Abstract
The ErbB2/3 heterodimer plays a critical role in breast cancer genesis and progression. EBP1, an ErbB3 binding protein, inhibits breast cancer growth but its effects on ErbB3 ligand mediated signal transduction or ErbB receptors is not known. We report here that ectopic expression of EBP1 in MCF-7 and AU565 breast cancer cell lines inhibited HRG induced proliferation. ErbB2 protein levels were substantially decreased in EBP1 transfectants, while ErbB3 levels were unchanged. HRG-induced AKT activation was attenuated in EBP1 stable transfectants and transfection of a constitutively activated AKT partially restored the growth response to HRG. Down-regulation of EBP1 expression in MCF7 cells by shRNA resulted in increased cell growth in response to HRG and increased Cyclin D1 and ErbB2 expression. These results suggest that EBP1, by down regulating ErbB signal transduction, attentuates HRG-mediated growth of breast cancer cells.
Keywords: EBP1, Heregulin, breast cancer, ErbB2
INTRODUCTION
A wealth of clinical data has demonstrated the aberrant expression of ErbB family members in breast cancer [1] [2]. The ErbB2 gene is amplified in 20–30% of breast carcinomas contributing to more aggressive disease [3]. The overexpression of ErbB2 has been successfully exploited therapeutically by use of the monoclonal antibody Trastuzumab and by tyrosine kinase inhibitors. ErbB3 is also overexpressed in many breast tumors [4]. Coexpression of ErbB2 and ErbB3 is significantly associated with decreased survival [5]. The ErbB2/ErbB3 receptor pair forms the most potent mitogenic receptor complex in vitro [6] and is key to the proliferation of human breast cancer cells that express these receptors [7].
Our laboratory has been interested in the role of ErbB3 as a regulator of growth and differentiation of human breast cancer cells. The ErbB3 receptor has impaired tyrosine kinase activity [8;9], necessitating its interactions with other proteins to exert its biological effects. Several proteins interact with ErbB3 to transduce its biological effects. For example, ErbB2 heterodimerizes with ErbB3 after HRG stimulation, leading to phosphorylation and activation of downstream substrates [10]. The RING finger E3 ubiquitin ligase neuregulin receptor degradation protein-1 (Nrdp1) associates with ErbB3 in an activation independent manner and is believed to be involved in ErbB3 trafficking or localization [11] [12]. Another ErbB3 binding protein (EBP1) was isolated in our laboratory during a yeast two-hybrid screen for ErbB3 interacting proteins [13]. Overexpression of EBP1 inhibits growth of both Estrogen Receptor (ER)positive and negative, ErbB2/3 expressing cell lines such as AU565, MCF-7, SKBR3 and MDA-MB-453 cells lines. EBP1 does not inhibit the growth of the MDA-MB-468 cell line which does not express ErbB2. In AU565 cells, ectopic expression of EBP1 promotes G2/M cell cycle arrest and cellular differentiation [14]. Overexpression of EBP1 inhibits the transcription of reporter genes controlled by Cyclin D1 Cyclin E and c-myc promoters and the transcription of endogenous E2F1 and c-myc genes via its binding to an E2F1 consensus element [15–17]. The interactions of EBP1 with histone deacetylase 2 (HDAC2), Rb and Sin3A are necessary for its ability to repress transcription[16;17] [18]. HRG increases binding of EBP1 to the E2F1 promoter complex and enhances EBP1-mediated repression of E2F1 regulated gene transcription [17]. Recent data also suggest that EBP1 is an RNA binding protein [19–21], that can affect protein translation.
While our previous work demonstrated that ectopic expression of EBP1 inhibits growth of human breast cancer cell lines, the effect of EBP1 on HRG induced signaling and proliferation was not examined. We hypothesized that EBP1 may specifically interfere with HRG induced growth signals. In this study, we determined that ectopic expression of EBP1 inhibited the HRG induced growth of MCF-7 and AU565 breast cancer cells. A decrease in AKT activation after HRG treatment was observed in EBP1 MCF-7 transfectants.
MATERIALS AND METHODS
Cell Culture
MCF-7 cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained at 37°C in a humidified atmosphere of 5% CO2 in air in RPMI 1640 (Biofluids, Rockville, MD) and 10% FBS (Sigma, St. Louis, MO).
Reagents
Heregulin β1 (HRGβ1) was obtained from R & D Systems Inc. (Minneapolis, MN), EGF from Sigma and Geneticin (G418) from Invitrogen (San Jose, CA).
Plasmids
A full-length EBP1 cDNA(GenBank NM006191) was generated by PCR with specific reverse and forward primers containing EcoRI and BamHII restriction sites using a pcDNA-EBP1 vector as a template (Xia et al, 2001). This cDNA includes all 3 possible translation initiation sites of EBP1 and encodes the largest form of the protein. The cDNA was subcloned into the BamHI-EcoR1 sites of the CMV10 vector (Sigma) which contains a 3×Flag epitope tag. The orientation and integrity of the cDNA insert was confirmed by automated DNA sequencing in the core laboratory of the University of Maryland School of Medicine using sequencing primers for the CMV10 plasmid (Sigma). A myristylated constitutively activated AKT plasmid cloned into a CMV 6 expression vector has been previously described [22].
Creation of stably transfected cell lines
To establish EBP1 overexpressing stable transfectants, subconfluent MCF-7 cells in 100-mm tissue culture dishes were transfected with 10µg of CMV-10, or CMV10- EBP1 expression plasmids using Fugene-6 according to the manufacturer's protocol. Cells were selected in G418 (500 µg/ml) for 5 weeks and mass cultures obtained. The AU565 epb1 cell line has been previously described [14].
For creation of EBP1 silenced MCF-7 cell lines, sh RNA targeted against the coding region beginning at nucleotide 476 (Genbank accession number U87954) (AAGCGACCAGGAUUAUAUUCU) was cloned into the pRNAT-U6.1 lentiviral vector (GenScript Corp, Scotch Plains, NJ). A synthetic oligo encoding this sequence was previously demonstrated to decrease EBP1 expression in prostate cancer cell lines [23]. Lentiviral particles were prepared using the Invtirogen ViraPower ™ system in 293FT cells as described by the manufacturer. MCF-7 cells were transduced with lentiviral stock and polybrene (6 µg/ml) and mass cultures were selected in G418 (500 µg/ml).
