International Cooperative Biodiversity Group project was supported by the Fogarty International Center, the National Cancer Institute, the National Science Foundation, the National Heart, Lung and Blood Institute, the National Institute of Mental Health, the Office of Dietary Supplements, and the Office of the Director of NIH, under Cooperative Agreement U01 TW000313 from the National Institutes of Health, and this support is gratefully acknowledged. We thank Mr. B. Bebout for obtaining the mass spectra and Mr. T. Glass for assistance with the NMR spectra. Field work essential for this project was conducted under a collaborative agreement between the Missouri Botanical Garden and the Parc Botanique et Zoologique de Tsimbazaza and a multilateral agreement between the ICBG partners, including the Centre National d’Applications des Recherches Pharmaceutiques. We gratefully acknowledge courtesies extended by the Government of Madagascar (Ministère des Eaux et Forêts).
References and Notes
- 1.Biodiversity Conservation and Drug Discovery in Madagascar, Part 26. For Part 25, see Yoder BJ, Cao S, Norris A, Miller JS, Ratovoson F, Razafitsalama J, Andriantsiferana R, Rasamison VE, Kingston DGI. J. Nat. Prod. 2007 doi: 10.1021/np060484y. in press (np060484y)
- 2.Valsaraj R, Pushpangadan P, Smitt UW, Adsersen A, Christensen SB, Sittie A, Nyman U, Nielsen C, Olsen CE. J. Nat. Prod. 1997;60:739–742. doi: 10.1021/np970010m. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava SK, Srivastava SD, Chouksey BK. Fitoterapia. 1999;70:390–394. [Google Scholar]
- 4.Conrad J, Vogler B, Klaiber I, Roos G, Walter U, Kraus W. Phytochemistry. 1998;48:647–650. [Google Scholar]
- 5.Kandil FE, Nassar MI. Phytochemistry. 1998;47:1567–1568. doi: 10.1016/s0031-9422(97)01078-9. [DOI] [PubMed] [Google Scholar]
- 6.Eldeen IMS, Elgorashi EE, Mulholland DA, Van Staden J. J. Ethnopharmacol. 2006;103:135–138. doi: 10.1016/j.jep.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Lee HS, Won NH, Kim KH, Lee H, Jun W, Lee K-W. Biol. Pharm. Bull. 2005;28:1639–1644. doi: 10.1248/bpb.28.1639. [DOI] [PubMed] [Google Scholar]
- 8.Scott AI. Interpretation of the Ultraviolet Spectra of Natural Products. Oxford: Pergamon; 1964. p. 158. [Google Scholar]
- 9.Zou YS, Hou AJ, Zhu GF, Chen YF, Sun HD, Zhao QS. Bioorg. Med. Chem. 2004;12:1947–1953. doi: 10.1016/j.bmc.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Louie KG, Behrens BC, Kinsella TJ, Hamilton TC, Grotzinger KR, McKoy WM, Winker MA, Ozols RF. Cancer Res. 1985;45:2110–2115. [PubMed] [Google Scholar]