Western blot Analysis
Total cell extracts were prepared by direct lysis of cells with lysis buffer [50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 250 mM NaCl, 1% Triton X-100, 0.5 mM DTT, and 1x Complete™ protease inhibitor]. Protein concentrations were measured using a detergent compatible kit (Bio-Rad). Proteins (30 µg per well) were resolved by SDS-PAGE, transferred to PVDF membranes, and immunoblotted as described [13]. The ErbB3 (C-17) antibody was from Santa Cruz (Santa Cruz, CA), the ErbB2 antibody from Calbiochem (La Jolla, CA), the EBP1 antibody from Upstate (Lake Placid, NY), the FLAG M2 and rabbit anti -actin antibodies from Sigma, the phospho AKT (Ser 473), amd total AKT, antibodies were from Cell Signaling (Beverly, MA) and the p-MAPK and MAPK antibodies were from Promega (Madison, WI)‥ Images were quantified using IMAGE-J software (NIH). Where indicated, blots were stripped in Restore Western blot Stripping Buffer™ (Pierce) as directed by the manufacturer and reprobed.
Cell Growth Assays
For studies assessing the effect of HRG or EGF on cell growth, cells (5× 103) were plated in 96 well plates in complete media. After a 24-hour attachment period, the medium was replaced with phenol-red free RPMI 1640 with 2% FBS and HRGβ1 or EGF at the indicated concentrations. Relative cell numbers were determined four days later using a Promega Proliferation Reagent as per manufacturer’s instructions with absorbance being read at 490nm using a Dynex plate reader.
Statistical Analysis
Data were analyzed using a two-tailed Students t-test and a p < 0.05 was deemed statistically significant.
RESULTS
EBP1 inhibits HRG-induced growth
As ErbB3 plays a key role in the proliferation of breast cancer cells, we sought to determine the effect of EBP1, which binds ErbB3, on cell proliferation induced by HRG, an ErbB3 ligand. An MCF-7 cell line stably transfected with FLAG-EBP1 was created (Fig. 1A, left panel). EBP1 expression was increased 2.1fold (Fig. 1A, right panel), as determined by densitometric tracing, in keeping with previous data indicating that high expression of EBP1 is incompatible with cell growth [14] [24]. MCF-7 vector control or EBP1 transfectants were plated in complete media and then switched to media containing 2% FBS in the presence or absence of increasing concentrations of HRG. HRG significantly (p<0.05) enhanced the growth of MCF-7 vector control cells with a maximal increase of 42% at 10 ng/ml as expected [25]. In contrast, the growth of cells transfected with EBP1 was unchanged by HRG at any of the concentrations tested (Fig. 1B). To test if the inhibition of HRG signaling occurred in other breast cancer cell lines, we tested the ability of AU565 cells stably transfected with EBP1 [14] to respond to HRG. EBP1 expression is increased two fold in this cell line [14]. AU565 vector control or EBP1 transfectants were plated in complete media and then switched to media containing 2% FBS in the presence or absence of increasing concentrations of HRG. HRG enhanced the growth of AU565 vector control cells with a maximal increase at 10 ng/ ml. Growth at higher concentrations was inhibited by HRG as previously reported [26]. In contrast, the growth of cells transfected with EBP1 was not significantly (p < 0.05) changed by HRG at any of the concentrations tested (Fig. 1C).
Fig. 1. Ectopic expression of EBP1 blocks HRG-induced proliferation.
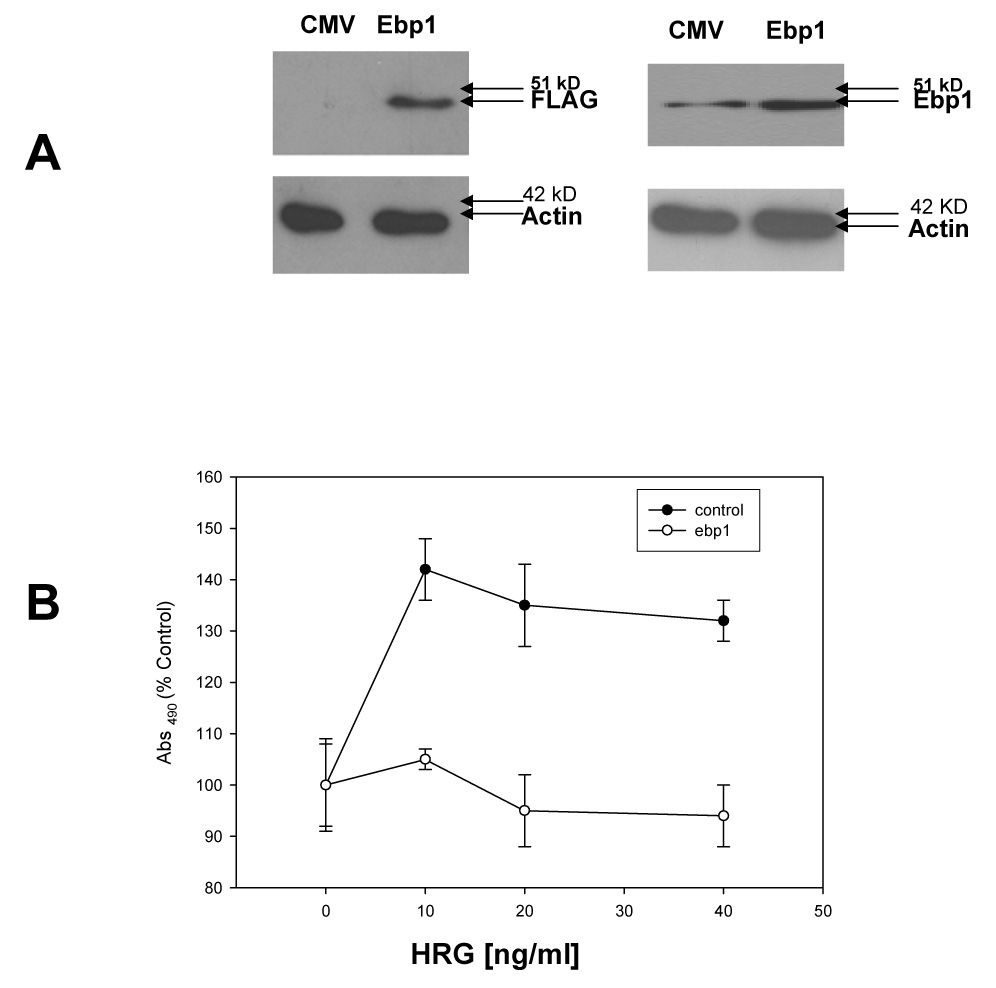
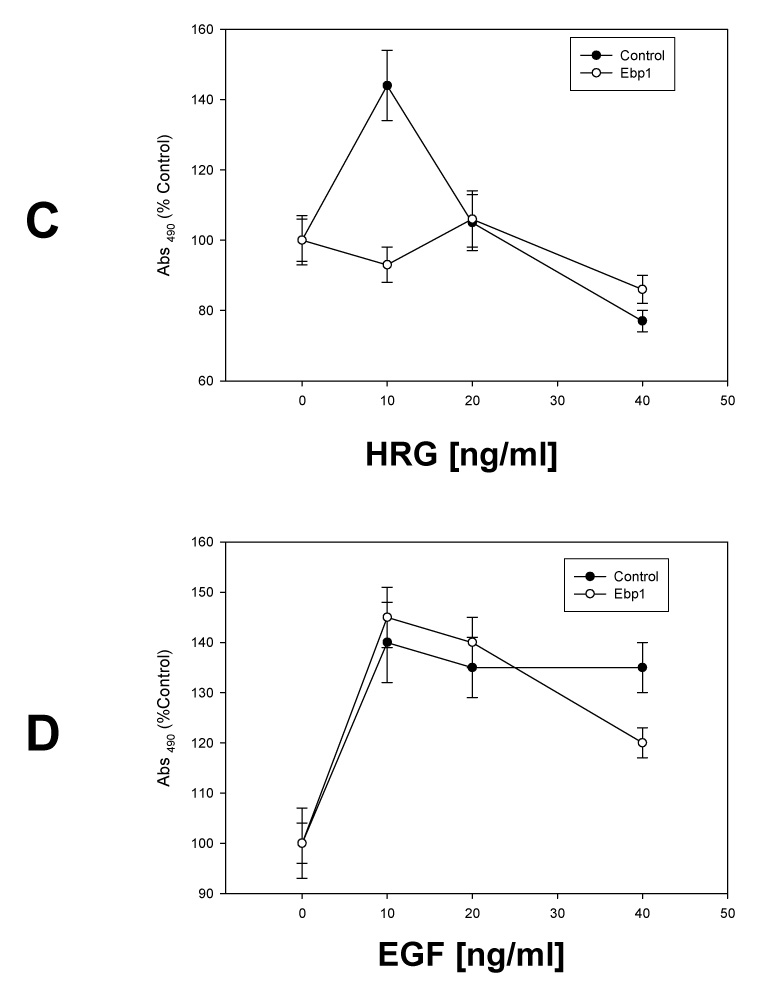
A) MCF-7 cells were stably transfected with a control Flag vector (CMV) or a FLAG-EBP1 vector (EBP1). Cells lysates were analyzed by Western blotting for FLAG and actin levels as indicated (left panel) or EBP1 and actin (right panel). B) MCF-7 vector control (Control) or EBP1 transfected cells (EBP1) were plated in 96 well plates in complete media overnight, and then switched to media with 2% FBS and the indicated concentrations of HRGβ1 as described in the Materials and Methods. Growth was determined 4 days later using a Promega Proliferation Assay. Each point represents the mean±S.D. for 6 wells. Data shown are representative of 3 experiments. C) AU565 vector control (Control) or EBP1 transfected cells (EBP1) were plated in 96 well plates in complete media overnight, and then switched to media with 2% FBS and the indicated concentrations of HRGβ1. Growth was determined 4 days later using a Promega Proliferation Assay. Each point represents the mean±S.D. for 6 wells and is representative of 2 experiments. D) MCF-7 vector control (Control) or EBP1 transfected cells (EBP1) were plated in 96 well plates in complete media overnight, and then switched to media with 2% FBS and the indicated concentrations of EGF. Growth was determined 4 days later using a Promega Proliferation Assay. Each point represents the mean ±S.D. for 6 wells and is representative of 2 experiments.
To determine if the inhibition of cell growth was specific for the HRG signaling pathway, we tested the ability of EBP1 to inhibit the growth response to EGF. MCF-7 vector control and EBP1 transfected cells were stimulated with EGF at increasing concentrations. MCF-7 control cells were able to respond to EGF as previously reported [27]. In contrast to the response to HRG, MCF-7 EBP1 transfected cells were growth stimulated by EGF to the same extent as control cells (Fig.1D).
To examine the mechanism underlying the EBP1 inhibition of HRG induced growth, we evaluated the effect of EBP1 expression on the protein levels of ErbB2 and ErbB3. There was no change in the level of ErbB3 (Fig. 2A) in the MCF-7-EBP1 transfectants when compared to vector controls. In contrast, ErbB2 protein was significantly decreased (Fig.2B). Similar results were observed in another group of independently derived MCF-EBP1 transfected cells (data not shown). ErbB2 protein was also decreased by 57% in AU565 cells (Fig. 2C).
Fig. 2. Effect of ectopic expression of EBP1 on ErbB3 or ErbB2 protein levels.
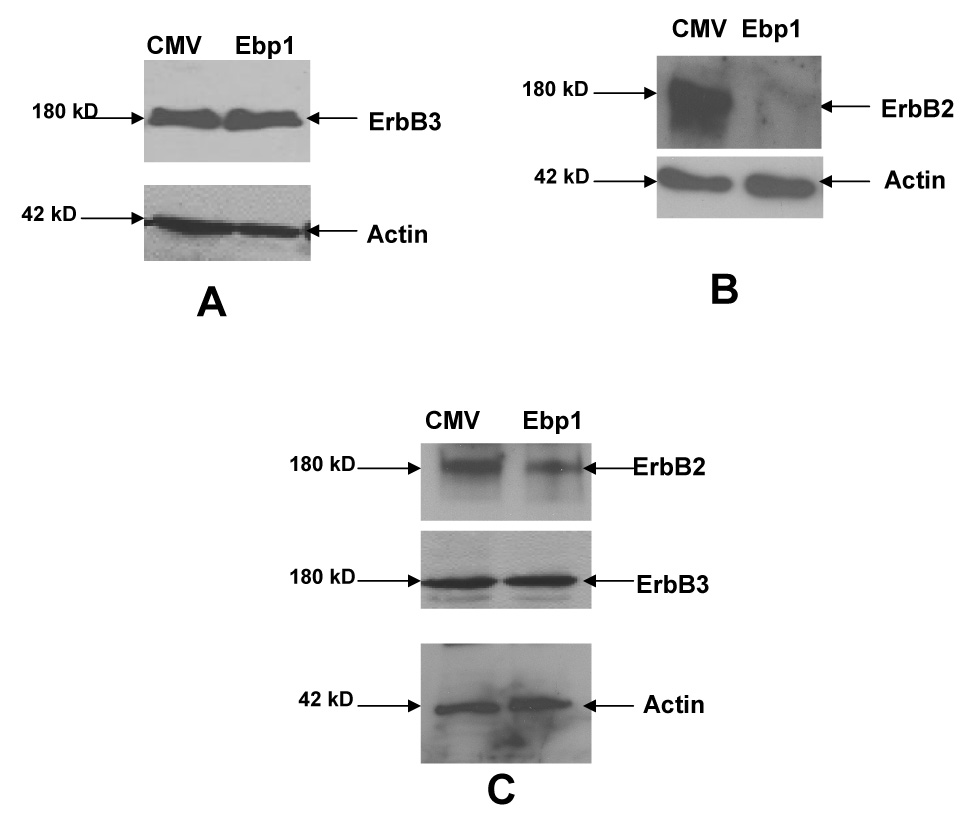
A,B) Lysates of logarithmically growing MCF-7 vector control cells (CMV) or EBP1 transfected cells (Ebp1) or C) AU565 cells were blotted with antibodies for ErbB3 or ErbB2 and actin as indicated. Data are representative of 3 experiments.
We next evaluated the effect of EBP1 overexpression on the activation of downstream signaling in response to HRG. HRG- induced activation of the ErbB2/ErbB3 heterodimer results in stimulation of the MEK/MAPK pathway [7]. HRG treatment caused a robust activation of MAPK in vector controls as determined using a phospho-specific MAPK antibody. In contrast, as previously reported for AU565 cells [14], MAPK was constitutively phosphorylated in EBP1 overexpressing cells. A small increase in MAPK phosphorylation was observed after HRG treatment in EBP1 transfectants (Fig.3A). HRG induced AKT phosphorylation in vector control MCF-7 cells at both 10 and 20 min after treatment as determined using a phospho specific AKT antibody. However, this response was attenuated in the EBP1 transfectants as compared to the vector controls (Fig.3B).
Fig. 3. Ebp1 affects HRG induced downstream signaling pathways.
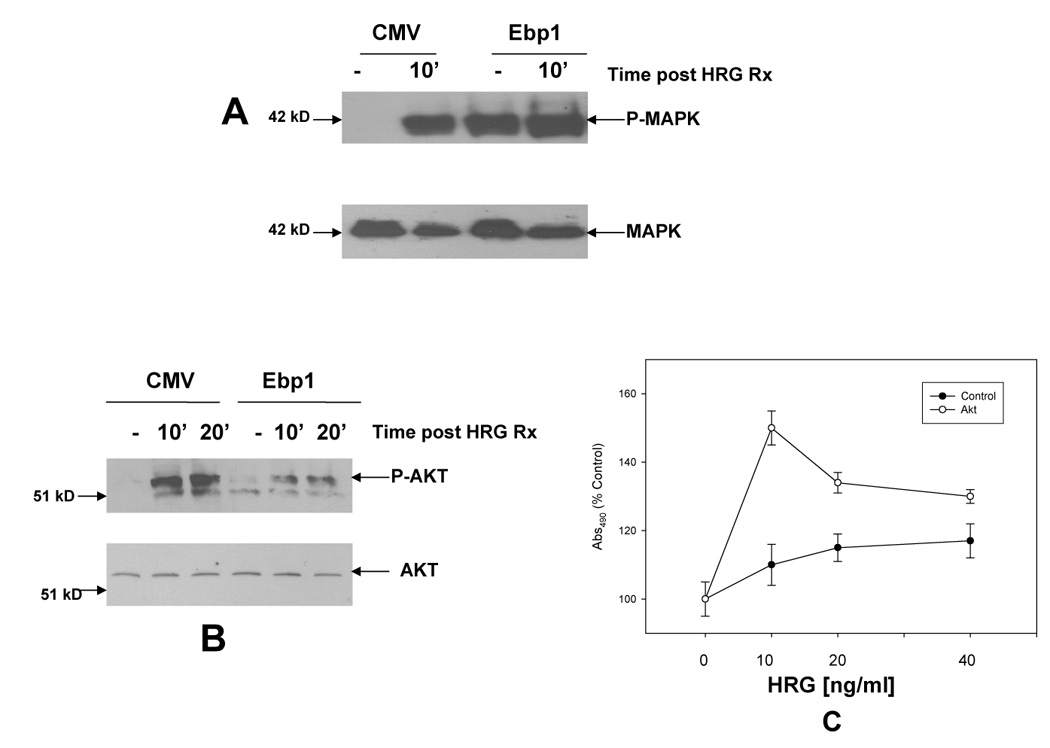
Serum starved MCF7 vector control (CMV) or EBP1 transfected cells (Ebp1) cells were stimulated with HRG (20 ng/ml) for the indicated times. Cells lysates were blotted with antibodies to A) phospho MAPK and total MAPK or B) phospho-Akt or total AKT. Data are representative of 3 experiments. C) MCF-7 ebp1 stably transfected cells were transiently transfected with a constitutively activated AKT in complete media. Eight hours after transfection, cells were refed with media with 2% serum and the indicated concentrations of HRG. Growth was assessed by MTT assay 3 days later. Data are representative of 2 experiments.
We next examined the role of the attenuation of AKT phosphorylation in the failure of MCF-7 EBP1 transfectants to grow in response to HRG. Transient transfection of a constitutively activated AKT resulted in a partial rescue of the HRG stimulated growth of EBP1 overexpressing cells (p<0.05) (Fig.3C).
ShRNA against EBP1 augments HRG induced cell proliferation
The contribution of EBP1 to HRG induced cell growth was evaluated by knockdown of endogenous EBP1 expression. As shown in Fig. 4A, transduction of MCF-7 cells with an shRNA vector targeted to EBP1 reduced EBP1 expression compared to a lentiviral control. The EBP1 knockdown cells grew about 30% faster than the vector controls in complete media (data not shown). To test the effects of ectopic expression of EBP1 on HRG-nduced cell growth, cells were plated in complete media and then switched to media containing 2% FBS and 10 ng/ml of HRG. Growth was assessed four days later. HRG increased the growth of vector controls 40% similar to parental MCF-7 cells [25]. In contrast, HRG increased the growth of EBP1 knock down cells 250% (Fig.4B).
Fig. 4. EBP1 shRNA promotes HRG stimulated cell growth.
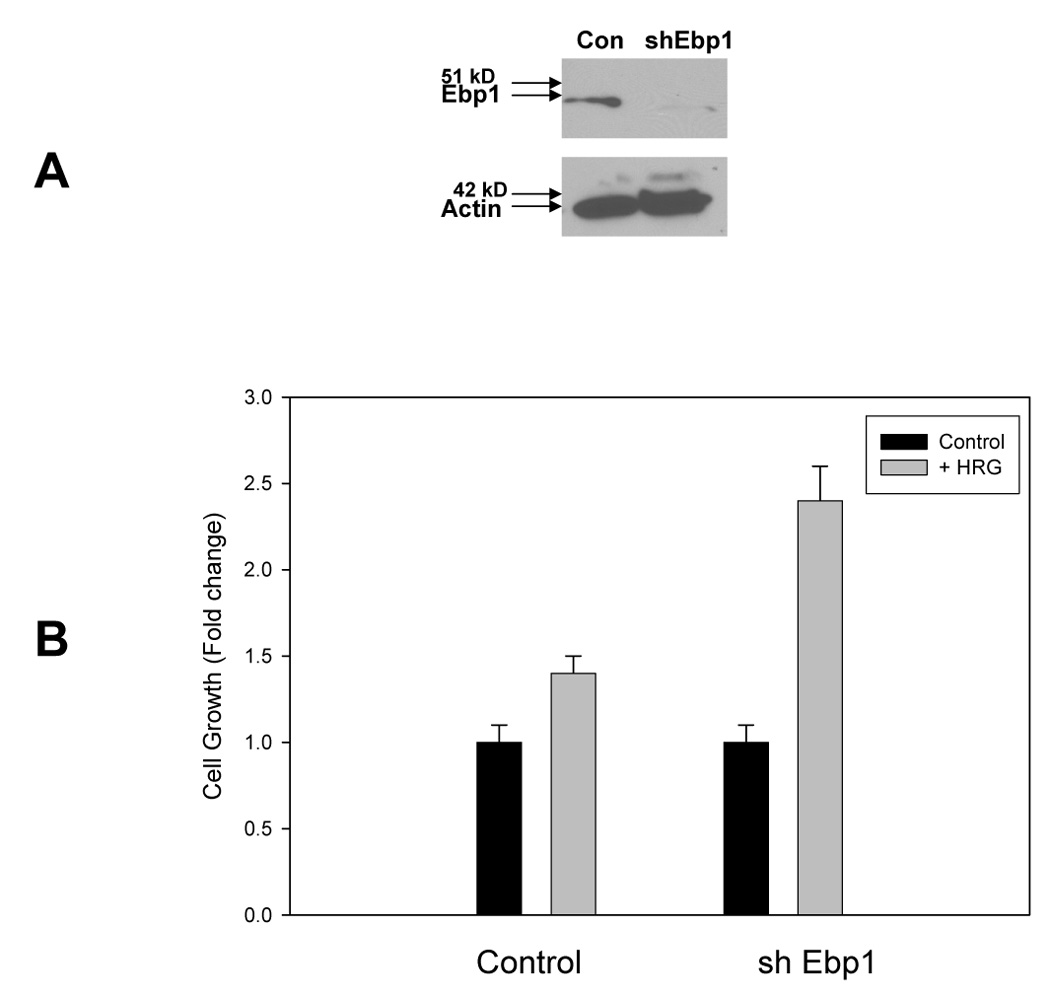
A) Lysates of MCF-7 cells stably transduced with a control lentivirus (Con) or an EBP1 targeted shRNA (sh Ebp1) were collected and resolved by SDS-PAGE. Western blots were probed for endogenous EBP1 or actin as indicated. B) MCF-7 cells transduced with a control lentivirus or a lentivirus with shRNA targeted to EBP1 were plated in 2% FBS and 10 ng/ml HRG as described in the Materials and Methods. Cell proliferation was measured 4 days later by a Promega Proliferation assay. Each bar represents the mean of 6 wells ± S.D. Data are representative of 3 experiments.
We further examined downstream effectors of HRG signaling. AKT was constitutively activated in the EBP1 knock down cells and HRG did not further enhance the phosphorylation (Fig.5A). As cyclin D1 is stimulated in HRG treated cells [28], we examined the protein levels of cyclin D1 in logarithmically growing control and EBP1 knockdown cells. Cyclin D1 levels were increased in cells in which EBP1 expression had been decreased as compared to lentivirus controls. As we had found that ErbB2 was decreased in EBP1 transfectants, we examined the levels of ErbB2 in EBP1 knock down cells. EBP1 knock down led to a 2.2 fold upregulation of ErbB2 expression when compared to actin controls (Fig. 5C).
Fig. 5. Changes in downstream signaling pathways in EBP1 knock down cells.
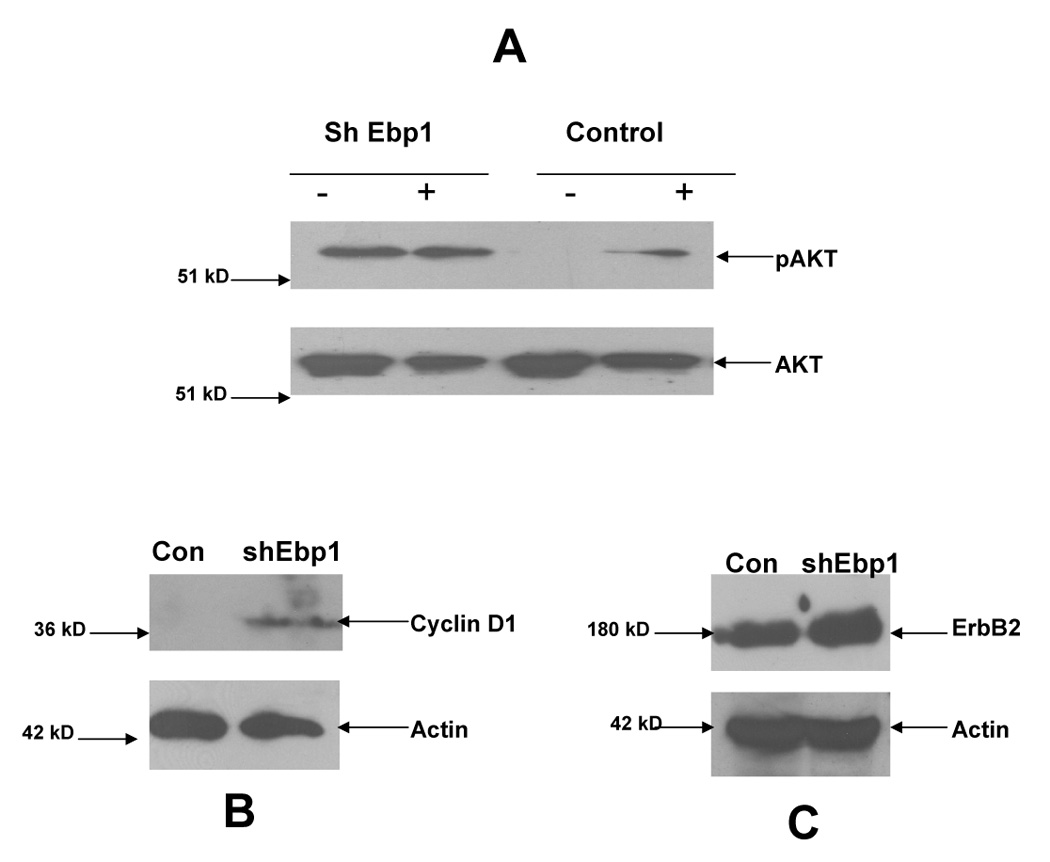
A) MCF-7 cells transduced with a lentivirus with shRNA targeted to EBP1 (sh Ebp1) or a control lentivirus (Con) were serum starved (−) and then stimulated with HRG (20 ng/ml) for 10 min (+). Cells lysates were blotted with antibodies to phospho-Akt or total AKT as indicated. B,C) Lysates of logarithmically growing MCF-7 cells transduced with a control lentivirus (Con) or a lentivirus targeted to EBP1 (shEbp1) were analyzed by Western blotting for Cyclin D or actin or ErbB2 and actin as indicated. Data are representative of 2 experiments.
DISCUSSION
Increasing data support the clinical importance of specific ErbB heterodimers and their interacting partners in breast cancer development [2]. We have previously shown that the ErbB3 binding protein EBP1 inhibited the growth of ErbB2/ ErbB3 expressing breast cancer cell lines [14]. However, the specific effect of EBP1 on HRG induced proliferation and down stream signaling was not assessed. We hypothesized that EBP1 negatively regulates HRG signaling, resulting in growth inhibition of breast cancer cells. Studies presented here show that ectopic expression of EBP1 diminished HRG induced cell proliferation in ErbB2/3 expressing AU565 and MCF-7 cells, while inhibition of EBP1 protein expression resulted in increased proliferation of MCF-7 cells in response to HRG. These data, along with our previously published work [14], demonstrate that EBP1 inhbits proliferation of cells that expresss both ErbB2 and ErbB3, regardless of ER status. Decreased cell growth was associated with a decrease in ErbB2 protein levels and attenuation of HRG-induced signaling. Our data suggest that EBP1 may function as a negative regulator of ErbB 2/3 heterodimer signaling.
Ectopic expression of EBP1 specifically decreased HRG induced stimulation of cell growth, as the ability of EGF to stimulate cell growth was not affected by EBP1 overexpression. To further examine the cause of this growth inhibition, we determined the levels of ErbB2 and ErbB3. The level of ErbB3 protein in EBP1 transfectants was unchanged from that of the vector control. This finding is in contrast to work of Jhabvala-Romera et al [29] and Yen et al [30] who showed that the ErbB modifying protein Ndrp1 induce decreases in the proteins levels of ErbB3. Surprisingly, levels of ErbB2 were greatly decreased in EBP1 overexpressing cells. The mechanism behind the decrease is unknown. It is possible that the binding of EBP1 to ErbB3 may inhibit ErbB2/3 heterodimers and destabilize ErbB2. In addition, EBP1 is an RNA binding protein[19] [21] that may either destabilize ErbB2 mRNA or decrease its translation. ErbB4 was undetectable in control cells in our hands, and EGFR levels were unaffected (data not shown).
We examined the role that MAPK and AKT signaling might play in the inability of EBP1 transfectants to respond to HRG. As previously demonstrated in AU565 cells [14], ectopic expression of EBP1 resulted in constitutive MAPK phosphorylation. Sustained activation of MAPK is a marker of HRG induced differentiation and inhibition of cell growth and this sustained MAPK activation may have contributed to the failure of EBP1 transfectants to grow in response to HRG. Pharmacological inhibition of MEK resulted in the inability of both EBP1 transfected and control cells to respond to HRG (data not shown). Thus, we were unable to directly test if the constitutive activation of MAPK was responsible for the inability of EBP1 transfectants to grow in response to HRG. As activation of AKT is a mediator of the growth stimulating effects of HRG in breast cancer cells [7;31], we also examined the ability of HRG to stimulate AKT phosphorylation. We found that the phosphorylation of AKT in response to HRG was decreased in EBP1 transfectants as compared to control cells. Thus, the reduced growth response to HRG in MCF-7 EBP1 transfectants may have been due in part to this attenuation of AKT signaling. Indeed, transfection of a constitutively activated AKT partially reversed the inhibitory effects of EBP1 on HRG induced cell growth. The enhanced response to HRG in EBP1 knock out cells, in which AKT was constitutively activated, support the hypothesis that inhibition of AKT activation by endogenous EBP1 may contribute to the normal attenuation of ErbB signal transduction.
A second mechanism by which EBP1 may inhibit HRG induced growth may be related to the ability of EBP1 to inhibit transcription of Cyclin D1 and Cyclin E genes [15;16]. Cyclin D1 and E are key regulators of breast cancer cell proliferation [32;33]. HRG- induced cell proliferation is mediated in part by increases in cyclin D1 levels[28]. EBP1 represses the activity of a cyclin D1 promoter luciferase reporter construct [15;16]. We have also demonstrated by ChIP analysis that EBP1 is recruited to the E2F1 promoter in response to HRG, where it can bind the transcriptional corepressors HDAC2 and Sin3A [18]. In addition, the ability of EBP1 to repress transcription is enhanced by HRG [17]. Thus, overexpression of EBP1 may result in recruitment of EBP1 to the Cyclin D1 promoter, inhibiting transcription and ultimately cell growth. It is possible that endogenous EBP1, whose transcriptional repression activity is regulated by HRG, may serve as a negative feed back mechanism to prevent unrestrained HRG signaling.
In summary, we demonstrate here that the ectopic expression of EBP1 in MCF-7 and AU565 cells resulted in the blocking of HRG mediated proliferation. In MCF_7 cells, this was accompanied by a decrease in AKT activation. Conversely, inhibiting EBP1 expression resulted in enhanced proliferation in response to HRG. These studies suggest that EBP1 may be a regulator of HRG signaling in ErbB2/3 expressing breast cancer cells and serve as a potential target for the development of anticancer agents.
Acknowledgments
This work was supported by NIH grants R01 CA76047 and R21 088882-01 and a grant from the Department of Pathology, University of Maryland School of Medicine (to AWH).
We thank Dr. Yun Qiu, Department of Pharmacology University of Maryland School of Medicine, for the AKT plasmid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holbro T, Civenni G, Hynes NE. The ErbB receptors and their role in cancer progression. Exp.Cell Res. 2003;284:99–110. doi: 10.1016/s0014-4827(02)00099-x. [DOI] [PubMed] [Google Scholar]
- 2.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat.Rev.Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 4.Lemoine NR, Barnes DM, Hollywood DP, Hughes CM, Smith P, Dublin E, Prigent SA, Gullick WJ, Hurst HC. Expression of the ERBB3 gene product in breast cancer. Br.J.Cancer. 1992;66:1116–1121. doi: 10.1038/bjc.1992.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiseman SM, Makretsov N, Nielsen TO, Gilks B, Yorida E, Cheang M, Turbin D, Gelmon K, Huntsman DG. Coexpression of the type 1 growth factor receptor family members HER-1, HER-2, and HER-3 has a synergistic negative prognostic effect on breast carcinoma survival. Cancer. 2005;103:1770–1777. doi: 10.1002/cncr.20970. [DOI] [PubMed] [Google Scholar]
- 6.Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Ratzkin BJ, Sela M, Yarden Y. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- 7.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, III, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc.Natl.Acad.Sci.U.S.A. 2003;100:8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraus MH, Issing W, Miki T, Popescu NC, Aaronson SA. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proc.Natl.Acad.Sci.U.S.A. 1989;86:9193–9197. doi: 10.1073/pnas.86.23.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plowman GD, Whitney GS, Neubauer MG, Green JM, McDonald VL, Todaro GJ, Shoyab M. Molecular cloning and expression of an additional epidermal growth factor receptor-related gene. Proc.Natl.Acad.Sci.U.S.A. 1990;87:4905–4909. doi: 10.1073/pnas.87.13.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinkas-Kramarski R, Shelly M, Glathe S, Ratzkin BJ, Yarden Y. Neu differentiation factor/neuregulin isoforms activate distinct receptor combinations. J.Biol.Chem. 1996;271:19029–19032. doi: 10.1074/jbc.271.32.19029. [DOI] [PubMed] [Google Scholar]
- 11.Diamonti AJ, Guy PM, Ivanof C, Wong K, Sweeney C, Carraway KL., III An RBCC protein implicated in maintenance of steady-state neuregulin receptor levels. Proc.Natl.Acad.Sci.U.S.A. 2002;99:2866–2871. doi: 10.1073/pnas.052709799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu XB, Goldberg AL. Nrdp1/FLRF is a ubiquitin ligase promoting ubiquitination and degradation of the epidermal growth factor receptor family member, ErbB3. Proc.Natl.Acad.Sci.U.S.A. 2002;99:14843–14848. doi: 10.1073/pnas.232580999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo JY, Wang XW, Rishi AK, Lessor T, Xia XM, Gustafson TA, Hamburger AW. Interaction of the PA2G4 (EBP1) protein with ErbB-3 and regulation of this binding by heregulin. Br.J.Cancer. 2000;82:683–690. doi: 10.1054/bjoc.1999.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lessor TJ, Yoo JY, Xia X, Woodford N, Hamburger AW. Ectopic expression of the ErbB-3 binding protein EBP1 inhibits growth and induces differentiation of human breast cancer cell lines. J.Cell Physiol. 2000;183:321–329. doi: 10.1002/(SICI)1097-4652(200006)183:3<321::AID-JCP4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 15.Xia X, Cheng A, Lessor T, Zhang Y, Hamburger AW. EBP1, an ErbB-3 binding protein, interacts with Rb and affects Rb transcriptional regulation. J. Cell Physiol. 2001;187:209–217. doi: 10.1002/jcp.1075. [DOI] [PubMed] [Google Scholar]
- 16.Zhang YX, Woodford N, Xia XM, Hamburger AW. Repression of E2F1-mediated transcription by the ErbB3 binding protein EBP1 involves histone deacetylases. Nucleic Acids Research. 2003;31:2168–2177. doi: 10.1093/nar/gkg318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Hamburger AW. Heregulin regulates the ability of the ErbB3-binding protein EBP1 to bind E2F promoter elements and repress E2F-mediated transcription. J.Biol.Chem. 2004;279:26126–26133. doi: 10.1074/jbc.M314305200. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Akinmade D, Hamburger AW. The ErbB3 binding protein EBP1 interacts with Sin3A to repress E2F1 and AR-mediated transcription. Nucleic Acids Res. 2005;33:6024–6033. doi: 10.1093/nar/gki903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bose SK, Sengupta TK, Bandyopadhyay S, Spicer EK. Identification of EBP1 as a component of cytoplasmic bcl-2 mRNP (messenger ribonucleoprotein particle) complexes. Biochem.J. 2006;396:99–107. doi: 10.1042/BJ20051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Squatrito M, Mancino M, Donzelli M, Areces LB, Draetta GF. EBP1 is a nucleolar growth-regulating protein that is part of pre-ribosomal ribonucleoprotein complexes. Oncogene. 2004;23:4454–4465. doi: 10.1038/sj.onc.1207579. [DOI] [PubMed] [Google Scholar]
- 21.Squatrito M, Mancino M, Sala L, Draetta GF. EBP1 is a dsRNA-binding protein associated with ribosomes that modulates eIF2alpha phosphorylation. Biochem.Biophys.Res.Commun. 2006;344:859–868. doi: 10.1016/j.bbrc.2006.03.205. [DOI] [PubMed] [Google Scholar]
- 22.Chen R, Kim O, Yang J, Sato K, Eisenmann KM, McCarthy J, Chen H, Qiu Y. Regulation of Akt/PKB activation by tyrosine phosphorylation. J.Biol.Chem. 2001;276:31858–31862. doi: 10.1074/jbc.C100271200. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Hamburger AW. Specificity and heregulin regulation of EBP1 (ErbB3 binding protein 1) mediated repression of androgen receptor signalling. Br.J.Cancer. 2005;92:140–146. doi: 10.1038/sj.bjc.6602257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang YX, Fondell JD, Wang QB, Xia XM, Cheng AW, Lu ML, Hamburger AW. Repression of androgen receptor mediated transcription by the ErbB-3 binding protein, EBP1. Oncogene. 2002;21:5609–5618. doi: 10.1038/sj.onc.1205638. [DOI] [PubMed] [Google Scholar]
- 25.Aguilar Z, Akita RW, Finn RS, Ramos BL, Pegram MD, Kabbinavar FF, Pietras RJ, Pisacane P, Sliwkowski MX, Slamon DJ. Biologic effects of heregulin/neu differentiation factor on normal and malignant human breast and ovarian epithelial cells. Oncogene. 1999;18:6050–6062. doi: 10.1038/sj.onc.1202993. [DOI] [PubMed] [Google Scholar]
- 26.Bacus SS, Gudkov AV, Zelnick CR, Chin D, Stern R, Stancovski I, Peles E, Ben-Baruch N, Farbstein H, Lupu R. Neu differentiation factor (heregulin) induces expression of intercellular adhesion molecule 1: implications for mammary tumors. Cancer Res. 1993;53:5251–5261. [PubMed] [Google Scholar]
- 27.van der BB, Rutteman GR, Blankenstein MA, de Laat SW, van Zoelen EJ. Mitogenic stimulation of human breast cancer cells in a growth factor-defined medium: synergistic action of insulin and estrogen. J.Cell Physiol. 1988;134:101–108. doi: 10.1002/jcp.1041340112. [DOI] [PubMed] [Google Scholar]
- 28.Neve RM, Holbro T, Hynes NE. Distinct roles for phosphoinositide 3-kinase, mitogen-activated protein kinase and p38 MAPK in mediating cell cycle progression of breast cancer cells. Oncogene. 2002;21:4567–4576. doi: 10.1038/sj.onc.1205555. [DOI] [PubMed] [Google Scholar]
- 29.Jhabvala-Romero F, Evans A, Guo S, Denton M, Clinton GM. Herstatin inhibits heregulin-mediated breast cancer cell growth and overcomes tamoxifen resistance in breast cancer cells that overexpress HER-2. Oncogene. 2003;22:8178–8186. doi: 10.1038/sj.onc.1206912. [DOI] [PubMed] [Google Scholar]
- 30.Yen L, Cao Z, Wu X, Ingalla ER, Baron C, Young LJ, Gregg JP, Cardiff RD, Borowsky AD, Sweeney C, Carraway KL., III Loss of Nrdp1 enhances ErbB2/ErbB3-dependent breast tumor cell growth. Cancer Res. 2006;66:11279–11286. doi: 10.1158/0008-5472.CAN-06-2319. [DOI] [PubMed] [Google Scholar]
- 31.Peles E, Lamprecht R, Ben-Levy R, Tzahar E, Yarden Y. Regulated coupling of the Neu receptor to phosphatidylinositol 3′-kinase and its release by oncogenic activation. J.Biol.Chem. 1992;267:12266–12274. [PubMed] [Google Scholar]
- 32.Hui R, Finney GL, Carroll JS, Lee CS, Musgrove EA, Sutherland RL. Constitutive overexpression of cyclin D1 but not cyclin E confers acute resistance to antiestrogens in T-47D breast cancer cells. Cancer Res. 2002;62:6916–6923. [PubMed] [Google Scholar]
- 33.McIntosh GG, Anderson JJ, Milton I, Steward M, Parr AH, Thomas MD, Henry JA, Angus B, Lennard TW, Horne CH. Determination of the prognostic value of cyclin D1 overexpression in breast cancer. Oncogene. 1995;11:885–891. [PubMed] [Google Scholar]


